Adsorption of Methyl Orange on a Novel Palygorskite/UiO-66 Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of UiO-66
2.1.2. Synthesis of Palygorskite/UiO-66 Composite
2.2. Materials Characterization
2.3. Adsorption Experiments
2.4. Theoretical Simulations
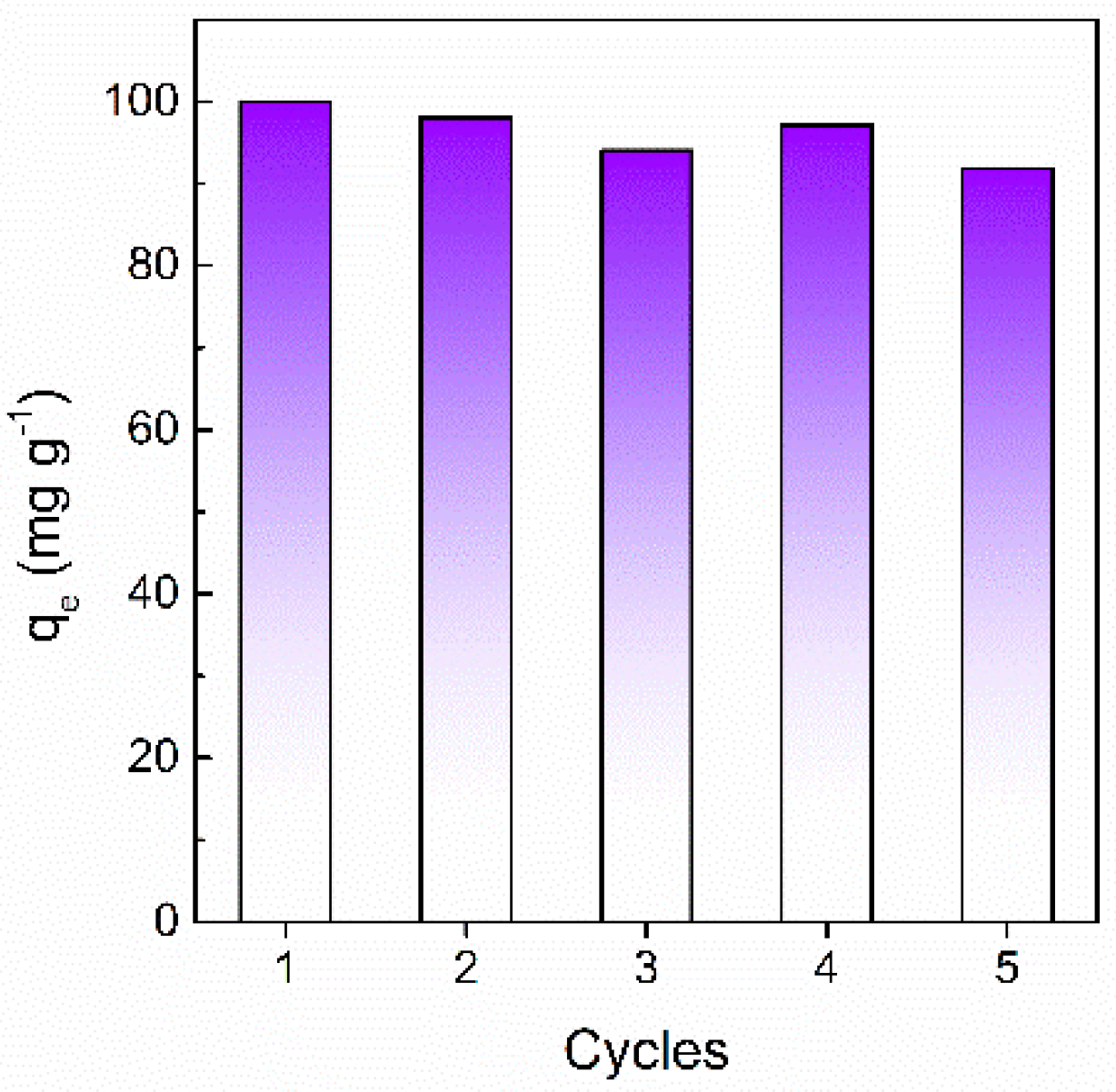
2.5. Recycling Experiments
3. Results and Discussion
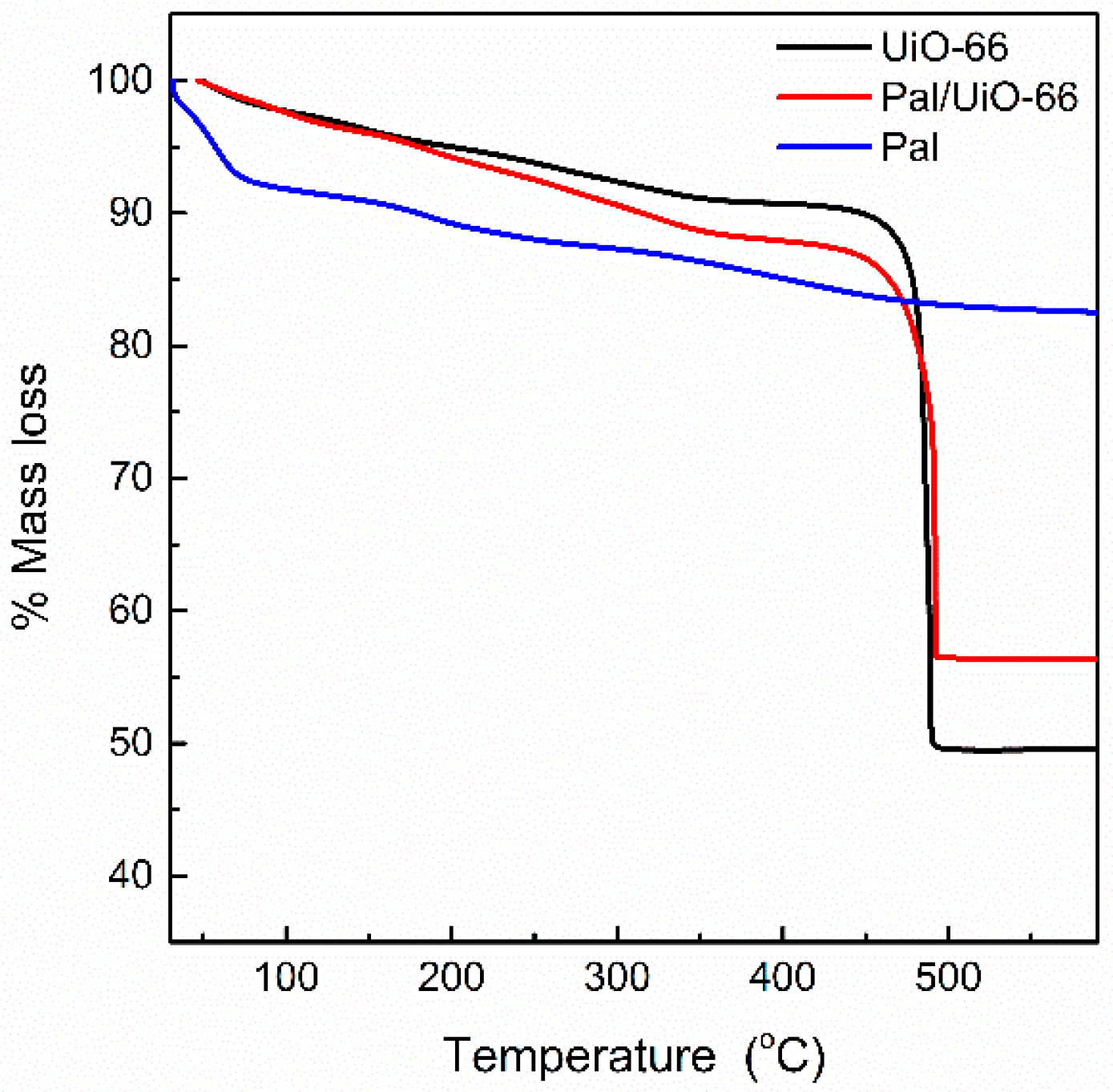
3.1. Materials Characterization
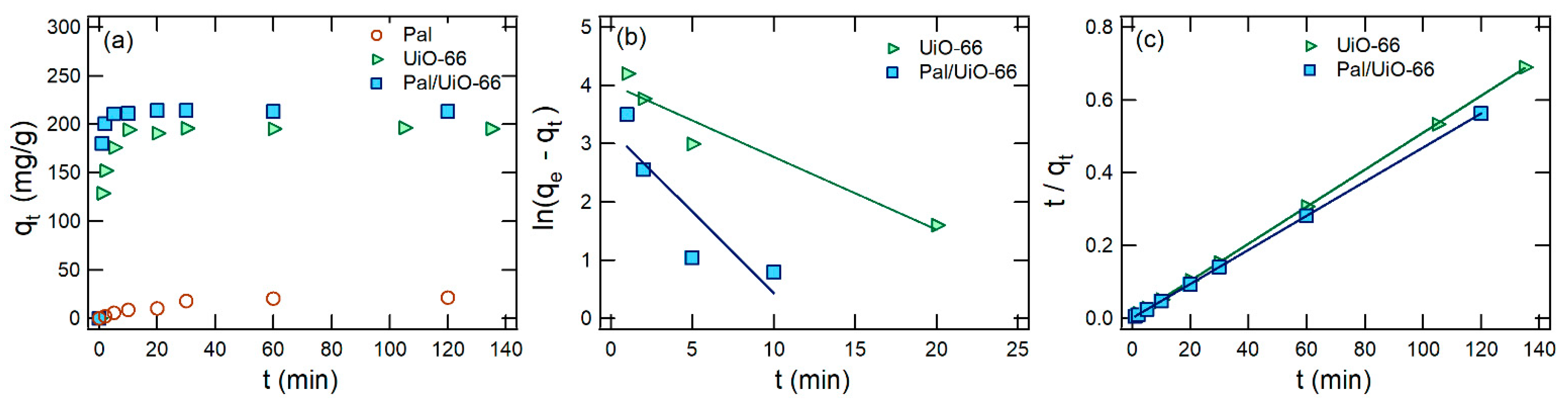
3.2. Dye Adsorption Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Swetha, S. Mixed Biosorbent of Agro Waste and Bacterial Biomass for the Separation of Pb(II) Ions from Water System. Chemosphere 2021, 277, 130236. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, M.; Wang, H.; Zeng, G.; Huang, D.; Cheng, M.; Liu, Y.; Xue, W.; Wang, Z. The Application of Different Typological and Structural MOFs-Based Materials for the Dyes Adsorption. Coord. Chem. Rev. 2019, 380, 471–483. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Loganathan, M.; Senthil Kumar, P.; Vo, D.-V.N. Cobalt and Nickel Oxides Supported Activated Carbon as an Effective Photocatalysts for the Degradation Methylene Blue Dye from Aquatic Environment. Sustain. Chem. Pharm. 2021, 21, 100406. [Google Scholar] [CrossRef]
- Renita, A.A.; Vardhan, K.H.; Kumar, P.S.; Ngueagni, P.T.; Abilarasu, A.; Nath, S.; Kumari, P.; Saravanan, R. Effective Removal of Malachite Green Dye from Aqueous Solution in Hybrid System Utilizing Agricultural Waste as Particle Electrodes. Chemosphere 2021, 273, 129634. [Google Scholar] [CrossRef]
