The Suppressive Activity of Water Mimosa Extract on Human Gastric Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction
2.3. Total Phenolic Content
2.4. MTT Assay
2.5. Morphological Identification Assay
2.6. Cell Migration Assay
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
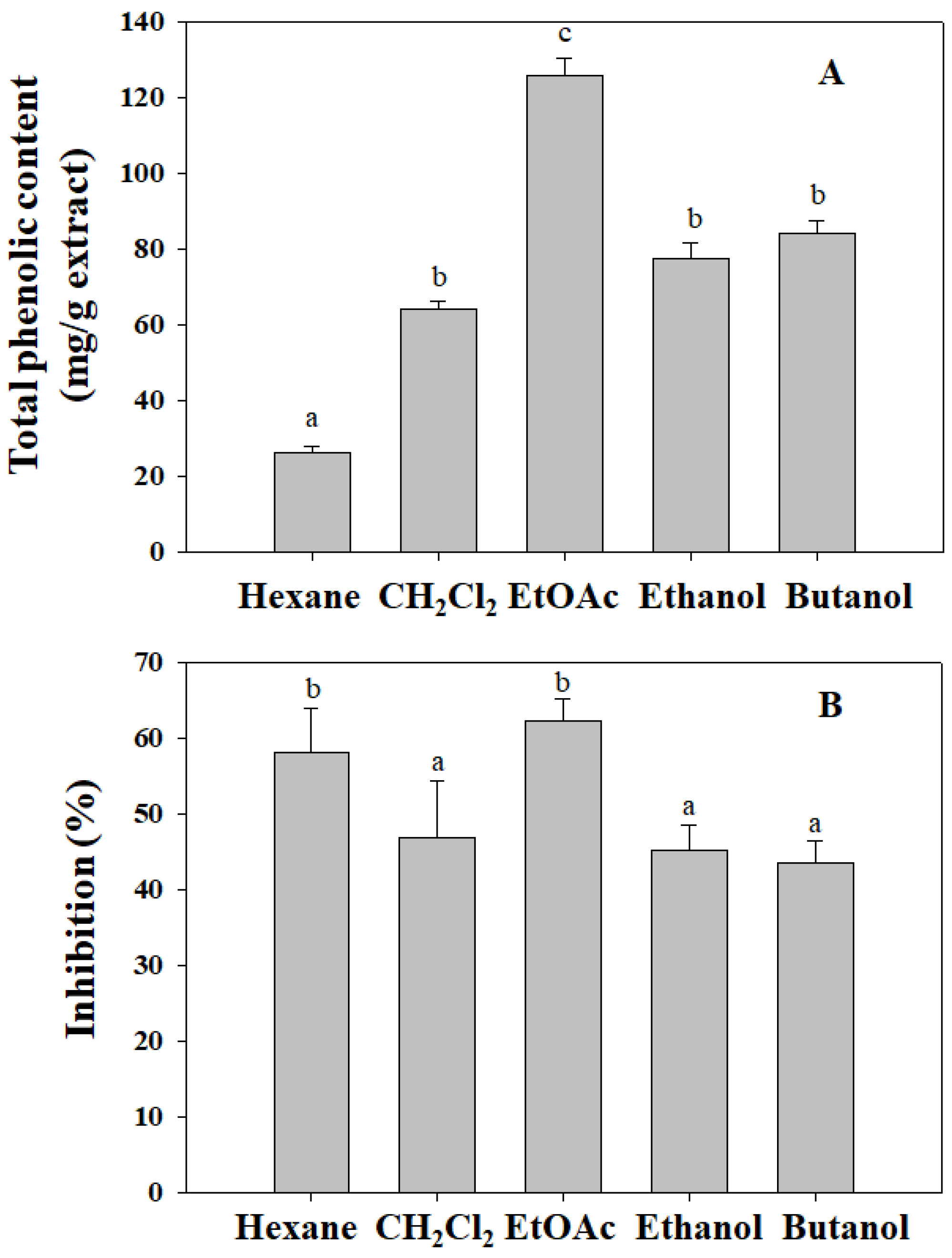
3.1. The TPC and Cell Proliferation Inhibition of N. oleracea Extracts
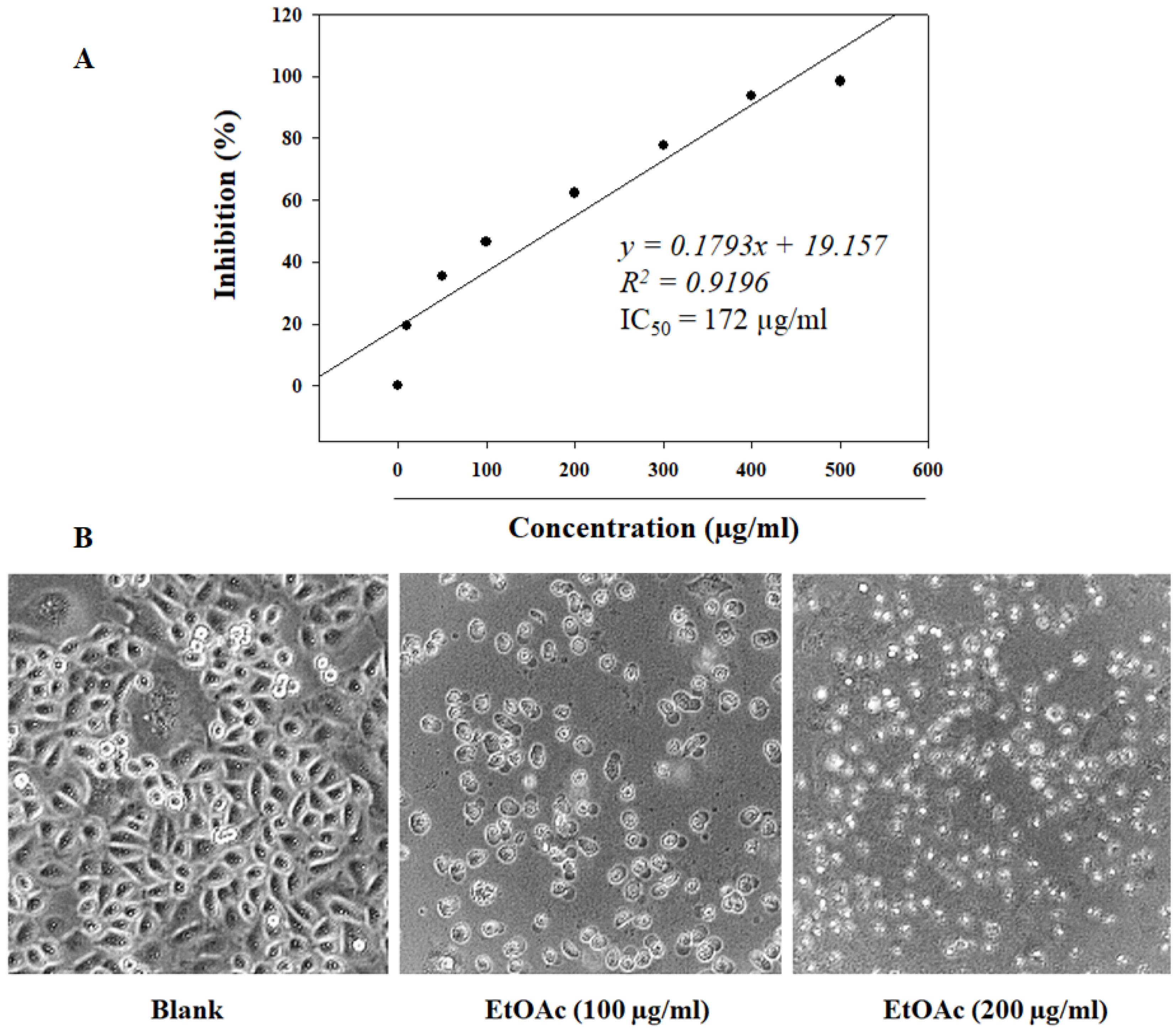
3.2. The Proliferation Inhibition of EtOAc Extract on BGC-823 Cells
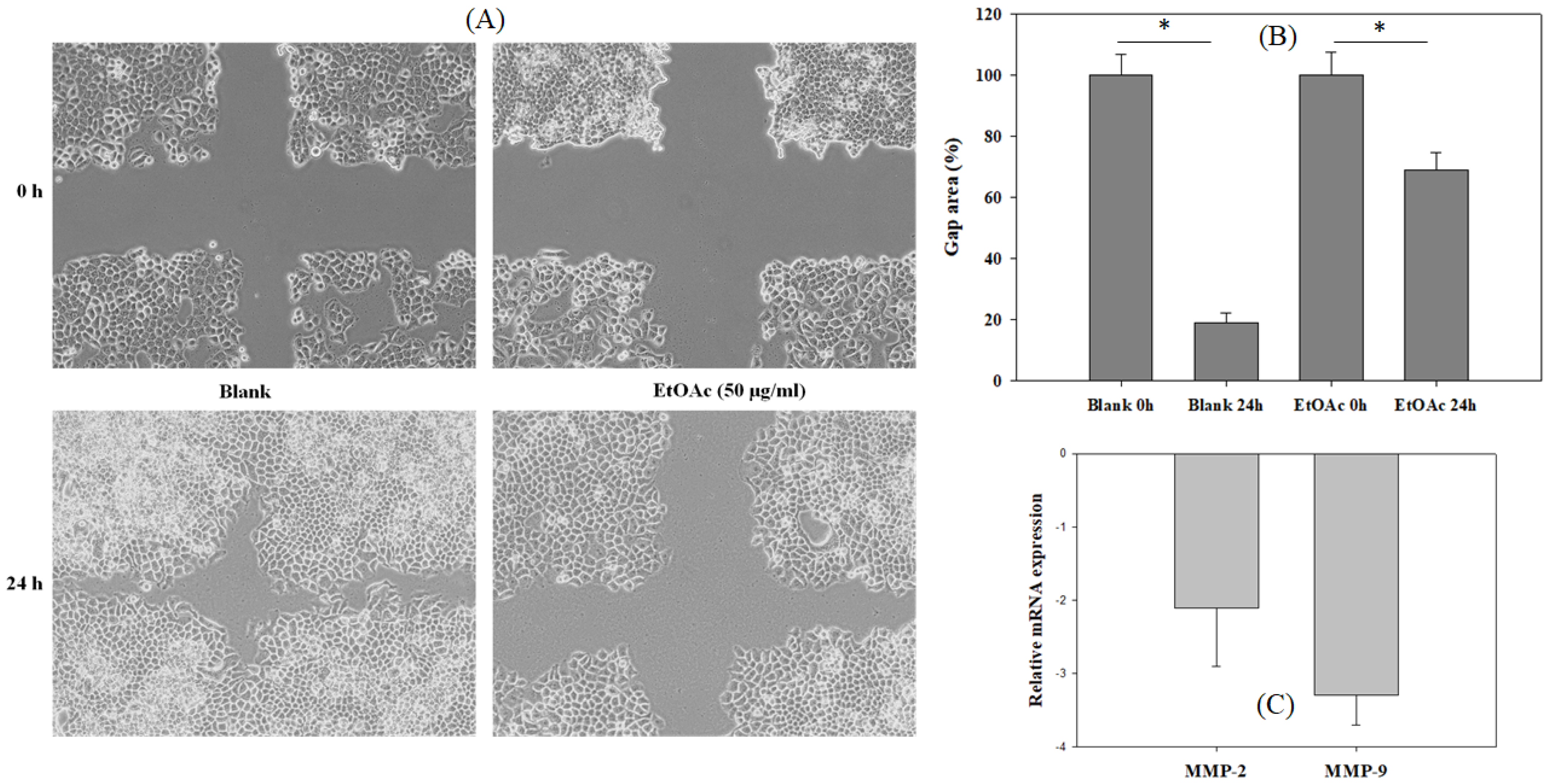
3.3. The Inhibition of EtOAc Extract on Cancer Cell Migration
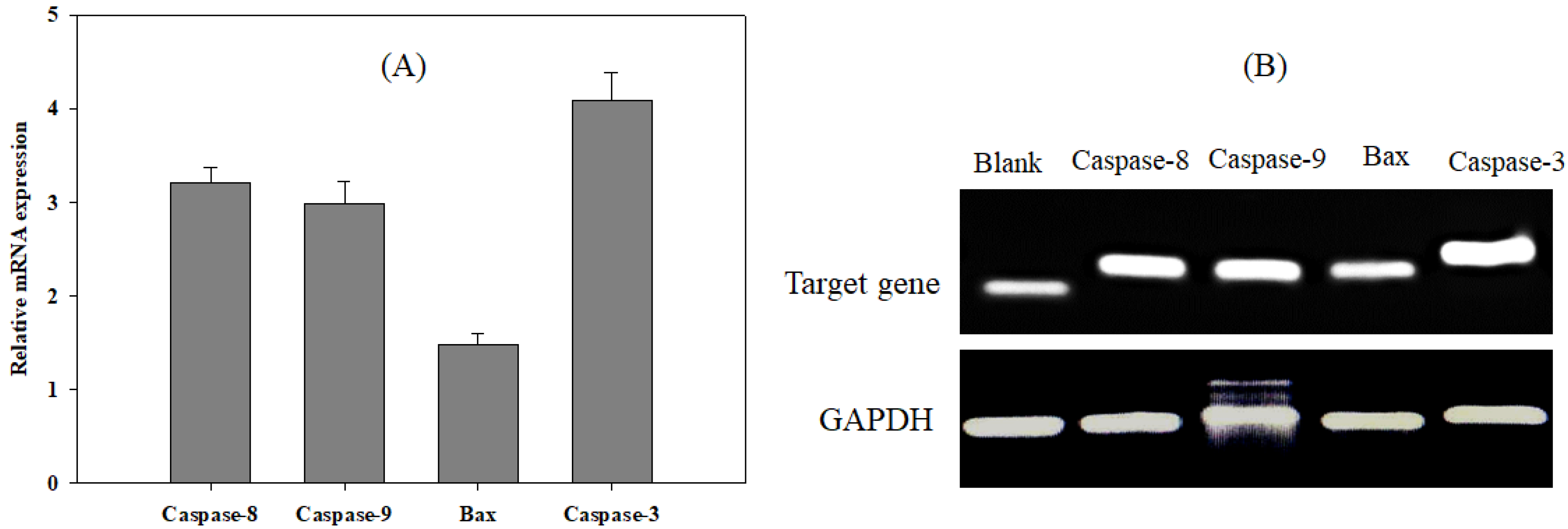
3.4. Effect of EtOAc Extract on Apoptotic Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, S.; Li, B.; Bai, Z.Z.; Wu, J.Q.; Xie, D.W.; Ma, Y.C.; Ma, X.X.; Zhao, J.H.; Guo, X.J. Clinical epidemiology of gastric cancer in Hehuang valley of China: A 10-year epidemiological study of gastric cancer. World J. Gastroenterol. 2014, 20, 10486–10494. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Shang, A.; Gan, R.Y.; Wu, D.T.; Atanasov, A.G.; Li, H.B. Phytochemicals for the prevention and treatment of gastric cancer: Effects and mechanisms. Int. J. Mol. Sci. 2020, 21, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izuishi, K.; Mori, H. Recent