Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts
Abstract
1. Introduction
2. Materials and Methods
2.1. Pumpkin Varieties
- “Melon Yellow” (belonging to the Cucurbita maxima species), is one of the oldest varieties. Its plants have shelling shoots up to 10 m long. It has large-scale fruit weighing between 15 and 30 kg, mostly spherical, ribbed, soft orange peel, only sometimes the fruit may be slightly flattened. The flesh of the fruit is compact, juicy, yellowish orange. This variety has an average dry matter, protein and vitamin content. Their content also depends to a large extent on the way in which the crop is grown. The quantity of these ingredients is higher in organic cultivation than conventional cultivation and, for nitrates, their concentration is smaller in organic farming,
- “Miranda” (belonging to the Cucurbita moschata species), is a variety of oil-free pumpkin seeds with no seed coat. The 3 to 4 spherical flattened fruits are formed on one plant of 3 to 4 kg. The fruit is light green, marble. As the skin grows, it color turns orange. Inside the fruit, apart from the tasty and thin flesh, is a lot of olive green, non-hulling seeds. They contain 25 to 50% fats and produce edible oil.
2.2. Probiotic Bacterial Strains and Growth Conditions
- The probiotic, human-origin bacterial strain Lacticaseibacillus rhamnosus Lock 0900 (former Lactobacillus rhamnosus Lock0900; patent number 209988) was obtained from a pure culture maintained at the Laboratory of Microbiology, Łódź University of Technology, Poland [22]
2.3. Functional Frozen Desserts Preparation
2.4. Microbiological Analysis
2.5. Acidity Analysis (pH)
2.6. Sugars Content
2.7. Total Carotenoids Content
2.8. Determination of Antioxidant Activity
2.8.1. DPPH Radical Scavenging Assay
2.8.2. ABTS Radical Scavenging Assay
2.9. Color Measurement
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results
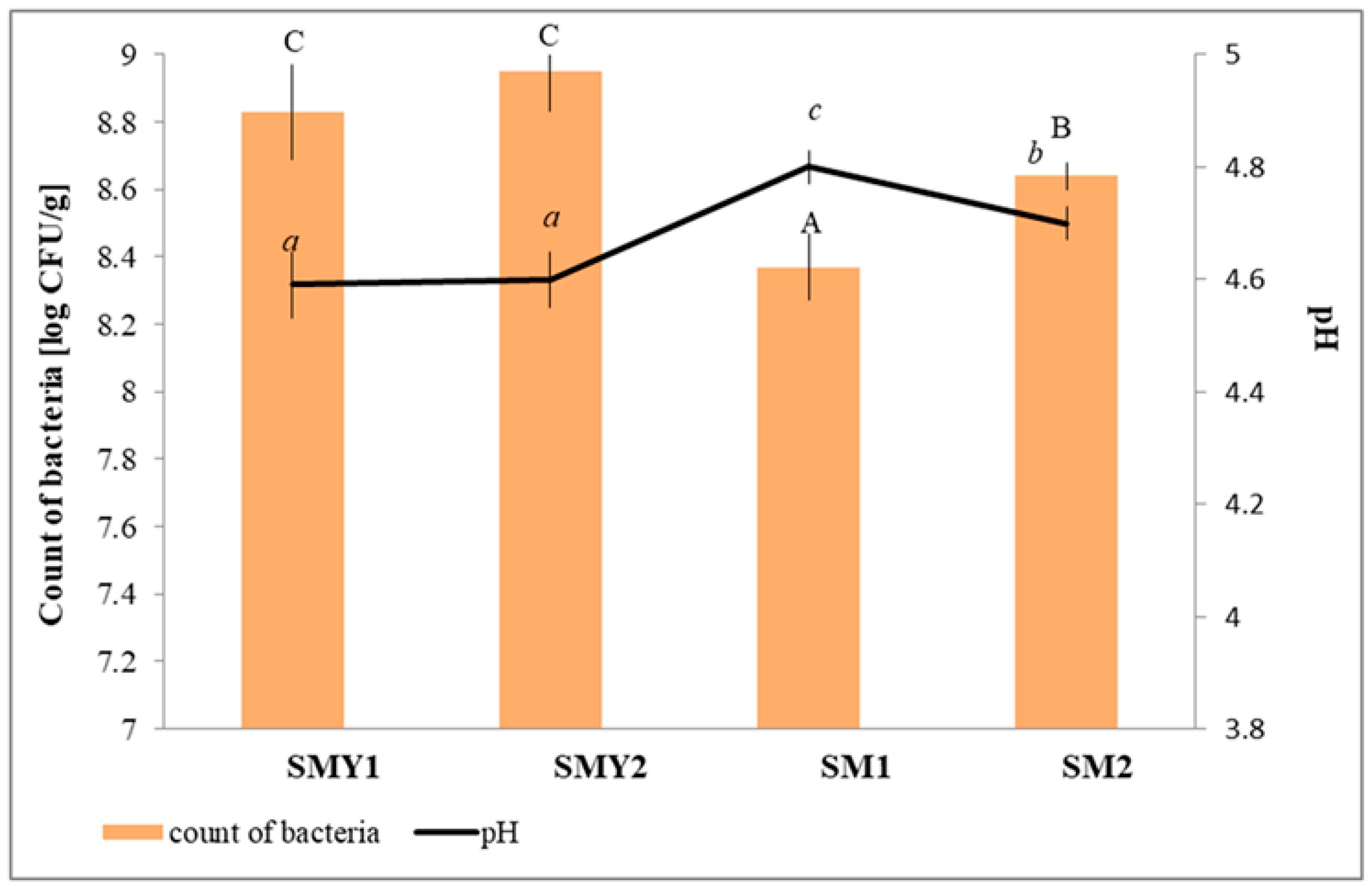
3.1. Microbiological Analysis and pH Changes
3.2. Sugars Content
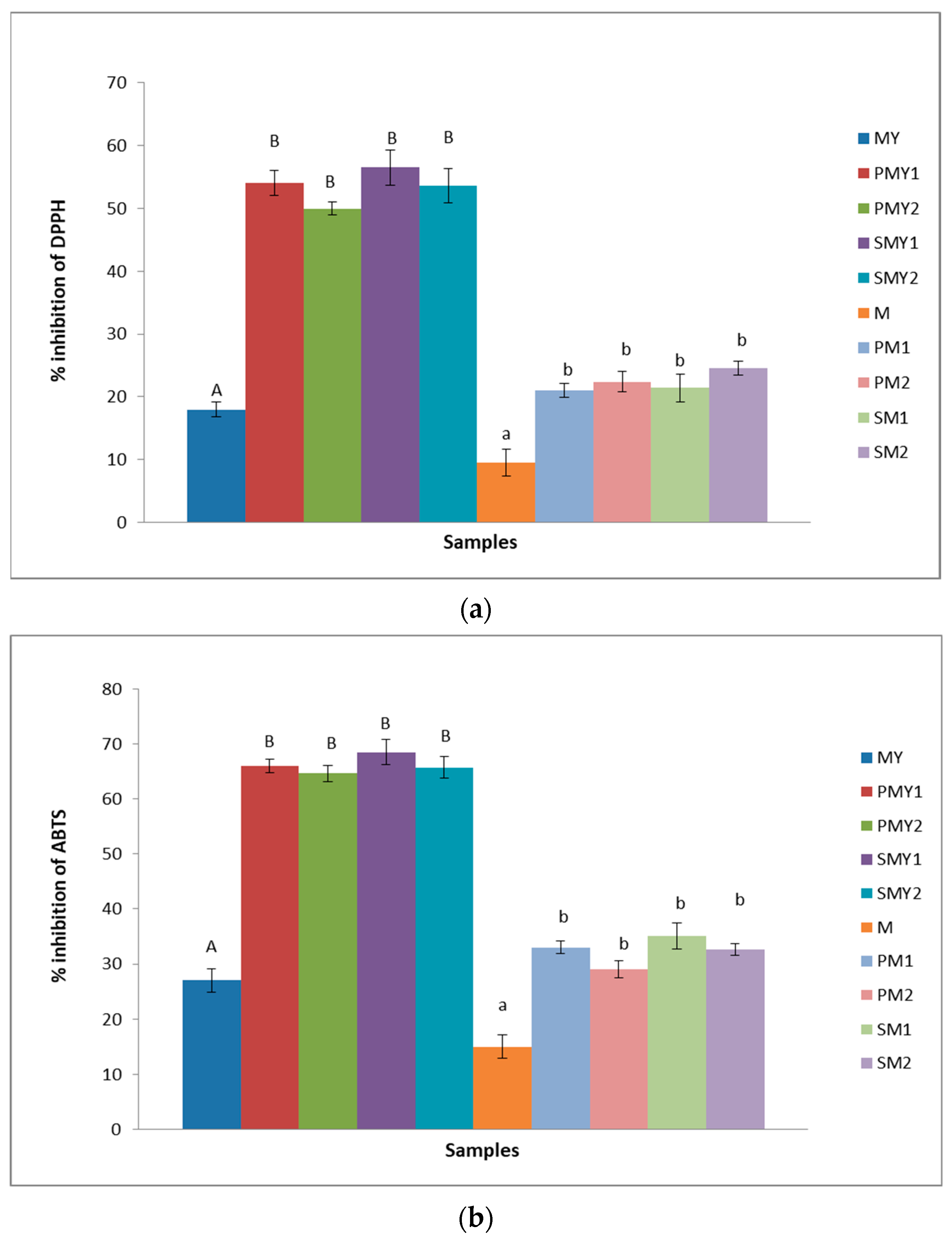
3.3. Determination of Antioxidant Activity
3.4. Color Measurement and Total Carotene Content
3.5. Sensory Characteristics of Functional Frozen Dessert
