Featured Application
This review can be used as a guide for comprehensive research linking polyphenols with antidiabetic activity and their application as functional food ingredients using nanotechnology.
Abstract
Background: Phenolic compounds are food-derived bioactive compounds well-known for their antioxidant and anti-inflammatory properties. They are in the spotlight for the management of diabetes due to their positive effects on glucose homeostasis. Materials and methods: We have performed a literature review on the main topics related to the application of phenolic compounds as functional food ingredients. This includes extraction and purification from vegetable sources and agro-industrial by-products, encapsulation to improve their solubility and bioavailability, and preclinical and clinical evidence linking these compounds with anti-diabetic activity. Objectives: (1) provide an understanding of the role of phenolic compounds on diabetes; (2) identify green technologies for phenolic compounds extraction from agri-food by-products following a biorefinery scheme; (3) underline the relevance of encapsulation techniques using nanotechnology to improve their bioavailability; (4) discuss the therapeutic efficacy of polyphenols. Results: This review compiles recent relevant research on phenolic compounds extraction from renewable resources, their purification from agri-food by-products, and encapsulation strategies using eco-friendly processes. It also highlights the preclinical and clinical evidence on phenolic compounds’ antidiabetic activity, giving insight into their mechanisms of action. Conclusions: This review explores the latest advances in polyphenols and how their benefits in glucose homeostasis can be applied toward improving the health of patients with diabetes and related conditions.
1. Introduction
According to the American Dietetic Association, “functional food is any modified food or food ingredient that may provide a health benefit beyond the traditional nutrients it contains” [1,2,3]. Functional foods present bioactive compounds with potential health advantages in preventing and controlling metabolic syndromes such as obesity and diabetes [4]. Over the past 20 years, phenolic compounds have been gathering attention as functional ingredients due to their benefits in lowering the risks of many chronic diseases related to oxidative stress, such as cardiovascular and neurogenerative diseases, cancer, and diabetes mellitus [5,6]. The clear evidence of the protective effects of phenolic compounds against diabetes has created new insights for developing polyphenol-rich functional foods and nutritional supplements [4,7].
Phenolic compounds are the most prominent family of antioxidant compounds in foods and constitute an essential part of the human diet [8]. These bioactive compounds represent a diverse group of secondary metabolites, commonly appearing in many foods such as fruits, vegetables, coffee, wine, tea, cocoa, or cereals [6]. With more than 8000 phenolic structure variants found in whole plant foods, phenolic compounds are subdivided into different groups based on the number of phenol rings and structural elements that bind these rings to one another, including phenolic acids, flavonoids, stilbenes, lignans, and polymeric lignans [9,10]. The phenolic acids are subdivided into two classes: hydroxybenzoic (e.g., gallic acid) and hydroxycinnamic (e.g., caffeic, ferulic, and coumaric acid) [10]. Flavonoids are flavones, flavanones, anthocyanidins, and flavanols. Stilbenes are present in low quantities in the human diet, the best-known compound being trans-resveratrol, primarily detected in grapes and red wine [10,11]. Tannins are a group of water-soluble phenolic compounds represented in two structural groups: gallotannins and ellagitannins of hydrolysable tannins [10]. A small group of phenolic compounds with two aromatic rings substituted with hydroxyls and linked by an aliphatic chain containing carbonyl groups is called diferuloylmethane, better known as curcumin in the dietary spice turmeric, which is widely used as a herbal remedy [10].
Phenolic compounds present several beneficial effects on blood glucose management in diabetes conditions (Figure 1). Indeed, these phenolic compounds have hypoglycemic effects such as lowering the intestinal absorption of dietary carbohydrates, modulation of enzymes involved in glucose metabolism, enhancement of β-cell function and insulin action, insulin secretion, and antioxidative and anti-inflammatory properties [11].
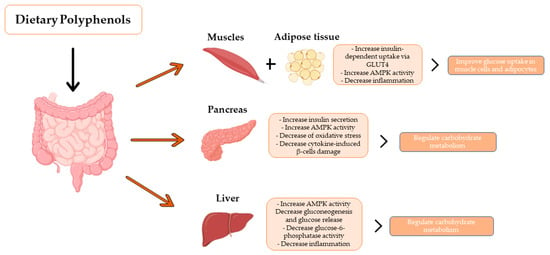
Figure 1.
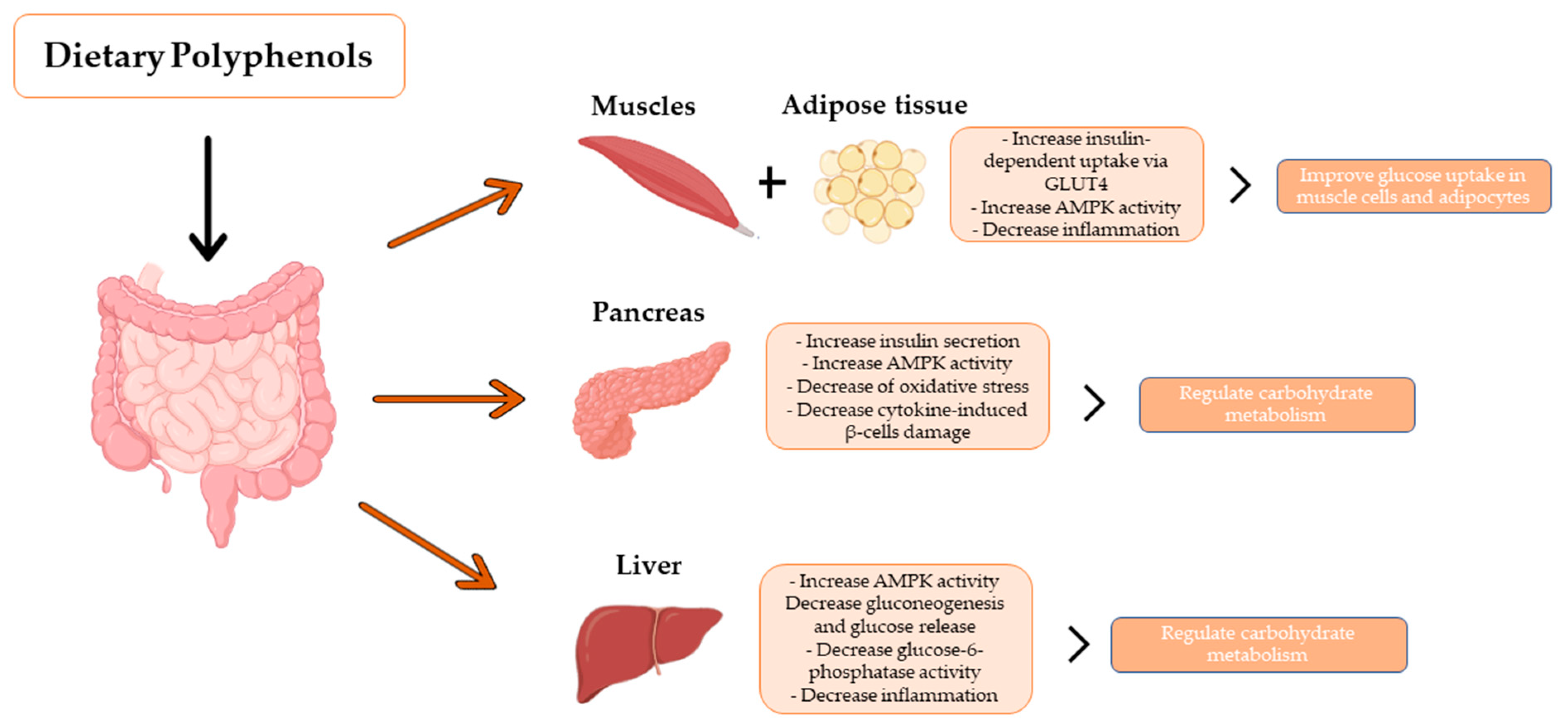
Beneficial effects of phenolic compounds on managing blood glucose in diabetes. The hypoglycemic effects of phenolic compounds are mainly attributed to reducing intestinal absorption of dietary carbohydrates, modulation of the enzymes involved in glucose metabolism, improvement of β-cell function and insulin action, and stimulation of insulin secretion (Adapted from [11,12]).
Phenolic compounds have potent antioxidant activity by endogenous and exogenous mechanisms that complement and add to the role of antioxidant vitamins and enzymes as a defence against oxidative stress induced by excessive production of reactive oxygen species (ROS) [9,10]. In diabetes, oxidative stress leads to the development and progression of micro and macrovascular complications [11]. High free radical production and a disabled antioxidant defence system promote an oxidant/antioxidant imbalance that can lead to long-term diabetic complications [13]. The inhibition of intracellular free radical formation provides a therapeutic strategy to prevent oxidative stress, and such pathological conditions [13]. Phenolic compounds inhibit ROS production by inactivating their producing enzymes. Examples are xanthine oxidase, cyclooxygenase, lipoxygenase, microsomal monooxygenase, glutathione-S-transferase, mitochondrial succinoxidase, and NADH oxidase [14]. On the other hand, some phenolic compounds such as green tea catechins decrease lipid peroxidation and increase plasma total antioxidant capacity [15], being their long-term administration advantageous against lipid and glucose metabolism disorders implicated in type II diabetes and minimising the risk of cardiovascular diseases [11,15].
In addition, studies have shown that phenolic compounds attenuate postprandial blood glucose response, making phenolic compounds promising compounds to tackle type II diabetes [16]. The hypoglycemic effects of phenolic compounds are mainly due to a reduction in the intestinal absorption of dietary carbohydrates, modulating the activity of enzymes involved in the glucose metabolism and improving the α-cell function and insulin action, stimulating insulin secretion, and the antioxidative and anti-inflammatory properties of these components [11]. Indeed, anthocyanins have been proven to act in carbohydrate metabolism as inhibitors of α-glucosidase and α-amylases activity, key enzymes for the digestion of dietary carbohydrates to glucose [17]. They can also inhibit enzymes that reduce blood glucose levels after starch-rich meals, making anthocyanins potential compounds for controlling type II diabetes [17,18]. Some studies have shown that compounds such as resveratrol can also regulate postprandial glycemia and avoid the development of glucose intolerance by improving the secretion of the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like polypeptide-1 (GLP-1), potentially used as antidiabetic agents [19]. Moreover, phenolic compounds may also affect peripheral glucose uptake. For example, quercetin, resveratrol, and epigallocatechin gallate (EGCG), one of the most abundant catechins in green tea, have been shown to improve insulin-dependent glucose uptake in muscle cells and adipocytes by translocation of glucose transporter, GLUT4 to the plasma membrane, induced by the AMP-activated protein kinase (AMPK) pathway [20].