strategies for treating stage IV gastric cancer: Roles of palliative gastrectomy, chemotherapy, and radiotherapy. J. Gastrointestin Liver Dis. 2016, 25, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V.; La Vecchia, C. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Murakami, A.; Koshimizu, K.; Ohigashi, H. Identification of pheophorbide a and its related compounds as possible anti-tumor promoters in the leaves of Neptunia oleracea. Biosci. Biotechnol. Biochem. 1996, 60, 1028–1030. [Google Scholar] [CrossRef]
- Bhumireddy, A.; Nellore, K.; Alapati, K.S. Anticancer activity of Neptunia oleracea methanolic extracts. Nat. Prod. Res. 2020, 36, 1053–1057. [Google Scholar] [CrossRef]
- Das, H.B.; Majumdar, K.; Datta, B.; Ray, D. Ethnobotanical uses of some plants by Tripuri and Reang tribes of Tripura. Nat. Prod. Radiance 2009, 8, 172–180. [Google Scholar]
- Lee, S.Y.; Mediani, A.; Ismail, I.S.; Maulidiani; Abas, F. Antioxidants and α-glucosidase inhibitors from Neptunia oleracea fractions using 1H NMR-based metabolomics approach and UHPLC-MS/MS analysis. BMC Complement. Ther. Med. 2019, 19, 7. [Google Scholar] [CrossRef]
- Le, P.; Ngo, D.; Vo, T. Optimization of extraction conditions for achieving high content and antioxidant activities of the total phenolic compounds of Phu Quoc sim fruit (Rhodomyrtus tomentosa (aiton) hasak.). J. Med. Mater. 2018, 23, 157–166. [Google Scholar]