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Research and Market Report: “Plant Based Food Market by Product Type (Dairy Alternatives, Meat Substitute, Plant-Based Eggs, Confectionery), Source (Soy Protein, Wheat Protein), and Distribution Channel (Business to Business and Business to Customers)—Global Forecast to 2027”. Available online: https://www.researchandmarkets.com/reports/5144605/plant-based-food-market-by-product-type-dairy?utm_source=CI&utm_medium=PressRelease&utm_code=8bdlgv&utm_campaign=1523437+-+Global+Plant+Based+Food+Market+Report+2020-2027%3a+Rising+Industry+Concentration+with+Growth+in+Mergers+and+Acquisitions+in+the+Plant-Based+Products+Space&utm_exec=chdo54prd (accessed on 25 January 2022).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Guan, Q.; Xiong, T.; Xie, M. Influence of Probiotic Fermented Fruit and Vegetables on Human Health and the Related Industrial Development Trend. Engineering 2021, 7, 212–218. [Google Scholar] [CrossRef]
- Murkovic, M.; Mülleder, U.; Neunteufl, H. Carotenoid Content in Different Varieties of Pumpkins. J. Food Compos. Anal. 2002, 15, 633–638. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Biesiada, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Wpływ sposobu przygotowania i warunków przechowywania przecierów, soków przecierowych i soków mętnych z owoców dyni olbrzymiej z dodatkiem owoców pigwowca i derenia na ich właściwości fizykochemiczne, Effect of production methods and storage conditions on the physicochemical properties of purees, puree juices and cloudy juices obtained from pumpkin enriched with japanese quince and cornelian cherry. Żywność. Nauka. Technol. Jakość Food. Sci. Technol. Qual. 2012, 3, 168–178. [Google Scholar]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Dimitrovski, D.; Dimitrovska-Vetadjoka, M.; Hristov, H.; Doneva-Shapceska, D. Developing probiotic pumpkin juice by fermentation with commercial probiotic strain Lactobacillus casei 431. J. Food Process. Preserv. 2021, 45, e15245. [Google Scholar] [CrossRef]
- Semjonovs, P.; Denina, I.; Fomina, A.; Sakirova, L.; Auzina, L.; Patetko, A.; Upite, D. Evaluation of Lactobacillus reuteri Strains for Pumpkin (Cucurbita pepo L.) Juice Fermentation. Biotechnology 2013, 12, 202. [Google Scholar] [CrossRef]