- Liang, J.; Ning, X.; Sun, J.; Song, J.; Lu, J.; Cai, H.; Hong, Y. Toxicity Evaluation of Textile Dyeing Effluent and Its Possible Relationship with Chemical Oxygen Demand. Ecotoxicol. Environ. Saf. 2018, 166, 56–62. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective Removal of Anionic Textile Dyes Using Adsorbent Synthesized from Coffee Waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Au, V.K.-M. Recent Advances in the Use of Metal-Organic Frameworks for Dye Adsorption. Front. Chem. 2020, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.-V.N. A Review on Cleaner Strategies for Extraction of Chitosan and Its Application in Toxic Pollutant Removal. Environ. Res. 2021, 196, 110996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; AlGarni, T.S.; Kumar, P.S.; Bhogal, S.; Kumar, A.; Sharma, S.; Naushad, M.; ALOthman, Z.A.; Stadler, F.J. Utilization of Ag2O–Al2O3–ZrO2 Decorated onto RGO as Adsorbent for the Removal of Congo Red from Aqueous Solution. Environ. Res. 2021, 197, 111179. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Al-Sehemi, A.G.; Mubashir, M.; Mukhtar, A.; Saqib, S.; Bustam, M.A.; Cheng, C.K.; Ibrahim, M.; Show, P.L. Adsorption Behavior of Mercury over Hydrated Lime: Experimental Investigation and Adsorption Process Characteristic Study. Chemosphere 2021, 271, 129504. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption Properties and Mechanisms of Palygorskite for Removal of Various Ionic Dyes from Water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Enhancing the Adsorption Capacities of Acid Dyes by Chitosan Nano Particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef]