Studies support that the disruption of gut microbiota equilibrium (dysbiosis) can be caused by age, diet, lack of exercise, stress, and drug consumption, associated with gastrointestinal and extra-intestinal cardiometabolic diseases, such as obesity and diabetes [21]. Furthermore, there is evidence that phenolic compounds can induce modulation of gene expression and the gut microbiota balance, which might have a protective effect on the gastrointestinal system [22]. More precisely, they can modulate the gut microbiota composition and function, impacting gut metabolism and immunity and fulfilling anti-inflammatory properties [21]. Growing evidence supports the involvement of gut microbiota dysbiosis in obesity and diabetes, and how dietary polyphenols have the ability to modulate the gut microbiota composition and function and interfere with metabolic pathways, bacterial quorum sensing, membrane permeability, and others [21,23]. The complex mechanisms underlying the interactions between the gut microbiota and functional food components, and their consequences on human health are still poorly understood and deserve further clarification.
Also, nutritional strategies tackling diabetes require further research, focusing on the bioavailability and bioactivity of phenolic compounds or functional foods with promising antidiabetic effects [4]. These knowledge gaps hinder the development of new specialised food products to tackle diabetes since the protection of the bioactive compounds is relevant to achieving their target site while maintaining the food product’s quality and pleasure [24]. For this reason, the functional foods market for patients with type II diabetes is still scarce. However, developing foods containing antidiabetic compounds may be a promising alternative for controlling blood sugar, reducing the risk of type II diabetes complications, and improving patients’ quality of life [25,26].
In order to prevent the degradation of these labile compounds when fortifying foods and to improve their bioavailability by increasing the solubility at the sites of absorption, it is necessary to develop encapsulation strategies for phenolic compounds. For this purpose, it is key to know the green technologies available for the extraction of these bioactive compounds from foods and by-products containing high concentrations and underutilized at present. However, it is of great relevance to understand the phenolic compounds’ antidiabetic effects and their mechanisms, so that effective encapsulation strategies can be developed. This review summarizes the most recent relevant research covering these topics: from phenolic extraction to their application as bioactive compounds for the management of diabetes and related disorders.
2. Phenolic Compounds Extraction from Renewable Resources within a Biorefinery Scheme
Environmental concerns over fossil fuel dependence and increasing demand for energy and chemicals require an urgent change in the economic production model [27]. Consequently, European Union policies highly encourage the search for renewable resources for sustainable development that contributes to a transition from a linear economy to a circular economy [28]. In this framework, residues are considered sources of new raw materials able to be reincorporated into the productive system, allowing the achievement of equilibrium between the economy, environment, and society [29].
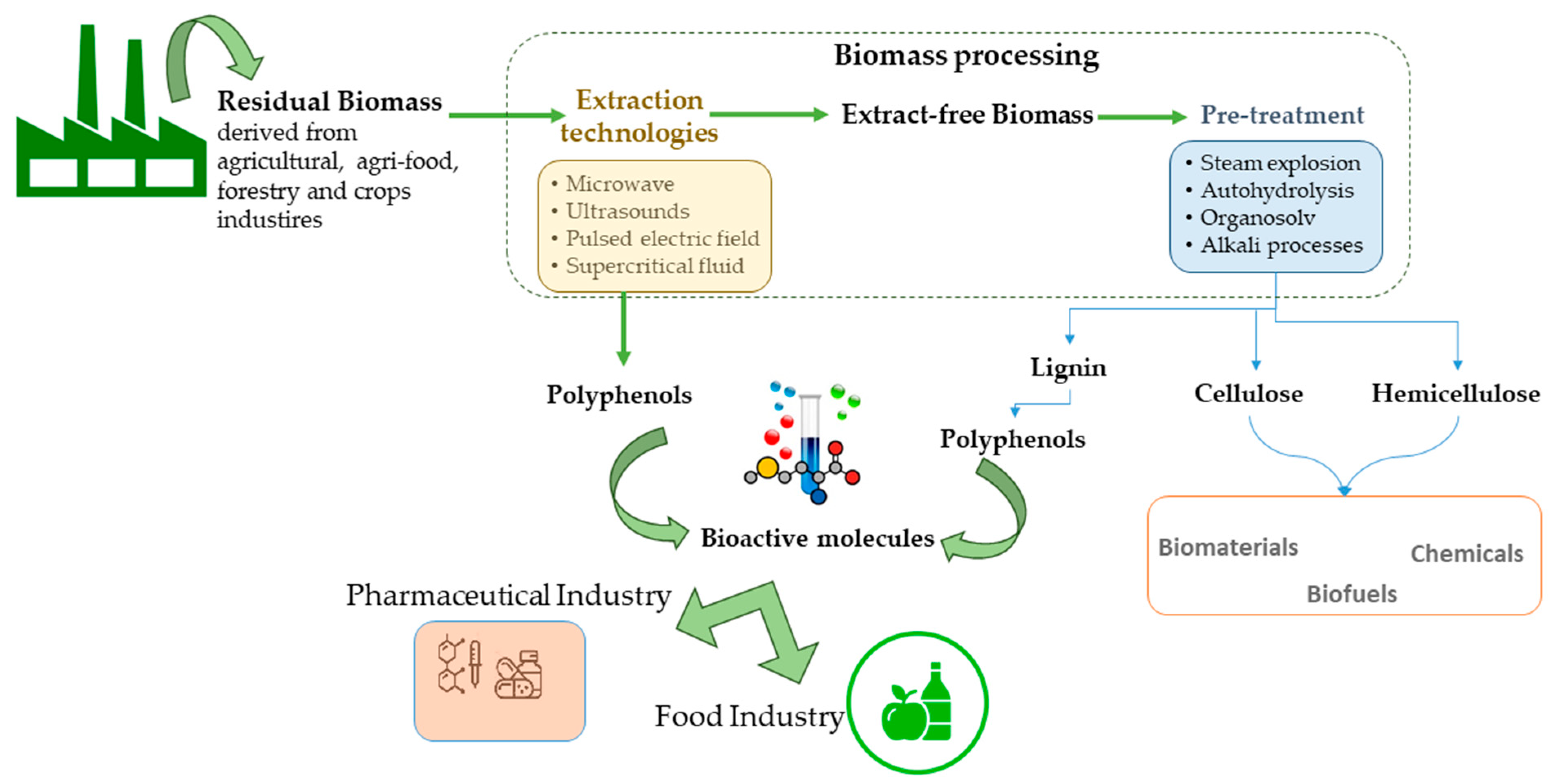
In this context, sectors such as the agriculture and agri-food industries are considered the highest producers of wastes and/or by-products worldwide. These residual biomasses are usually under-valorised and discarded, causing environmental damage. Nevertheless, it is known that wastes generated in these industries (such as citrus processing, winemaking, olive oil production, and seaweed hydrocolloid industry) are important sources of a wide range of bioactive compounds such as phenolic compounds. The extraction of phenolic compounds within a biorefinery scheme is the desired formula to obtain a broad spectrum of marketable products and to attain an integral valorisation of these residues (Figure 2). For the feasibility of biorefineries based on agri-food residues, one first stage of extraction of these biomolecules with pharmaceutical and/or food applications is a priority, followed by the manufacturing of biofuels, materials of chemicals. In addition, this cascading biomass processing has to fulfill green chemistry principles to satisfy the increasing demand for environmentally friendly and safe processes [30]. Moreover, this first step of biorefinery aims to meet requirements such as a cost-effective, versatile and straightforward process for extracting a wide range of compounds [31]. Therefore, the extraction of phenolic compounds from wastes implies a double benefit: reducing costs and environmental problems related to waste management and, on the other hand, improving the feasibility of biorefineries and increasing the value-added compounds that can be obtained [32].
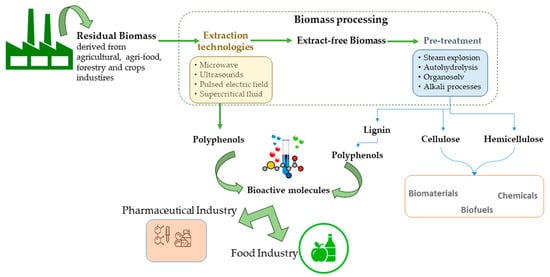
Figure 2.
Polyphenolic compound extraction from residual biomass within a biorefinery scheme.
Generally, lignocellulosic-based wastes can include agri-food and forestry residues, dedicated crops, and short rotation crops. Lignocellulosic biomasses are composed of non-structural components (protein, ashes, extractives) and structural components (mainly cellulose, hemicellulose, and lignin). Residues from food and agricultural industries usually include peels, seeds, hulls, and shells rich in non-structural components (such as phenolic compounds). Traditionally, biomass processing includes physical (extrusion, milling), physicochemical (steam explosion, AFEX), and chemical (e.g., alkali, autohydrolysis, organosolv, acid) treatments. Nevertheless, harsh conditions are necessary for breaking down the recalcitrant lignocellulosic structure to improve the enzymatic access to cellulose. This approach can hinder the integral valorisation of lignocellulosic biomass since more susceptible fractions (such as hemicellulose and extractives) can be degraded. In this context, milder conditions are employed first to extract thermolabile biomolecules, such as some phenolic compounds present in non-structural components of residual biomass [33].