- Szydłowska, A.; Kołożyn-Krajewska, D. Development of potentially probiotic and synbiotic pumpkin frozen desserts. CyTA-J. Food 2019, 17, 251–259. [Google Scholar] [CrossRef]
- Du, B.; Song, Y.; Hu, X.; Liao, X.; Ni, Y.; Li, Q. Oligosaccharides prepared by acid hydrolysis of polysaccharides from pumpkin (Cucurbita moschata) pulp and their prebiotic activities. Int. J. Food Sci. Technol. 2011, 46, 982–987. [Google Scholar] [CrossRef]
- Paredes-Toledo, J. Roasted chickpeas as a probiotic carrier to improve L. plantarum 299v survival during storage. LWT 2021, 146, 111471. [Google Scholar] [CrossRef]
- Genevois, C.; Pieniazek, F.; Messina, V.; Flores, S.; de Escalada, P.M. Bioconversion of pumpkin by-products in novel supplements supporting Lactobacillus casei. LWT 2019, 105, 23–29. [Google Scholar] [CrossRef]
- Ristic-Medic, D.; Petrovic, S.; Arsic, A.; Vucic, V. Liver disease and COVID-19: The link with oxidative stress, antioxidants and nutrition. World J. Gastroenterol. 2021, 27, 5682–5699. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Mayol, I.; Guerra-Valle, M.; Casas-Forero, N.; Sobral, M.C.; Viegas, O.; Alarcón-Enos, J.; Mplvo Ferreira, I.; Pinho, O. Western Dietary Pattern Antioxidant Intakes and Oxidative Stress: Importance During the SARS-CoV-2/COVID-19 Pandemic. Adv. Nutr. 2021, 12, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska, A.; Kołożyn-Krajewska, D. Applying potencially probiotic bacterial strains to pumpkin pulp fermentation. Żywność. Nauka. Technol. Jakość Food. Sci. Technol. Qual. 2010, 17, 109–119. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Szydłowska, A. Frozen Dessert and Its Preparation. Polish Patent 213822 B1, 31 May 2013. [Google Scholar]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcher, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. LWT 2020, 125, 109205. [Google Scholar] [CrossRef]
- Waithera Kuria, M.; Wafula Matofarim, J.; Masani Nduko, J. Physicochemical, antioxidant, and sensory properties of functional mango (Mangifera indica L.) leather fermented by lactic acid bacteria. J. Agric. Food Res. 2021, 6, 100206. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef]
- Hassan, M.F.Y.; Barakat, H. Effect of Carrot and Pumpkin Pulps Adding on Chemical, Rheological, Nutritional and Organoleptic Properties of Ice Cream. Food Nutr. Sci. 2018, 9, 969–982. [Google Scholar] [CrossRef][Green Version]