- Mazloum-Ardakani, M.; Barazesh, B.; Moshtaghioun, S.M.; Sheikhha, M.H. Designing and optimization of an electrochemical substitute for the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell viability assay. Sci. Rep. 2019, 9, 14966. [Google Scholar]
- Kwak, Y.; Ju, J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr. Res. Pract. 2015, 9, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motadi, L.R.; Choene, M.S.; Mthembu, N.N. Anticancer properties of Tulbaghia violacea regulate the expression of p53-dependent mechanisms in cancer cell lines. Sci. Rep. 2020, 10, 12924. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Shen, Y.; Li, Q.; Wang, Q.; Feng, L. Apoptosis and inhibition of proliferation of cancer cells induced by cordycepin. Oncol. Lett. 2015, 10, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in food: Cancer prevention and apoptosis induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Ghazanfari, T.; Yaraee, R.; Shams, J.; Rahmati, B.; Radjabian, T.; Hakimzadeh, H. Cytotoxic effect of four herbal medicines on gastric cancer (AGS) cell line. Food Agr. Immunol. 2013, 24, 1–7. [Google Scholar] [CrossRef]
- Han, H.; Chen, G.Z.; Zhou, S.K.; Xu, R.R.; Wu, C.L. In vitro anti-tumor activity in SGC-7901 human gastric cancer cells treated with dandelion extract. Trop. J. Pharm. Res. 2018, 17, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nair, M.G. Labdane diterpenes in Curcuma mangga rhizomes inhibit lipid peroxidation, cyclooxygenase enzymes and human tumour cell proliferation. Food Chem. 2011, 124, 527–532. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, Y.; Xiao, Q.; Yin, L.; Yang, L.; He, W. Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Sig. Transduct. Target Ther. 2019, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, J.; Bai, J.; Tong, R.; An, F.; Jiao, P.; He, L.; Zeng, D.; Long, E.; Yan, J.; et al. The application of natural products in cancer therapy by targeting apoptosis pathways. Curr. Drug Metab. 2018, 19, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Sandoghchian Shotorbani, S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar]
- Chen, Q.; Kang, J.; Fu, C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target. Ther. 2018, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The most competent plant-derived natural products for targeting apoptosis in cancer therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef]
- Wahle, K.W.; Brown, I.; Rotondo, D.; Heys, S.D. Plant phenolics in the prevention and treatment of cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51. [Google Scholar] [PubMed]