2.1. Techniques for Phenolic Compounds Extraction from Agri-Food By-Products
In this context, literature collects many research studies that have evaluated the extraction processes to obtain phenolic compounds and characterised their bioactivities (namely, antioxidant and antibacterial features) [34]. This antioxidant activity is related to a broad spectrum of health effects, including anti-allergenic, cardioprotective, antiatherogenic, antimutagenic, and anti-inflammatory [35]. Organic solvents (such as ethanol) are usually employed for phenolic compound extraction, achieving attractive yields. For instance, 32% of the extract was recovered from eucalyptus leaves (T = 50 °C; time = 225 min and ethanol-water = 56%), containing 92 mg/g of total phenolic compounds and 53 mg/g of total flavonoid compounds [31]. Currently, significant efforts have been devoted to developing suitable methods of biomolecule extraction from agri-food wastes focusing on alternatives to the conventional extraction processes. These traditional solvents (such as methanol, acetone, or hexane) present several disadvantages such as toxicity, volatility, and flammability, which limit their application. Therefore, emerging technologies (such as microwave-assisted, ultrasound-assisted, pulsed electric field, and ohmic heating) have been combined with alternative solvents to enhance the extraction of biomolecules from renewable resources. Recently, the use of smart solvents as deep eutectic solvents (DES) for the recovery of bioactive compounds from agri-food residues has been reviewed by Gullón and coworkers [36]. These avant-garde solvents have attracted great expectations due to their physicochemical features, low toxicity, and biodegradability. Moreover, DES combined with innovative techniques allows successful isolation of target biomolecules compared to conventional techniques of extraction. Therefore, techniques for phenolic compound extraction can be classified into: (i) conventional methods such as maceration, liquid-liquid, and solid-liquid extraction, which have been the most used for many years since they have the advantage of being relatively simple and effective with a broad tailoring window in the extraction of phenolic compounds at a low cost, (ii) non-conventional methods, developed to increase the yield of polyphenol extraction. These methods focus on using fewer organic solvents or alternative ones and improve the selectivity of phenolic compounds [37,38,39,40].
Some of the most used non-conventional methods (not an exhaustive list) are ultrasound-assisted extraction (UAE). This method uses ultrasound energy to increase the mass transfer through the cavitation mechanism. As a result, fragmentation, erosion, and pore formation occur in the cellular matrix, thus favouring interactions between the analytes and the solvent [37,41,42,43]. The microwave-assisted extraction (MAE) is based on electromagnetic radiation to quickly rupture the cell membrane due to the increased temperature caused by the radiation [41,44]. Finally, supercritical fluid extraction (SFE) is based on the use of fluids that can exceed their specific critical pressure, such as CO2. This characteristic allows these fluids to have properties of both liquids and gases, such as lower surface tension and high diffusivity. These properties allow supercritical fluids (SF) to spread easily and penetrate faster within the solid matrix resulting in an efficient and highly selectivity extraction [38,45,46,47,48]. A summary of the advantages, drawbacks and their application to extract phenolic compounds from by-products is shown in Table 1.

Table 1.
Green methods for polyphenol extraction from agri-food by-products and wastes.
While these non-conventional methods have shown excellent results, the use of non-organic solvents is still required most of the time. However, they are required in lower volumes usually. The growing interest (and need) to improve these methods and reduce the use of organic solvents furthermore has led to investigating the use of safer and or natural alternatives, such as the following.
2.1.1. Natural Deep Eutectic Solvents
In recent years ionic liquids (IL) and deep eutectic solvents (DES) have been revisited for their capacity to replace organic solvents in the extraction of natural compounds. Choi et al. (2011) [49] hypothesised that some metabolites that occur naturally in cells could act as a third type of liquid. When studied, these metabolites showed similar properties to synthetic IL and DES; these metabolites are referred to as natural deep eutectic solvents (NADES). NADES are formed by mixing two or three hydrogen bond donor metabolites such as amino acids, choline, malic, citric acid, and lactic acid with organic salts that act as hydrogen bond acceptors. This combination results in stable intramolecular hydrogen bonds, and the mix can contain up to 50% water (v/v). NADES’ numerous structural variations and adjustable physicochemical properties such as viscosity, surface tension, and polarity offer a vast opportunity for their use in extracting phenolic compounds [50,51,52]. NADES are non-toxic, biodegradable, sustainable, low cost, and reduce oxidative degradation, making them a good and green alternative to conventional and some non-conventional methods [39,40,50]. Moreover, NADES, jointly with other non-conventional methods, has been studied to extract phenolic compounds from by-products. A recent study by Popovic et al. (2022) [53] on the extraction of anthocyanins from cherry pomace showed that NADES in conjunction with ultra-fast MAE was 62% more efficient than with the conventional solvent (50% EtOH). Another study on grape skins showed that NADES in conjunction with UAE proved to be more effective (47%) than methanol in extracting phenolic compounds and anthocyanins [54]. However, these authors agree that further studies are needed to optimise NADES in the extraction according to the target phenolic compounds, scale up the method, and recover and or recycle NADES after extraction [55,56,57].
2.1.2. Surfactant Extraction and Colloidal Gas Aphrons
Surfactants are amphiphilic molecules with both hydrophobic and hydrophilic components with the capacity to form micelles when the concentration is at or above the critical micelle concentration. The formed micelles can aid in the separation and extraction by establishing chemical and physical interactions with hydrophilic and hydrophobic substances demonstrating the capacity to interact and/or self-associate with phenolic compounds facilitating their separation [55,56,57]. In a study conducted by Sharma, Kori, and Parmar (2015) [58], the authors analysed the capacity of surfactants to extract phenolic compounds from different juices; the results showed that surfactants extracted up to 35% more compared to ethanol. Even though the extraction with surfactants was successful, the authors emphasised the need for further research on the interactional behaviour and the scaling-up process.
Another approach studied for surfactant-based extraction is the use of colloidal gas aphrons (CGA). These are surfactant-stabilised microbubbles (10–100 μm) generated by the intense stirring of a surfactant solution at high speeds (>8000 rpm). Due to their unique structure, CGA presents relevant properties such as molecule adsorbance to the air-water interface of the microbubbles by electrostatic and/or hydrophobic interactions with the surfactant giving them selectivity in the separation. The small bubble size gives place to a larger interfacial area per unit volume and thus a larger capacity of molecule adsorption; they can also be easily separated from the bulk liquid without mechanical aid due to their buoyancy [59,60,61].
Moreover, CGA is an easily scalable, controllable, and cost-effective process since it is easy to pump from one point to another [62]. CGA have been used to separate phenolic compounds from artichokes residues using Tween 20 and cetyl trimethyl ammonium bromide (CTAB), showing that the first exhibited the highest recovery of phenolic compounds [63]. Another study demonstrated the use of Tween 20 to be efficient in the extraction of phenolic compounds from grape pomace, with a particular affinity for anthocyanins in addition to improving phenolic compound solubility, thus possibly their bioavailability and absorption [64]. Further studies are needed in this field to understand better the interactions between the surfactants and the phenolic compounds, their stability as a system, and their possible application in food, cosmetics, and/or medicinal products.
2.1.3. Enzyme Assisted Extraction (EAE)
The use of enzymes in the agri-food sector is not something new; however, they are an option for extracting phenolic compounds since they can rupture the cell wall, thus increasing the analyte extraction. Pectinolytic enzymes are the most widely used to increase juice yield, especially in the juice industry. For their use in extracting phenolic compounds, parameters such as pH, time, temperature, and enzyme concentration need to be optimised for each substrate and process. One of the main drawbacks of EAE is the high cost of enzymes, which can represent a limitation for their implementation at the industrial level [65,66,67].
Studies on the effect of enzymes in the extraction of phenolic compounds from grape marc have shown that tannase and pectinase increase the phenolic compounds′ content and antioxidant activity; however, cellulase did not show any release of phenolic compounds, thus no improvement in the antioxidant capacity [68]. In a recent study [69], enzymatic extraction was applied to obtain non-extractable phenolic compounds (NEP) from sweet cherry pomace. Their results showed that enzymes positively affected the extraction of proanthocyanidin and phenolic compounds with high molecular weight. In addition, the enzyme extraction showed to extract up to four times more phenolic compounds than conventional methods.
The emerging use of natural solvents and non-organic solvents used alone or in conjunction with non-conventional methods is moving forward in safer and sustainable processes, revealing promising results for the extraction of phenolic compounds. Although further research is needed to understand better their mechanisms, advantages, limitations, and industrial success, their implementation will certainly lead to more eco-friendly and effective extraction of phenolic compounds from by-products.
2.2. Co-Production of Phenolic Compounds within a Biorefinery Scheme
Recently, some authors have proposed sequential processes for biomass fractionation to obtain more than one product, such as biofuels, phenolic compounds, and oligosaccharides. For instance, aqueous solutions or hydrothermal treatment (severity of 3.08) were also employed to co-produce oligosaccharides with potential use as prebiotics and antioxidant compounds from chestnut shells [32]. In this study, the hemicellulosic hydrolysates were composed of phenolic and flavonoid compounds (13.2% of pyrogallol derived from lignin and extractives). On the other hand, a cascading biorefinery scheme was proposed by del Río and co-workers [30] for the full use of Sargassum muticum, where liquid and solid biofuels were produced after suitable extraction of bioactive molecules. The proposed extraction methods (microwave hydro diffusion and gravity followed by autohydrolysis) recovered an extract containing 85% of fucoidan as fucooligosaccharides with increased phloroglucinol and Trolox equivalent antioxidant capacity of 9.73 g/100 g of extract and 31.1 g/100 g of extract, respectively. In addition, extract-free S. muticum was employed to produce 15.6 g/L of bioethanol and remained solid after fermentation, providing a calorific power of 10 MJ/kg. Alternatively, microwave hydrothermal treatment at a severity of 3.41 was employed for the extraction of oligomer and phenolic compounds (2.85 g/L of TE via ABTS assay) and bioethanol production (20.5 g/L) from pretreated S. muticum [71].