- Aleksandrzak-Piekarczyk, T.; Koryszewska-Bagińska, A.; Bardowski, J. Genome sequence of the probiotic strain Lactobacillus rhamnosus (Formerly Lactobacillus casei) LOCK 900. Genome Announc. 2013, 1, e00640-13. [Google Scholar] [CrossRef]
- Zielińska, D.; Rzepkowska, A.; Radawska, A.; Zieliński, K. In vitro screening of selected probiotic properties of Lactobacillus strains isolated from traditional fermented cabbage and cucumber. Curr. Microbiol. 2015, 70, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Długosz, E.; Zawistowska-Deniziak, A. Functional properties of food origin Lactobacillus in the gastrointestinal ecosystem—In vitro study. Probiotics Antimicrob. Proteins 2019, 11, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Łepecka, A.; Ołdak, A.; Długosz, E.; Kołożyn-Krajewska, D. Growth and adhesion inhibition of pathogenic bacteria by live and heat-killed food-origin Lactobacillus strains or their supernatants. FEMS Microbiol. Lett. 2021, 368, fnab024. [Google Scholar] [CrossRef] [PubMed]
- NF ISO 15214; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria. Colony Count Technique at 30°C. ISO: London, UK, 1998.
- PN-90/A-75101/07; Processed Fruit and Vegetable Products—Sample Preparation and Physico-Chemical Testing Methods—Determination of Sugar and Sugar-Free Extract Content. The Polish Committee for Standardization: Warsaw, Poland, 1990.
- PN-90/-75101/12; Fruit and Vegetable Products. Determination of Total Carotenoids and β-Carotene. The Polish Committee for Standardization: Warsaw, Poland, 1990.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Feistauer Gomes, C.; Ribeiro Sarkis, J.; Damasceno Ferreira Marczak, L. Ohmic blanching of Tetsukabuto pumpkin: Effects on peroxidase inactivation kinetics and color changes. J. Food Eng. 2018, 233, 74–80. [Google Scholar] [CrossRef]
- Mendoza, F.; Dejmek, P.; Aguilera, H.J. Calibrated color measurements of agricultural foods using image analysis. Postharvest Biol. Technol. 2006, 41, 285–295. [Google Scholar] [CrossRef]
- Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. Available online: https://www.iso.org/standard/58042.html (accessed on 22 January 2022).