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449. [Google Scholar] [CrossRef] [PubMed]
- Devi, O.S.; Singh, T.N.; Singh, L.J.; Singh, T.P. Biological Importance and Phytochemistry of Neptunia oleracea Lour: A Mini Review. Asian J. Chem. 2021, 33, 2276–2280. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.Q.; Nguyen, H.N.M.; Ngo, D.-H.; Phan, P.-H.; Vo, T.S. The Suppressive Activity of Water Mimosa Extract on Human Gastric Cancer Cells. Appl. Sci. 2022, 12, 6817. https://doi.org/10.3390/app12136817
Nguyen TQ, Nguyen HNM, Ngo D-H, Phan P-H, Vo TS. The Suppressive Activity of Water Mimosa Extract on Human Gastric Cancer Cells. Applied Sciences. 2022; 12(13):6817. https://doi.org/10.3390/app12136817
Chicago/Turabian StyleNguyen, Thanh Quang, Hoang Nhat Minh Nguyen, Dai-Hung Ngo, Phuoc-Hien Phan, and Thanh Sang Vo. 2022. "The Suppressive Activity of Water Mimosa Extract on Human Gastric Cancer Cells" Applied Sciences 12, no. 13: 6817. https://doi.org/10.3390/app12136817
APA StyleNguyen, T. Q., Nguyen, H. N. M., Ngo, D.-H., Phan, P.-H., & Vo, T. S. (2022). The Suppressive Activity of Water Mimosa Extract on Human Gastric Cancer Cells. Applied Sciences, 12(13), 6817. https://doi.org/10.3390/app12136817