Furthermore, olive agro-industrial wastes have been extensively evaluated as sources of bioactive compounds. Olive tree (Olea europaea) pruning and olive mill leaves were submitted to different extraction processes to obtain total phenolic and flavonoid contents with antioxidant activity being the most abundant benzaldehyde, followed by p-vinylguaiacol and o-guaiacol [32]. Pinus by-products as a source of bioactive phenolic compounds using green and sustainable valorisation techniques were recently reviewed by Ferreira-Santos and collaborators [72]. They included emerging technologies of extraction (ultrasounds, supercritical fluids, pressurised liquids, and electric fields) to obtain bioactive compounds within a biorefinery concept [73]. Avocado waste can also be processed to obtain bioenergy and bioproducts using a single or integrated process [74]. Avocado industrial residues were also evaluated for the production of oligosaccharides and polyphenolics, obtaining 3.48 g gallic acid equivalents/100 g and 10.80 g Trolox equivalents/100 g of residue, using autohydrolysis treatment at 150 °C [75]. Ultrasound treatment was also employed for bioactive molecule extraction from avocado peels under optimised conditions (ethanol 38.46%, 44.06 min and 50 °C), leading to an extract mainly composed of hydroxybenzoic and hydroxycinnamic acids and flavonoids, such as flavanols, flavanonols, flavones, flavanones and chalcone, phenylethanoids and lignans [76].
Alternative to chemical extraction, biotechnological production of phenolic acids has attracted significant interest [77]. Recently, a high resveratrol concentration (151 mg/L) was obtained by simultaneous saccharification and fermentation of hydrothermally treated eucalyptus wood using a recombinant and thermotolerant Saccharomyces cerevisiae strain [78].
3. Encapsulation Techniques for Improving Phenolic Compounds Functionality
The stability and functionality of phenolic compounds can be disrupted by chemical or structural modifications caused by food processing and storage conditions, such as light, temperature, pH, oxygen, or interactions with other food constituents, reducing their bioavailability and bioactivity in the target tissues [79,80].
Minimising the drawbacks of using phenolic compounds (such as bitterness), augmenting the antioxidant stability, and improving the phenolic bioavailability are essential to preserve their functionality and allow their commercialisation as food products to tackle chronic diseases such as diabetes [81,82]. Phenolic compounds can be stabilised through different techniques, including chemical reactions and non-covalent complex formation [83]. In addition to stabilisation strategies, much research has been carried out on the encapsulation of phenolic compounds to prevent unwanted degradation and improve their bioavailability by increasing the solubility at the sites of absorption [84,85]. In particular, nano-encapsulation of phenolic compounds can protect them from chemical degradation that can lead to low absorption [86,87]. Additionally, the encapsulation of phenolics offers other benefits, such as masking their unpleasant taste and protecting them against processing conditions and modifications during storage [79].
There are several methods of encapsulation that can be applied to natural phenolic compounds [88]. However, choosing the best method requires consideration of the chemical structure of phenolic compounds, their solubility, compatibility with coating/encapsulating agents, and their thermophysical stability, among others [88]. Considering that the final products are for human consumption, all encapsulation methods must be performed using both food-grade solvents and wall materials without documented toxicity [89]. These technologies include liposome and micelle entrapment, coacervation, spray-drying, inclusion complexation, freeze-drying, yeast encapsulation, emulsion, and co-crystallisation [89,90]. The spray drying technique is one of the most popular encapsulation techniques in the food and pharmaceutical industries due to its simplicity and low cost [91,92,93]. This encapsulation technique involves the formation of microcapsules in which a mixture of phenolic compounds is formulated with carriers in solution or suspension and then atomised in a hot air stream to obtain a dry powder [94,95]. However, in the case of heat-sensitive bioactive compounds, the process conditions must be selected with extreme care. Typical wall materials that have been applied for spray drying are polysaccharides (maltodextrins, cyclodextrins, and gum arabic), proteins (soybean proteins, whey proteins, and sodium caseinate), chitosan, gelatin, and modified starch [96]. This technology is a valuable method for encapsulating antioxidants, including resveratrol, quercetin, flavonoids, or tannic acid [93].
Encapsulated Phenolic Compounds as Antidiabetic Agents
Several phenolic compounds have been encapsulated to improve their suitability as a tool in managing diabetes and CVDs. The following paragraphs provide a summary of the strategies followed and the main outcomes of each approach. Table 2 summarizes the materials and encapsulation strategies that have been proven effective for the encapsulation of some of the most studied.

Table 2.
Encapsulation technologies for different phenolic compounds and their positive effects observed in in vitro/in vivo studies.
Curcumin, long known for its medicinal uses, is a polyphenol derived from turmeric (Curcuma longa) and belongs to the subgroup of curcuminoids [107]. This polyphenol shows anti-inflammatory, antioxidant, anti-cancer, and hypolipidemic effects [107,108]. Due to its low hydrophilicity, nano-encapsulation is an excellent solution to enhance its solubility [109]. There are reports of various encapsulation approaches to the use of curcumin to treat diabetes and related diseases [108,109]. A good example is an encapsulation of curcumin in poly(gamma-benzyl-1-glutamate)-poly(ethyleneglycol)-poly(gamma-benzylglutamate) nanoparticles prepared by ring-opening polymerisation, which enhanced its bioactivity and water solubility [97]. This nanosystem demonstrated a potent effect in attenuating diabetic cardiomyopathy, which might lead to heart failure and myocardial ischaemia [97], by allowing curcumin to cross-regulate receptors responsible for calcium-sensing (CaSR) and the endogenous cystathionine γ-lyase/hydrogen sulphide system (CSE/H2S) [97]. Furthermore, a formulation of curcumin encapsulated in polybutylcyanoacrylate-based nanoparticles, prepared by anionic emulsion polymerisation, has been used to treat diabetic peripheral neuropathy, which usually occurs as a consequence of type II diabetes and leads to neuropathy [98]. Oral ingestion of this curcumin nanoformulation significantly lowered plasma serum levels of total cholesterol, C reactive protein, interleukin-6, and triglycerides, and increased HDL cholesterol [98]. In addition, the curcumin-loaded nanoformulation effectively alleviated diabetic neuropathy-induced pain in rats [98]. In another in vivo study, curcumin nanoparticles (CNPs), prepared by a re-precipitation method, were embedded into a thermosensitive hydrogel containing matrix metalloproteinase 9 (MMP9) to provide a safe and efficient system to promote skin wound healing in diabetic patients [99]. It has been demonstrated that such a structure can induce controlled drug release at the wound bed and promote efficacy in healing skin wounds in diabetic rat models by decreasing ROS and promoting cell migration to restore normal skin structures and function [99].
Berberine, an essential benzylisoquinoline alkaloid from Coptis chinensis, has been used to treat diabetes, cancer, hyperlipidaemia, and cognitive heart failure [109,110]. Nanoformulations of berberine have been demonstrated to ameliorate its bioavailability. A nanoformulation of berberine encapsulated in polyethylene glycol-co-lactic acid-glycolic acid (PEG-PLGA) nanoparticles, designed to be administered orally, was successfully tested in vitro to reduce intracellular levels of proprotein convertase subtilisin/kexin type 9 (PCSK9) in hepatocytes, which contributes to lower plasma LDL-cholesterol levels [101].
Naringenin is a type of flavone found primarily in citrus fruits and vegetables with potential antioxidant, blood-sugar-lowering, anti-inflammatory, and anti-cancer properties [111]. Naringenin encapsulated in chitosan-coated alginate core-shell nanoparticles, ranging in size from 158 to 300 nm, improved the solubility of the flavonoid. The nanoformulation had excellent entrapment efficiency (90%) and allowed controlled release. In an in vivo assay, these naringenin nanoparticles showed a high anti-diabetic effect with low toxicity when administered orally to diabetic rat models [100]. Proanthocyanidins are flavonoids found in many plants such as cranberries, blueberries, and grape seeds and have various pharmacological properties such as antioxidant, anticancer, antidiabetic, neuroprotective, and antimicrobial activities [112,113]. A cinnamon extract rich in proanthocyanidins was encapsulated by complex coacervation combining gelatin and five different polysaccharides (gum arabic, pectin, cashew gum, carboxymethylcellulose, and K-carrageenan) [102]. The complex coacervation method successfully produced microparticles of cinnamon extract that exhibited high encapsulation efficiency and encapsulation yield [102]. Additionally, this encapsulation method protected the extract components, improving the antioxidant capacity and inhibiting the digestive enzymes α-amylase and α-glycosidase [102].
Quercetin is a flavonoid found in citrus fruits and tea with antioxidant and anti-inflammatory properties [114]. It was reported to lower blood pressure, insulin resistance, and cholesterol levels [114]. In an in vivo anti-diabetic study, focused on improving the therapeutic profile of quercetin as an anti-diabetic drug, quercetin was effectively encapsulated in succinyl-chitosan core-shell-corona nanoparticles produced by ionic cross-linking [103]. Results obtained with these biocompatible and biodegradable nanoparticles showed that the nanoformulation enabled pH-sensitive controlled release of quercetin with no detected toxicity [103]. Furthermore, in diabetic rat models, after oral administration of free quercetin and these quercetin nanoparticles, encapsulated quercetin was found to have a significantly stronger hypoglycaemic effect and effective maintenance of glucose homeostasis compared to free quercetin [103]. In a similar study, PLGA nanoparticles loaded with quercetin were prepared by the emulsion-diffusion-evaporation method and characterised with a drug entrapment efficiency higher than 86% [104]. This report showed that encapsulation in PLGA nanocapsules improved the oral bioavailability of quercetin (523% increase) compared to a free quercetin suspension, allowing diabetic rats to be dosed every five days instead of daily to achieve a similar effect [104]. In a different approach, quercetin nanorods reduced protein oxidation, lipid peroxidation, and hexokinase activity in alloxan-induced diabetic mice. The liver function and kidney function of the mice improved. The authors found increased levels of antioxidant enzymes (SOD, GSH, and catalase) in the groups treated with quercetin nanorods. A comet assay showed a reduction in DNA damage, suggesting that quercetin nanorods have great potential in treating diabetes and its associated complications [105].