- ISO 8586-2; Sensory Analysis. General Guidance for the Selection, Training and Monitoring of Assessors–Part 2: Experts. ISO: London, UK, 1994.
- Granato, D.; Putnik, P.; Kovacevic, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Da Cruz, A.G.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in Chemometrics: Food Authentication, Microbiology, and Effects of Processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef]
- Colombo Pimentel, T.; Iara Gomes de Oliveira, L.; Carvalho de Souza, R.; Magnani, M. Probiotic non-dairy frozen dessert: Technological and sensory aspects and industrial challenges. Trends Food Sci. Technol. 2021, 107, 381–388. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Janak, K.; Vidanarachchi, J.K.; Silva Rocha, R.; Balthaza, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- Dronkers, T.M.G.; Ouwehand, A.C.; Rijkers, G.T. Data on global analysis of clinical trials with probiotics. Data Brief 2020, 32, 106269. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Pellegrino, P. A critical evaluation of the factors affecting the survival and persistence of beneficial bacteria in healthy adults. Benef. Microbes 2021, 12, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Genevois, C.; Flores, S.; de Escalada, P.M. By product from pumpkin (Cucurbita moschata Duchesne ex poiret) as a substrate and vegetable matrix to contain Lactobacillus casei. J. Funct. Foods 2016, 23, 210–221. [Google Scholar] [CrossRef]
- Krawęcka, A.; Libera, J.; Latoch, A. The Use of the Probiotic Lactiplantibacillus plantarum 299v in the Technology of Non-Dairy Ice Cream Based on Avocado. Foods 2021, 10, 2492. [Google Scholar] [CrossRef] [PubMed]
- Väkeväinen, K.; Rinkinen, N.; Willman, R.-M.; Lappi, J.; Raninen, K.; Kårlund, A.; Mikkonen, S.; Plumed-Ferrer, C.; Kolehmainen, M. Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability. Foods 2021, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Machado, C.C.; Carlos Fernandes, M.T.; Saori Ishii Mauro, C.; Silva Farinazzo, F.; Prudencio, S.H.; Garcia, S. Probiotic Juçara and Banana Sorbet: Cell Viability, Antioxidant Activity during Storage and Sensory Acceptability by Children. J. Culin. Sci. Technol. 2021, 19, 460–474. [Google Scholar] [CrossRef]
- De Bellis, P.; Angelo, S.; Lavermicocca, P. Probiotic bacteria and plant-based matrices: An association with improved health-promoting features. J. Funct. Foods 2021, 87, 104821. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Content of bioactive compounds and antioxidant capacity of pumpkin puree enriched with japanese quince, cornelian cherry, strawberry and apples. Acta Sci. Pol. Technol. Aliment. 2011, 10, 51–60. [Google Scholar]
- Azevedo-Meleiro, H.C.; Rodriguez-Amaya, B.D. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef] [PubMed]
- Provesi, J.G.; Odebrecht Dias, C.; Amante, E.R. Changes in carotenoids during processing and storage of pumpkin puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Khaneghah, A.M.; Sant’Ana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Nawirska–Olszańska, A. Chapter 5, Characteristic of investigated pumpkin varieties, Przydatność owoców dyni jako surowca do przetwórstwa spożywczego. In The Usefulness of Pumpkin Fruit as a Raw Material for Food Processing; Dziuba, E., Ed.; Uniwersytet Przyrodniczy we Wrocławiu: Wrocław, Poland, 2011; pp. 23–28. [Google Scholar]