Resveratrol is a polyphenol found in grapes and nuts that has several pharmacological effects, including anti-inflammatory, antioxidant, platelet-enhancing, analgesic, neuroprotective, cardioprotective, and anti-aging [108]. Some studies have found that resveratrol has promising properties for treating T2D [115]. Resveratrol-loaded nanoliposomes were prepared to alleviate diabetes in diabetic rat models. Compared to free resveratrol, the nanoformulation increased the expression of ROS-inactivating enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), in diabetic pancreatic β TC cells and significantly decreased elevated glucose levels simultaneously with increasing insulin levels [106]. In another study, resveratrol nanocapsules, prepared by interfacial deposition, were tested for their effect on insulin resistance in mice with induced metabolic syndrome, using the quantitative insulin sensitivity check index (QUICKI) as a diagnostic tool [116]. The treatment with resveratrol nanocapsules successfully regulated insulin and glucose levels and controlled the QUICKI index within the healthy range. Additionally, systolic and diastolic blood pressure were also regulated in mice treated with resveratrol nanocapsules [116].
Tannic acid is a specific form of tannin, a polyphenol compound, found in all above-ground plant tissues. It has been well studied for its antioxidant, antimutagenic, and anticarcinogenic properties [117,118]. An in vitro study using a gastrointestinal tract model, investigated the inhibitory effect of tannic acid on α-amylase as a tool to control and prevent diabetes mellitus and obesity [119]. The study demonstrated that tannic acid inhibits α-amylase very effectively, showing better results than a commercial starch blocker (Phase 2 Starch Blocker). However, the authors noted that inhibition of α-amylase in the gastrointestinal tract is complex when administered orally due to the non-specific and reversible non-competitive interactions with other proteins [119]. To protect tannic acid from non-specific interactions during oral administration, the authors encapsulated the bioactive in calcium alginate microspheres. The results showed that this delivery system could protect the tannic acid from proteins in the stomach, provide a controlled release, and efficiently inhibit α-amylase activity in the small intestine [119].
In another study, a polyphenol-rich herbal formulation was microencapsulated by a freeze-drying process using different combinations of gum arabic, gelatin, and maltodextrin as wall materials [82]. The antioxidant activity, bioavailability, and anti-diabetic potential (In vitro inhibition of α-amylase and α-glucosidase) of the obtained microcapsules were tested and compared with the free extract. Amongst all tested products, microencapsulates containing a combination of 5% gum arabic and 5% maltodextrin and formulations with a wall composed of 10% gum Arabic enabled the best bioavailability ratios (50% and 40%, respectively). These two formulations also had the best results in terms of preserving antioxidant activity and the highest in vitro anti-diabetic potential due to a high potency for inhibiting α-amylase and α-glucosidase activities. In addition, these formulations were tested for acute toxicity in mice with no signs of toxicity at a dose of 2000 mg/kg administered by gavage [82].
4. The Antidiabetic Effects of Dietary Phenolic Compounds: Preclinical and Clinical Evidence
Preclinical experiments using different animal models of type 1 or type 2 diabetes (T1D or T2D, respectively) have demonstrated the benefits of dietary phenolic compounds on glucose homeostasis. For instance, the polyphenol resveratrol partially prevents and reverts the onset of diabetes [120] in NOD mice, a non-obese polygenic model for autoimmune T1D, and improves glucose tolerance and insulin sensitivity in a hypoinsulinemic diabetic animal model induced by streptozotocin (STZ) [121]. Moreover, studies conducted in T2D animal models, e.g., hyperinsulinemic and hyperglycemic mice fed hypercaloric diets, i.e., diet-induced obese mice, have demonstrated that dietary supplementation with polyphenol-rich cranberry extract induces both preventive [122] and therapeutic [123] benefits on the glucose metabolism such as improved glucose oral tolerance and insulin sensitivity and reduced glucose-induced hyperinsulinemia. Additionally, preclinical trials conducted in genetic models of T2D have also demonstrated the glucoregulatory properties of phenolic compounds. For instance, dietary supplementation with aspalathin, which belongs to the dihydrochalcone subgroup of phenolic compounds, improves glucose tolerance in hyperphagic and overweight leptin-deficient type 2 diabetic mice (ob/ob) [124]. Moreover, interventional studies in spontaneous animal models of T2D such as obese mice with a homozygous mutation in leptin receptor (db/db mice) or non-obese Goto kakizaki rats have revealed that the phenolic compound resveratrol preserves the abundance of pancreatic β cells, reduces fasting blood glucose levels and improves glucose tolerance [125,126]. Moreover, green tea phenolic compounds reduce fasting glucose and insulin levels and improve insulin sensitivity in obese and T2D Zucker fatty rats [127].
The glucoregulatory effects of phenolic compounds have also been documented in humans. In healthy individuals, the intake of a drink rich in phenolic compounds from red grape pomace reduces postprandial insulin and increases insulin sensitivity [128]. In a cross-over controlled randomised interventional trial conducted on subjects with metabolic syndrome, it has been demonstrated that eight weeks of polyphenol dietary supplementation improved whole-body glucose clearance after an oral glucose load and enhanced early insulin secretion [129]. Moreover, grape phenolic compounds dietary supplementation prevents the fructose-induced decrease in hepatic insulin sensitivity and glucose infusion rate in healthy obese and overweight subjects with a risk of T2D [130]. A 2-month resveratrol intervention also reduced fasting glucose and oxidative stress in T1D patients [131]. Nevertheless, phenolic compounds such as resveratrol do not show additive benefits with the glucose-lowering medication metformin [132], which highlights the clinical applicability of phenolic compounds in drug naïve diabetic patients or individuals with prediabetes.
Table 3 highlights the pleiotropic effects of dietary intervention with phenolic compounds. Indeed, these molecules induce diverse benefits including the restoration of postprandial insulin secretion, the improvement of hepatic insulin sensitivity, or the reduction of oxidative stress and dyslipidemia. Importantly, the clinical relevance of these compounds is supported by studies in humans. Table 3 also indicates important information on different dietary interventions conducted in humans and rodents such as the duration and the dose employed.

Table 3.
Clinical and preclinical evidence of the benefits of phenolic compounds in glucose homeostasis.
Molecular Mechanisms Underlying the Benefits of Phenolic Compounds in Glucose Homeostasis
The mechanisms through which dietary phenolic compounds can reduce postprandial glycemia have been explored in several in vivo and in vitro preclinical trials. These molecules induce benefits on glucose homeostasis through diverse mechanisms including the modulation of glucose metabolism in muscle and liver by dietary phenolic compounds that reach blood circulation, and the regulation of immune and endocrine pathways in the gut that also have an impact on glucose homeostasis.
Table 4 details the in vivo or in vitro approaches conducted to identify the molecular pathways involved in the glucose-related benefits of phenolic compounds.

Table 4.
Molecular mechanisms involved in the glucoregulatory effects of phenolic compounds. A focus on muscle, liver, and intestine.
In muscle and liver, it has been described that phenolic compounds enhance glucose uptake and reduce endogenous glucose production [124,125,126].
For instance, the incubation of rat skeletal myoblast cell cultures with aspalthin or carnosol, a rosemary extract polyphenol, induces the translocation of the glucose transport GLUT4 to the cell surface, increasing glucose uptake by activating the AMPK signalling and independently of the insulin cascade [124,142]. Nevertheless, through muscle ex vivo approaches, other investigations have identified insulin-mediated glucose uptake mechanisms induced by green tea phenolic compounds [127]. Moreover, phenolic compounds such as resveratrol enhance the glycogen depots in C2C12 myotubes pretreated with fatty acids [137]. Furthermore, in human hepatic cells, epicatechin (the main cocoa flavanol) and phenolic cocoa extract activate the AKT-mediated insulin cascade to inhibit gluconeogenic enzyme expression, phosphoenolpyruvate carboxykinase (PEPCK), leading to reduce glucose production [143].
Overall, the benefits of phenolic compounds in the aforementioned glucose-related metabolism routes are associated with the antioxidative and anti-inflammatory properties of these compounds that contribute to preserving functional pancreatic β-cells and improving insulin-dependent and independent pathways in muscle and liver.
Experiments with rat insulinoma cells or RIN cells demonstrated that phenolic compounds such as genistein, quercetin, apigenin, luteolin, and sulfuretin prevent the cytokine-induced cytotoxicity and the impaired glucose-induced insulin secretion in β-cells by inhibiting the NFκβ cascade [144,145,146]. In vivo experiments combined with in vitro approaches conducted in NOD mice have also demonstrated that resveratrol reduces insulitis by blocking the migration of IL17-producing T cells and CD11b+ F4/80hi macrophages from spleen and pancreatic lymph nodes to the pancreas through a mechanism that involves the downregulation of the chemokine (C-C motif) receptor 6 (CCR6) [120].
Moreover, natural phenolic compounds such as curcumin or resveratrol prevent the fatty acid-induced proinflammatory cytokine and reactive oxygen species (ROS) production in skeletal muscle C2C12 cells by suppressing the phosphorylation of IKKα-IKKβ, and JNK [137,139]. In cultures of human hepatocytes (HepG2 cells), resveratrol attenuates the high glucose-induced ROS production by inducing the expression of oxidant genes through a mechanism that involves the downregulation of the methylation of the nuclear factor erythroid 2–related factor 2 (Nrf2) promoter [138]. Moreover, phenolic compounds limit the enhanced proinflammatory response in the hepatocytes associated with high adiposity. Indeed, the natural polyphenol gallic acid reduces the expression of the proinflammatory cytokines TNFα and IL1β in the co-cultures of macrophages and lipid-laden hepatocytes [70].
On the other hand, it is well established that the excessive intake of saturated fats and simple sugars increases intestinal permeability and proinflammatory pathways, leading to systemic inflammation and insulin resistance in tissues such as muscle and liver [148]. Thus, beyond the direct effects of dietary phenolic compounds on the pancreas, liver, and muscle, the glucoregulatory benefits of phenolic compounds may be derived from their capacity to modulate intestinal immunity. Although it has not been extensively explored, some studies demonstrated that polyphenol-dietary supplementation in diet-induced obese mice with impaired insulin sensitivity and glucose tolerance limits their enhanced intestinal oxidative stress, inflammation, and gut barrier permeability. These effects in turn could contribute to alleviating the proinflammatory response in the liver and improve glucose tolerance and insulin sensitivity [122,123]. In vitro studies with the human colonic Caco-2 epithelial cell line co-cultured with differentiated THP-1 cells reveal that, mechanistically, under inflammatory conditions, phenolic compounds provide protective effects on gut mucosa by restoring the expression of tight junction proteins and by reducing the intestinal epithelial cell apoptosis [140]. Furthermore, phenolic compounds such as the oligomeric proanthocyanidins polymers derived from blueberry polyphenolic extract increase colonic mucin-secreting goblet cells required to create the protective mucus layer in diet-induced obese mice [133]. Moreover, phenolic compounds can modulate the abundance of intestinal immune cells. Indeed, the plant-derived phenolic compounds carnosol and curcumin inhibit the inflammatory-induced metabolic adaptation of human dendritic cells by activating the energy sensor AMPK [141], and apple condensed tannins increase the abundance of TCR γδ intraepithelial lymphocytes strengthening the intestinal tolerogenic properties [149].
On the other hand, strategies based on the gut hormone GLP-1 have been designed to treat T2D due to their benefits on postprandial glycemia by increasing insulin secretion and improving insulin sensitivity [150]. Studies in humans, mice, or GLP-1 secreting cells have also demonstrated that phenolic compounds such as delphinidin 3-rutinoside, an anthocyanin derivative, curcumin, and resveratrol stimulate GLP-1 secretion, reducing postprandial glycemia [20,132,137,138,149]. The role of phenolic compounds as GLP-1 secretagogues is mediated by the G protein-coupled receptors (GPCRs) GPR40 and GPR120 and the bitter taste receptors Tas2Rs [151] and is dependent on the GLP-1 receptor [19].
Furthermore, phenolic compounds’ modulation of intestinal immune and GLP-1-mediated routes may be preceded by their effects on gut microbiota. Indeed, phenolic compounds reaching the colon can be metabolised by intestinal bacteria [152], generating metabolites that beneficially modulate the functionality of enterocytes and immune or enteroendocrine cells. Faecal microbiota transplant experiments conducted in diet-induced obese mice have demonstrated that the protective effects of phenolic compounds derived from the fruit Myrciaria dubia on metabolic syndrome are dependent on the gut microbiota [136]. Currently, identifying key intestinal bacterial species showing metabolic adaptations to dietary phenolic compounds is an early investigation stage nevertheless, some studies provide evidence in this regard. For instance, polyphenol-rich cranberry extract intervention in diet-induced obese mice increases the abundance of Akkermansia muciniphila [122,123], which induces protective effects on the gut barrier, preventing systemic inflammation [153], enhancing GLP-1 secretion [154], and reducing postprandial glycemia. In addition, the microbial metabolite urolithin A derived from dietary phenolic compounds improves gut barrier function and limits intestinal inflammation in the colon by a mechanism mediated by the aryl hydrocarbon receptor (AhR) and the nuclear factor erythroid 2–related factor 2 (Nrf2) [155]. Further details of how these in vivo and in vitro approaches have been conducted are indicated in Table 4.
5. Conclusions
Phenolic compounds are secondary metabolites contained in many food products essential to the human diet. These compounds present antioxidant and anti-inflammatory properties and are being studied due to their positive effects on blood glucose management in diabetes conditions. The food origin of phenolic compounds has focused scientific research on their extraction from agri-food residues and wastes, including peels, seeds, hulls, and shells rich in phenolic compounds. Moreover, alternative technologies to conventional chemical extraction methods are been widely developed for phenolic compounds purification from these residues. In addition, progress in micro- and nanoencapsulation methodologies has shown they can improve phenolic compounds bioavailability and preserve their biological activities during digestion. Reviewed studies even suggest that micro- and nano-encapsulated polyphenolic compounds could induce beneficial effects in the treatment and prevention of type 2 diabetes and its associated complications. Encapsulation of phenolic compounds can also mask the undesirable taste of some plant extracts, clearing the way for the development of future functional foods, nutraceuticals, or orally administered polyphenol-based drugs with preventive and therapeutic effects against diabetes.
Funding
The research leading to these results received funding from the European Union’s H2020 Research and Innovation Programme under the Maria Sklodowska-Curie grant agreement no. 778388 (H2020 MSCA-RISE-2017) project Food for Diabetes and Cognition (FODIAC), the project cLabel+ (POCI-01-0247-FEDER-046080) co-financed by Compete 2020, Lisbon 2020, Portugal 2020 and the European Union, through the European Regional Development Fund (ERDF), and Bacteriophage-releasing nanostructured smart packaging materials for the control of food-borne pathogens–PACKTERIOPHAGE (POCI-01-0145-FEDER-032594). The Ministry of Science and Innovation (MICIN) of Spain funded AR through the grant RYC2020-030690-I.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernandes, S.S.; Coelho, M.S.; de las Mercedes Salas-Mellado, M. Bioactive Compounds as Ingredients of Functional Foods: Polyphenols, Carotenoids, Peptides From Animal and Plant Sources New. In Bioactive Compounds: Health Benefits and Potential Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 129–142. ISBN 9780128147757. [Google Scholar]
- Arnoldi, A. Functional Foods, Cardiovascular Disease, and Diabetes, 1st ed.; Arnoldi, A., Ed.; Woodhead Publishing Limited: Cambridge, UK; CRC Press LLC: Boca Raton, FL, USA, 2004; ISBN 9781855739499. [Google Scholar]
- Bloch, A.; Thomson, C.A. Position of the American Dietetic Association. Phytochemicals and Functional Foods. J. Am. Diet. Assoc. 1995, 95, 493–496. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Rehman, K.; Al-Gubory, K.H.; Laher, I.; Akash, M.S.H. Dietary Polyphenols in the Prevention and Treatment of Diabetes Mellitus. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer: Cham, Switzerland, 2018; pp. 377–395. ISBN 9783319676258. [Google Scholar]
- Chikezie, P.C.; Ojiako, O.A.; Ogbuji, A.C. Oxidative Stress in Diabetes Mellitus. Int. J. Biol. Chem. 2015, 9, 92–109. [Google Scholar] [CrossRef]
- Dembinska-Kiec, A.; Mykkänen, O.; Kiec-Wilk, B.; Mykkänen, H. Antioxidant Phytochemicals against Type 2 Diabetes. Br. J. Nutr. 2008, 99, ES109–ES117. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Williamson, G. A Review of the Health Effects of Green Tea Catechins in In Vivo Animal Models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef] [PubMed]
- Prpa, E.J.; Bajka, B.H.; Ellis, P.R.; Butterworth, P.J.; Corpe, C.P.; Hall, W.L. A Systematic Review of in Vitro Studies Evaluating the Inhibitory Effects of Polyphenol-Rich Fruit Extracts on Carbohydrate Digestive Enzymes Activity: A Focus on Culinary Fruits Consumed in Europe. Crit. Rev. Food Sci. Nutr. 2020, 61, 3783–3803. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The Inhibitory Effects of Berry Polyphenols on Digestive Enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Dao, T.M.A.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthélemy, S.; Barra, Y.; et al. Resveratrol Increases Glucose Induced GLP-1 Secretion in Mice: A Mechanism Which Contributes to the Glycemic Control. PLoS ONE 2011, 6, e20700. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Steinberg, G.R. SIRT1 Takes a Backseat to AMPK in the Regulation of Insulin Sensitivity by Resveratrol. Diabetes 2010, 59, 551–553. [Google Scholar] [CrossRef]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of Gut Microbiota with Functional Food Components and Nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Tutelyan, V.A.; Sharafetdinov, K.K.; Plotnikova, O.A.; Vorobiova, I.S.; Kochetkova, A.A.; Krul, E.S.; Ouwehand, A.C.; Mendelson, G.J. Innovative Approaches in the Development of Specialized Food Products of Optimized Composition for Patients with Type 2 Diabetes. J. Diabetes Metab. 2016, 7. [Google Scholar] [CrossRef]
- Tutelyan, V.A.; Sharafetdinov, K.K.; Lapik, I.A.; Vorobyeva, I.S.; Sukhanov, B.P. Priorities in the Development of Specialized Food Products with Optimized Composition for Patients with Type 2 Diabetes Mellitus. Vopr. Pitan. 2014, 83, 41–51. [Google Scholar] [PubMed]
- Kochetkova, A.A.; Vorobyeva, I.S.; Vorobyeva, V.M.; Sharafetdinov, K.K.; Plotnikova, O.A.; Pilipenko, V.V.; Alexeeva, R.I.; Sasunova, A.N. Specialized Food Products with Modified Carbohydrate Profile for Dietary Correction of Diet of Patients with Type 2 Diabetes. Vopr. Pitan. 2018, 87, 76–88. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- del Río, P.G.; Gullón, B.; Wu, J.; Saddler, J.; Garrote, G.; Romaní, A. Current Breakthroughs in the Hardwood Biorefineries: Hydrothermal Processing for the Co-Production of Xylooligosaccharides and Bioethanol. Bioresour. Technol. 2022, 343, 126100. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A Critical Review on the Development Stage of Biorefinery Systems towards the Management of Apple Processing-Derived Waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- del Río, P.G.; Flórez-Fernández, N.; Álvarez-Viñas, M.; Torres, M.D.; Romaní, A.; Domínguez, H.; Garrote, G. Evaluation of Sustainable Technologies for the Processing of Sargassum Muticum: Cascade Biorefinery Schemes. Green Chem. 2021, 23, 7001–7015. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Optimization of Solvent Extraction of Antioxidants from Eucalyptus Globulus Leaves by Response Surface Methodology: Characterization and Assessment of Their Bioactive Properties. Ind. Crops Prod. 2017, 108, 649–659. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal Treatment of Chestnut Shells (Castanea sativa) to Produce Oligosaccharides and Antioxidant Compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Rocha, C.M.R.; Michelin, M.; Domingues, L.; Teixeira, J.A. Chapter 20—Valorization of Lignocellulosic-Based Wastes. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Gnansounou, E., Khanal, S.K., Raveendran, S.B.T.-C.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–410. ISBN 978-0-444-64321-6. [Google Scholar]