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Fereidoon, S., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2018; pp. 273–286. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of carotenoids and other bioactive molecules in various pumpkin fruits (Cucurbita maxima Duchesne) cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plant 2019, 8, 96. [Google Scholar] [CrossRef]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kahala, M.; Marsol-Vall, A.; Blasco, L.; Järvenpää, E.; Rosenvald, S.; Virtanen, M.; Tarvainen, M.; Yang, B. Impact of lactic acid fermentation on sensory and chemical quality of dairy analogues prepared from lupine (Lupinus angustifolius L.) seeds. Food Chem. 2021, 346, 128852. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, M.; Mujumdar, A.S. Effect of Various Pretreatments on the Quality of Vacuum-Fried Carrot Chips. Dry. Technol. 2006, 24, 1481–1486. [Google Scholar] [CrossRef]
- Itle, R.A.; Kabelka, E.A. Correlation Between L*a*b* Color Space Values and Carotenoid Content in Pumpkins and Squash (Cucurbita spp.). Hortscience 2009, 44, 633–637. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Velioglu, Y.Z. Effects of cooking methods on chlorophylls, pheophytins and color of selected green vegetables. Int. J. Food Sci. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- CIE. Commission Internationale de L’Éclairage, Colorimetry, 2nd ed., Publication CIE No. 15.2, 78 + vi pp., paperbound, price $28; Central Bureau of the CIE, Vienna, 1987. Available in the U.S. from the U.S. National Committee, CIE, c/o National Bureau of Standards, Gaithersburg, Maryland 20899. Special price to USNC members of $22.00. Color Res. Appl. 1988, 13, 64–65. [Google Scholar] [CrossRef]
- Danowska-Oziewicz, M.; Narwojsz, A.; Draszanowska, A.; Marat, N. The effects of cooking method on selected quality traits of broccoli and green asparagus. Int. J. Food Sci. Technol. 2020, 55, 127–135. [Google Scholar] [CrossRef]
- Sipple, L.R.; Racette, C.M.; Schiano, A.N.; Drake, M.A. Consumer perception of ice cream and frozen desserts in the “better-for-you” category. J. Dairy Sci. 2022, 105, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Ice Cream and Sorbet/Other Nutritional Information. Available online: https://www.coldstonecreamery.com/nutrition/pdf/CSC_Nutrition%20Info-Ice%20Cream_Sorbet.pdf (accessed on 20 January 2022).


| Sample Code | Sample Name | Pumpkin Cultivar | Applied Bacterial Strain |
|---|---|---|---|
| MY | Pumpkin flesh | “Melon Yellow” | - |
| PPMY | Pumpkin pulp | “Melon Yellow” | - |
| MY1 | Fermented pumpkin pulp | “Melon Yellow” | Lactobacillus rhamnosus Lock 0900 |
| MY2 | Fermented pumpkin pulp | “Miranda” | Lacticaseibacillus casei O14 |
| SMY1 | Pumpkin sorbet | “Melon Yellow” | Lactobacillus rhamnosus Lock 0900 |
| SMY2 | Pumpkin sorbet | “Melon Yellow” | Lacticaseibacillus casei O14 |
| M | Pumpkin flesh | “Miranda” | - |
| PPM | Pumpkin pulp | “Miranda” | - |
| M1 | Fermented pumpkin pulp | “Melon Yellow” | Lactobacillus rhamnosus Lock 0900 |
| M2 | Fermented pumpkin pulp | “Miranda” | Lacticaseibacillus casei O14 |
| SM1 | Pumpkin sorbet | “Miranda” | Lactobacillus rhamnosus Lock 0900 |
| SM2 | Pumpkin sorbet | “Miranda” | Lacticaseibacillus casei O14 |
| Sample Code | pH | Count of Bacteria [log CFU/g] |
|---|---|---|
| MY | 6.95 ± 0.03 c | - |