- Paula, V.; Pedro, S.I.; Campos, M.G.; Delgado, T.; Estevinho, L.M.; Anjos, O. Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Appl. Sci. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Goyal, A.; Algın Yapar, E.; Cavalu, S. Bioactive Compounds and Nanodelivery Perspectives for Treatment of Cardiovascular Diseases. Appl. Sci. 2021, 11, 11031. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food by-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Kelly, N.P.; Kelly, A.L.; O’Mahony, J.A. Strategies for Enrichment and Purification of Polyphenols from Fruit-Based Materials. Trends Food Sci. Technol. 2019, 83, 248–258. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New Perspective in Extraction of Plant Biologically Active Compounds by Green Solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Espino, M.; de los Ángeles Fernández, M.; Gomez, F.J.V.; Silva, M.F. Natural Designer Solvents for Greening Analytical Chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of High Added-Value Components from Food Wastes: Conventional, Emerging Technologies and Commercialized Applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated during Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Kala, H.; Mehta, R.; Sen, K.; Tandey, R.; Mandal, V. Critical Analysis of Research Trends and Issues in Microwave Assisted Extraction of Phenolics: Have We Really Done Enough. TrAC Trends Anal. Chem. 2016, 85. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food by-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef] [PubMed]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of Polyphenols and Vitamins in Wine-Making by-Products by Supercritical Fluid Extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Abbott, A.P.; Cullis, P.M.; Gibson, M.J.; Harris, R.C.; Raven, E. Extraction of Glycerol from Biodiesel into a Eutectic Based Ionic Liquid. Green Chem. 2007, 9, 868–887. [Google Scholar] [CrossRef]
- Fernández, M.d.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial by-Products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-Use Green Polyphenolic Extracts from Food by-Products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents—Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojcic Redovnikovic, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Characteristic Features of Surfactants. In Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–38. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Micelle Formation by Surfactants. In Surfactants and Interfacial Phenomena; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 123–201. [Google Scholar]
- Spigno, G.; Amendola, D.; Dahmoune, F.; Jauregi, P. Colloidal Gas Aphrons Based Separation Process for the Purification and Fractionation of Natural Phenolic Extracts. Food Bioprod. Process. 2015, 94, 434–442. [Google Scholar] [CrossRef]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant Mediated Extraction of Total Phenolic Contents (TPC) and Antioxidants from Fruits Juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Sebba, F. Foams and Bioliquid Foams, Aphrons; Wiley: Chichester, UK, 1987; ISBN 0471916854. [Google Scholar]
- Jauregi, P.; Varley, J. Colloidal Gas Aphrons: A Novel Approach to Protein Recovery. Biotechnol. Bioeng. 1998, 59, 471–481. [Google Scholar] [CrossRef]
- Jauregi, P.; Mitchell, G.R.; Varley, J. Colloidal Gas Aphrons (CGA): Dispersion and Structural Features. AIChE J. 2000, 46, 24–36. [Google Scholar] [CrossRef]
- Jauregi, P.; Dermiki, M. Separation of Value-Added Bioproducts by Colloidal Gas Aphrons (CGA) Flotation and Applications in the Recovery of Value-Added Food Products. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Woodhead Publishing: Sawston, UK, 2010; pp. 284–313. [Google Scholar] [CrossRef]
- Noriega, D.; Zuñiga, M.E.; Soto, C.; MohdMaidin, N.; Michael, N.; Jauregi, P. Colloidal Gas Aphrons Separation to Obtain Polyphenol Rich Fractions from Artichoke Agro-Industrial Discards. Food Bioprod. Process. 2018, 110, 50–59. [Google Scholar] [CrossRef]
- Mohd Maidin, N.; Michael, N.; Oruna-Concha, M.J.; Jauregi, P. Polyphenols Extracted from Red Grape Pomace by a Surfactant Based Method Show Enhanced Collagenase and Elastase Inhibitory Activity. J. Chem. Technol. Biotechnol. 2018, 93, 1916–1924. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Kuhlman, B.; Hansen, J.; Jørgensen, B.; du Toit, W.; Moore, J.P. The Effect of Enzyme Treatment on Polyphenol and Cell Wall Polysaccharide Extraction from the Grape Berry and Subsequent Sensory Attributes in Cabernet Sauvignon Wines. Food Chem. 2022, 385, 132645. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in Polyphenol and Polysaccharide Content of Grape Seed Extract and Grape Pomace after Enzymatic Treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-Assisted Extraction of Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- del Río, P.G.; Gullón, B.; Pérez-Pérez, A.; Romaní, A.; Garrote, G. Microwave Hydrothermal Processing of the Invasive Macroalgae Sargassum Muticum within a Green Biorefinery Scheme. Bioresour. Technol. 2021, 340, 125733. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- García-Vargas, M.C.; Contreras, M.D.; Castro, E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Appl. Sci. 2020, 10, 8195. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; del Río, P.G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal Treatment of Avocado Peel Waste for the Simultaneous Recovery of Oligosaccharides and Antioxidant Phenolics. Bioresour. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Gullón, B.; Teixeira, J.A.; Botelho, C.M.; Yáñez, R. Exploiting the Potential of Bioactive Molecules Extracted by Ultrasounds from Avocado Peels—Food and Nutraceutical Applications. Antioxidants 2021, 10, 1475. [Google Scholar] [CrossRef]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef]
- Costa, C.E.; Møller-Hansen, I.; Romaní, A.; Teixeira, J.A.; Borodina, I.; Domingues, L. Resveratrol Production from Hydrothermally Pretreated Eucalyptus Wood Using Recombinant Industrial Saccharomyces Cerevisiae Strains. ACS Synth. Biol. 2021, 10, 1895–1903. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available Technologies on Improving the Stability of Polyphenols in Food Processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A Review on Advanced Microencapsulation Technology to Enhance Bioavailability of Phenolic Compounds: Based on Its Activity in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Spigoni, V.; Mena, P.; Fantuzzi, F.; Tassott, M.; Brighenti, F.; Bonadonna, R.C.; Del Rio, D.; Dei Cas, A. Bioavailability of Bergamot (Citrus bergamia) Flavanones and Biological Activity of Their Circulating Metabolites in Human pro-Angiogenic Cells. Nutrients 2017, 9, 1328. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Song, Y. Microencapsulation and Characterization of Natural Polyphenols from PHF Extract. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.R.; Xiang, R.; Liu, X.M.; Zhu, M.J. The Effects of Thermal Processing and β-Cyclodextrin on Extractable Polyphenols in Mulberry Juice-Enriched Dried Minced Pork Slices. LWT 2019, 116, 108503. [Google Scholar] [CrossRef]
- Rahim, R.A.; Jayusman, P.A.; Muhammad, N.; Ahmad, F.; Mokhtar, N.; Mohamed, I.N.; Mohamed, N.; Shuid, A.N. Recent Advances in Nanoencapsulation Systems Using Plga of Bioactive Phenolics for Protection against Chronic Diseases. Int. J. Environ. Res. Public Health 2019, 16, 4962. [Google Scholar] [CrossRef]
- Tsume, Y.; Incecayir, T.; Song, X.; Hilfinger, J.M.; Amidon, G.L. The Development of Orally Administrable Gemcitabine Prodrugs with D-Enantiomer Amino Acids: Enhanced Membrane Permeability and Enzymatic Stability. Eur. J. Pharm. Biopharm. 2014, 86, 514–523. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; López-Carballo, G.; Gavara, R.; Muriel Galet, V.; Guarda, A.; Galotto, M.J. Improving Polyphenolic Thermal Stability of Aristotelia Chilensis Fruit Extract by Encapsulation within Electrospun Cyclodextrin Capsules. J. Food Process. Preserv. 2019, 43, e14044. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Rodríguez-Marín, M.L.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent Advances in Microencapsulation of Natural Sources of Antimicrobial Compounds Used in Food—A Review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of Chitosan Nanoparticles to Enhance Absorption and Bioavailability of Tea Polyphenols: A Review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Rambaran, T.F. Nanopolyphenols: A Review of Their Encapsulation and Anti-Diabetic Effects. SN Appl. Sci. 2020, 2, 1335. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Pereira, S.V.; Siqueira, S.; Salomão, W.F.; Freitas, L.A.P. Curcuminoid Content and Antioxidant Activity in Spray Dried Microparticles Containing Turmeric Extract. Food Res. Int. 2013, 50, 657–663. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Wang, J.; Martínez-Hernández, A.; de Lamo-Castellví, S.; Romero, M.P.; Kaade, W.; Ferrando, M.; Güell, C. Low-Energy Membrane-Based Processes to Concentrate and Encapsulate Polyphenols from Carob Pulp. J. Food Eng. 2020, 281, 109996. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Gruppi, A.; Vieira, M.V.; Matos, G.S.; Vicente, A.A.; Teixeira, J.A.C.; Fuciños, P.; Spigno, G.; Pastrana, L.M. How Additive Manufacturing Can Boost the Bioactivity of Baked Functional Foods. J. Food Eng. 2021, 294, 110394. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a Tool to Counteract the Typical Low Bioavailability of Polyphenols in the Management of Diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Tong, F.; Chai, R.; Jiang, H.; Dong, B. In Vitro/Vivo Drug Release and Anti-Diabetic Cardiomyopathy Properties of Curcumin/PBLG-PEG-PBLG Nanoparticles. Int. J. Nanomed. 2018, 13, 1945–1962. [Google Scholar] [CrossRef]