| PPMY | 6.92 ± 0.10 c | - |
| MY1 | 4.50 ± 0.03 a | 9.48 ± 0.07 c |
| MY2 | 4.53 ± 0.03 a | 9.41 ± 0.09 c |
| SMY1 | 4.57 ± 0.05 a | 8.69 ± 0.11 a |
| SMY2 | 4.71 ± 0.04 b | 8.82 ± 0.05 b |
| Sample Code | pH | Count of Bacteria [log CFU/g] |
|---|---|---|
| M | 6.51 ± 0.03 d | - |
| PPM | 6.53 ± 0.07 d | - |
| M1 | 4.70 ± 0.09 b | 9.26 ± 0.12 d |
| M2 | 4.45 ± 0.08 a | 9.16 ± 0.09 c |
| SM1 | 4.80 ± 0.03 c | 8.35 ± 0.10 a |
| SM2 | 4.61 ± 0.03 b | 8.94 ± 0.04 b |
| Sample Code | Reducing Sugars [%] | Total Sugars [%] | Saccharose [%] |
|---|---|---|---|
| MY | 2.30 ± 0.07 e | 3.12 ± 0.10 d | 0.78 ± 0.06 c |
| PPMY | 2.11 ± 0.10 d | 2.56 ± 0.05 c | 0.44 ± 0.06 b |
| MY1 | 1.43 ± 0.06 b | 1.84 ± 0.05 b | 0.35 ± 0.06 a |
| MY2 | 1.28 ± 0.05 a | 1.75 ± 0.11 a | 0.45 ± 0.07 b |
| SMY1 | 1.39 ± 0.09 b | 15.20 ± 0.08 e | 13.10 ± 0.10 d |
| SMY2 | 1.31 ± 0.09 a | 15.62 ± 0.05 f | 13.50 ± 0.07 e |
| Sample Code | Reducing Sugars [%] | Total Sugars [%] | Saccharose [%] |
|---|---|---|---|
| M | 1.25 ± 0.07 d | 2.05 ± 0.06 d | 0.76 ± 0.04 c |
| PPM | 1.00 ± 0.03 c | 1.80 ± 0.03 c | 0.76 ± 0.11 c |
| M1 | 0.80 ± 0.12 b | 1.45 ± 0.06 b | 0.62 ± 0.05 b |
| M2 | 0.75 ± 0.06 a,b | 1.31 ± 0.05 a | 0.53 ± 0.07 a |
| SM1 | 0.76 ± 0.10 a,b | 12.10 ± 0.06 f | 10.77 ± 0.12 e |
| SM2 | 0.69 ± 0.09 a | 11.20 ± 0.10 e | 9.98 ± 0.07 d |
| Sample Code | Total Carotenoids (µg/g FW) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| MY | 4.30 ± 0.65 e | 47.01 ± 2.10 b | 10.96 ± 1.14 c,d | 45.53 ± 0.15 d | - |
| PPMY | 3.90 ± 0.41 e | 45.91 ± 1.03 b | 12.49 ± 1.12 d | 47.82 ± 2.10 d,e | 2.96 |
| MY1 | 2.90 ± 0.11 c | 41.77 ± 1.23 a | 12.58 ± 0.09 d | 43.95 ± 1.11 d | 5.74 |
| MY2 | 3.20 ± 0.09 d | 42.60 ± 1.51 a | 10.24 ± 0.02 c | 26.75 ± 0.04 b | 19.30 |
| SMY1 | 1.80 ± 0.09 a | 50.34 ± 0.34 c | 9.92 ± 0.12 a, b | 35.68 ± 0.81 c | 10.45 |
| SMY2 | 2.60 ± 0.03 b | 54.25 ± 0.23 d | 4.93 ± 0.15 a | 20.16 ± 0.13 a | 27.06 |
| Sample Code | Total Carotenoids (µg/g FW) | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| M | 1.75 ± 1.42 e,f | 44.5 ± 2.04 c | 14.01 ± 1.23 e | 39.65 ± 0.91 d | - |
| PPM | 1.50 ± 0.35 e | 43.81 ± 1.78 c | 12.21 ± 0.01 d | 41.50 ± 1.11 d | 2.67 |
| M1 | 1.00 ± 0.10 c | 40.00 ± 0.11 a | 8.13 ± 0.12 c | 34.37 ± 0.09 c | 9.15 |
| M2 | 1.15 ± 0.08 d | 42.54 ± 0.54 b | 7.50 ± 0.07 b | 33.24 ± 0.06 b | 9.34 |
| SM1 | 0.65 ± 0.12 a | 50.75 ± 1.39 d | 3.26 ± 0.09 a | 32.45 ± 0.09 a | 14.40 |
| SM2 | 0.95 ± 0.11 b | 50.10 ± 2.33 d | 3.21 ± 0.11 a | 34.20 ± 0.11 c | 13.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydłowska, A.; Zielińska, D.; Kołożyn-Krajewska, D. Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts. Appl. Sci. 2022, 12, 8063. https://doi.org/10.3390/app12168063
Szydłowska A, Zielińska D, Kołożyn-Krajewska D. Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts. Applied Sciences. 2022; 12(16):8063. https://doi.org/10.3390/app12168063
Chicago/Turabian StyleSzydłowska, Aleksandra, Dorota Zielińska, and Danuta Kołożyn-Krajewska. 2022. "Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts" Applied Sciences 12, no. 16: 8063. https://doi.org/10.3390/app12168063
APA StyleSzydłowska, A., Zielińska, D., & Kołożyn-Krajewska, D. (2022). Effect of Pumpkin Cultivar on the Selected Quality Parameters of Functional Non-Dairy Frozen Desserts. Applied Sciences, 12(16), 8063. https://doi.org/10.3390/app12168063