- Jia, T.; Rao, J.; Zou, L.; Zhao, S.; Yi, Z.; Wu, B.; Li, L.; Yuan, H.; Shi, L.; Zhang, C.; et al. Nanoparticle-Encapsulated Curcumin Inhibits Diabetic Neuropathic Pain Involving the P2Y12 Receptor in the Dorsal Root Ganglia. Front. Neurosci. 2018, 11, 755. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate Coated Chitosan Core-Shell Nanoparticles for Efficient Oral Delivery of Naringenin in Diabetic Animals—An in Vitro and in Vivo Approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Ochin, C.C.; Garelnabi, M. Berberine Encapsulated PLGA-PEG Nanoparticles Modulate PCSK-9 in HepG2 Cells. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 61–70. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.B.; Thomazini, M.; Echalar Barrientos, M.A.; Nalin, C.M.; Ferro-Furtado, R.; Genovese, M.I.; Favaro-Trindade, C.S. Functional Properties and Encapsulation of a Proanthocyanidin-Rich Cinnamon Extract (Cinnamomum zeylanicum) by Complex Coacervation Using Gelatin and Different Polysaccharides. Food Hydrocoll. 2018, 77, 297–306. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. Preparation, Characterization and in Vivo Evaluation of PH Sensitive, Safe Quercetin-Succinylated Chitosan-Alginate Core-Shell-Corona Nanoparticle for Diabetes Treatment. Carbohydr. Polym. 2018, 182, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, D.; Nikalaje, S.K.; Mittal, A.; Chand, M.; Kumar, N. Development of Quercetin Nanoformulation and in Vivo Evaluation Using Streptozotocin Induced Diabetic Rat Model. Drug Deliv. Transl. Res. 2012, 2, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Abdullah, K.M.; Singh, B.R.; Naqvi, A.H.; Naseem, I. Ameliorative Effect of Quercetin Nanorods on Diabetic Mice: Mechanistic and Therapeutic Strategies. RSC Adv. 2016, 6, 55092–55103. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Aktaş, Y. Nanoliposomal Resveratrol as a Novel Approach to Treatment of Diabetes Mellitus. J. Nanosci. Nanotechnol. 2017, 18, 3856–3864. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Bahramsoltani, R.; Marques, A.M.; Naseri, R.; Rahimi, R.; Haratipour, P.; Panah, A.I.; Farzaei, M.H.; Abdollahi, M. A Systematic Review of Nano Formulation of Natural Products for the Treatment of Inflammatory Bowel Disease: Drug Delivery and Pharmacological Targets. DARU J. Pharm. Sci. 2018, 26, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.O.; Michael, O.S.; Rathee, S.; Singh, K.R.; Ajayi, O.O.; Adetunji, J.B.; Ojha, A.; Singh, J.; Singh, R.P. Potentialities of Nanomaterials for the Management and Treatment of Metabolic Syndrome: A New Insight. Mater. Today Adv. 2022, 13, 100198. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of Natural Products for Management of Metabolic Syndrome. Int. J. Nanomed. 2019, 14, 5303. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and Coptidis Rhizoma as Potential Anticancer Agents: Recent Updates and Future Perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant Properties, Radical Scavenging Activity and Biomolecule Protection Capacity of Flavonoid Naringenin and Its Glycoside Naringin: A Comparative Study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Terauchi, M. The Diverse Efficacy of Food-Derived Proanthocyanidins for Middle-Aged and Elderly Women. Nutrients 2020, 12, 3833. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol Treatment as an Adjunct to Pharmacological Management in Type 2 Diabetes Mellitus-Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Shahraki, A.; Bahadorikhalili, S.; Hashemzaei, M.; Shahraki, J.; Tajrobekar, O. Effects of Resveratrol Nanocapsules on the Quantitative Insulin Sensitivity Check Index in Insulin Resistance: A Study on Metabolic Syndrome Induce Mice. SN Appl. Sci. 2020, 2, 962. [Google Scholar] [CrossRef]
- Yan, W.; Shi, M.; Dong, C.; Liu, L.; Gao, C. Applications of Tannic Acid in Membrane Technologies: A Review. Adv. Colloid Interface Sci. 2020, 284, 102267. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Zhao, W.; Iyer, V.; Flores, F.P.; Donhowe, E.; Kong, F. Microencapsulation of Tannic Acid for Oral Administration to Inhibit Carbohydrate Digestion in the Gastrointestinal Tract. Food Funct. 2013, 4, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Yang, H.; Tartar, D.M.; Gao, B.; Luo, X.; Ye, S.Q.; Zaghouani, H.; Fang, D. Prevention and Treatment of Diabetes with Resveratrol in a Non-Obese Mouse Model of Type 1 Diabetes. Diabetologia 2011, 54, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, Á.; Santamaría, B.; Mas-Gutierrez, J.A.; Rada, P.; Fernández-Millán, E.; Pardo, V.; Álvarez, C.; Cuadrado, A.; Ros, M.; Serrano, M.; et al. Resveratrol Treatment Restores Peripheral Insulin Sensitivity in Diabetic Mice in a Sirt1-Independent Manner. Mol. Nutr. Food Res. 2015, 59, 1431–1442. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia Spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.A.; Desjardins, Y.; Roy, D.; et al. A Polyphenol-Rich Cranberry Extract Reverses Insulin Resistance and Hepatic Steatosis Independently of Body Weight Loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin Improves Hyperglycemia and Glucose Intolerance in Obese Diabetic Ob/Ob Mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef]
- Do, G.-M.; Jung, U.J.; Park, H.-J.; Kwon, E.-Y.; Jeon, S.-M.; McGregor, R.A.; Choi, M.-S. Resveratrol Ameliorates Diabetes-Related Metabolic Changes via Activation of AMP-Activated Protein Kinase and Its Downstream Targets in Db/Db Mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef]
- Szkudelska, K.; Deniziak, M.; Hertig, I.; Wojciechowicz, T. Effects of resveratrol in Goto-Kakizaki rat, a model of type 2 diabetes. Nutrients 2019, 11, 2488. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, Y.; Zhou, J.; Xiao, L.; Johnson, M.; Qu, X. Green Tea Polyphenols Ameliorate Metabolic Abnormalities and Insulin Resistance by Enhancing Insulin Signalling in Skeletal Muscle of Zucker Fatty Rats. Clin. Sci. 2020, 134, 1167–1180. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape Pomace Polyphenols Improve Insulin Response to a Standard Meal in Healthy Individuals: A Pilot Study. Clin. Nutr. 2019, 38, 2727–2734. [Google Scholar] [CrossRef]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-Rich Diets Improve Glucose Metabolism in People at High Cardiometabolic Risk: A Controlled Randomised Intervention Trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape Polyphenols Prevent Fructose-Induced Oxidative Stress and Insulin Resistance in First-Degree Relatives of Type 2 Diabetic Patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects with Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.-C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild Blueberry Proanthocyanidins Shape Distinct Gut Microbiota Profile and Influence Glucose Homeostasis and Intestinal Phenotypes in High-Fat High-Sucrose Fed Mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin Improves Glucose Tolerance via Stimulation of Glucagon-like Peptide-1 Secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef]
- Fujii, Y.; Osaki, N.; Hase, T.; Shimotoyodome, A. Ingestion of Coffee Polyphenols Increases Postprandial Release of the Active Glucagon-like Peptide-1 (GLP-1(7-36)) Amide in C57BL/6J Mice. J. Nutr. Sci. 2015, 4, e9. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol Ameliorates Metabolic Disorders and Insulin Resistance in High-Fat Diet-Fed Mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef]
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Babaei Khorzoughi, R.; Namvarjah, F.; Izadi, P.; Meshkani, R. Resveratrol Alleviates Non-Alcoholic Fatty Liver Disease through Epigenetic Modification of the Nrf2 Signaling Pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Regulating JNK/NF-KB Pathway and ROS Production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, M.; Yang, J.; Pan, H.; Yu, M.; Ji, F. Curcumin Improves Epithelial Barrier Integrity of Caco-2 Monolayers by Inhibiting Endoplasmic Reticulum Stress and Subsequent Apoptosis. Gastroenterol. Res. Pract. 2021, 2021, 5570796. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Fletcher, J.M.; Dunne, A. Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase-1. Front. Immunol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Baron, D.; Vlachogiannis, I.A.; MacPherson, R.E.K.; Tsiani, E. Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation. Int. J. Mol. Sci. 2018, 19, 1321. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa Flavonoids Improve Insulin Signalling and Modulate Glucose Production via AKT and AMPK in HepG2 Cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Kwon, K.-B.; Song, M.-Y.; Han, M.-J.; Lee, J.-H.; Lee, Y.-R.; Lee, J.-H.; Ryu, D.-G.; Park, B.-H.; Park, J.-W. Flavonoids Protect against Cytokine-Induced Pancreatic Beta-Cell Damage through Suppression of Nuclear Factor KappaB Activation. Pancreas 2007, 35, e1–e9. [Google Scholar] [CrossRef]
- Kim, E.-K.; Kwon, K.-B.; Song, M.-Y.; Seo, S.-W.; Park, S.-J.; Ka, S.-O.; Na, L.; Kim, K.-A.; Ryu, D.-G.; So, H.-S.; et al. Genistein Protects Pancreatic Beta Cells against Cytokine-Mediated Toxicity. Mol. Cell. Endocrinol. 2007, 278, 18–28. [Google Scholar] [CrossRef]
- Song, M.-Y.; Jeong, G.-S.; Kwon, K.-B.; Ka, S.-O.; Jang, H.-Y.; Park, J.-W.; Kim, Y.-C.; Park, B.-H. Sulfuretin Protects against Cytokine-Induced Beta-Cell Damage and Prevents Streptozotocin-Induced Diabetes. Exp. Mol. Med. 2010, 42, 628–638. [Google Scholar] [CrossRef]
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar] [CrossRef]
- Basson, A.R.; Chen, C.; Sagl, F.; Trotter, A.; Bederman, I.; Gomez-Nguyen, A.; Sundrud, M.S.; Ilic, S.; Cominelli, F.; Rodriguez-Palacios, A. Regulation of Intestinal Inflammation by Dietary Fats. Front. Immunol. 2021, 11, 604989. [Google Scholar] [CrossRef]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R.; et al. Dietary Unripe Apple Polyphenol Inhibits the Development of Food Allergies in Murine Models. FEBS Lett. 2005, 579, 4485–4491. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of Dietary Polyphenols and Gut Microbiota: Microbial Metabolism of Polyphenols, Influence on the Gut Microbiota, and Implications on Host Health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia Muciniphila Secretes a Glucagon-like Peptide-1-Inducing Protein That Improves Glucose Homeostasis and Ameliorates Metabolic Disease in Mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).