- Belaid, K.D.; Kacha, S.; Kameche, M.; Derriche, Z. Adsorption Kinetics of Some Textile Dyes onto Granular Activated Carbon. J. Environ. Chem. Eng. 2013, 1, 496–503. [Google Scholar] [CrossRef]
- Li, C.-P.; Zhou, H.; Chen, J.; Wang, J.-J.; Du, M.; Zhou, W. A Highly Efficient Coordination Polymer for Selective Trapping and Sensing of Perrhenate/Pertechnetate. ACS Appl. Mater. Interfaces 2020, 12, 15246–15254. [Google Scholar] [CrossRef]
- Abidi, N.; Errais, E.; Duplay, J.; Berez, A.; Jrad, A.; Schäfer, G.; Ghazi, M.; Semhi, K.; Trabelsi-Ayadi, M. Treatment of Dye-Containing Effluent by Natural Clay. J. Clean. Prod. 2015, 86, 432–440. [Google Scholar] [CrossRef]
- Lagaly, G. Colloid Clay Science. Colloid Clay Science. Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; Volume 1, pp. 141–245. ISBN 978-0-08-044183-2. [Google Scholar]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of Chemical Modification of Palygorskite and Sepiolite by 3-Aminopropyltriethoxisilane on Adsorption of Cationic and Anionic Dyes. Appl. Clay Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Middea, A.; Spinelli, L.S.; Souza, F.G., Jr.; Neumann, R.; Fernandes, T.L.A.P.; Gomes, O.d.F.M. Preparation and Characterization of an Organo-Palygorskite-Fe3O4 Nanomaterial for Removal of Anionic Dyes from Wastewater. Appl. Clay Sci. 2017, 139, 45–53. [Google Scholar] [CrossRef]
- García-Romero, E.; Suárez, M. On the Chemical Composition of Sepiolite and Palygorskite. Clays Clay Miner. 2010, 58, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Nikolopoulou, A.; Christoforidis, K.C.; Fernández-Garcia, M.; Li, H.; Shu, Y.; Sato, T. Palygorskite-TiO2 Nanocomposites: Part 2. Photocatalytic Activities in Decomposing Air and Organic Pollutants. Appl. Clay Sci. 2013, 83–84, 198–202. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Shu, Y.; Sato, T.; Katsuki, H. Three-Phase Nanocomposites of Two Nanoclays and TiO2: Synthesis, Characterization and Photacatalytic Activities. Appl. Catal. B Environ. 2014, 147, 526–533. [Google Scholar] [CrossRef]
- Papoulis, D.; Panagiotaras, D.; Tsigrou, P.; Christoforidis, K.C.; Petit, C.; Apostolopoulou, A.; Stathatos, E.; Komarneni, S.; Koukouvelas, I. Halloysite and Sepiolite-TiO2 nanocomposites: Synthesis Characterization and Photocatalytic Activity in Three Aquatic Wastes. Mater. Sci. Semicond. Process. 2018, 85, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, A.; Wu, J.; Zhao, J.; Yan, H. Adsorption Behaviors and Mechanisms of Methyl Orange on Heat-Treated Palygorskite Clays. Ind. Eng. Chem. Res. 2012, 51, 14026–14036. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Feng, X.; Yu, W.; Zhang, D.; Fu, J. Palygorskite-Cerium Oxide Filled Rubber Nanocomposites. Appl. Clay Sci. 2012, 67–68, 44–49. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Gregg, A.; Moss, B.; Kafizas, A.; Petit, C. The Effect of Materials Architecture in TiO2/MOF Composites on CO2 Photoreduction and Charge Transfer. Small 2019, 15, e1805473. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 Capture and Photocatalytic Reduction Using Bifunctional TiO2/MOF Nanocomposites under UV–Vis Irradiation. Appl. Catal. B Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, Z.; Song, J.; Song, J.; Pan, F.; Zhang, P.; Cao, X. Elevated Pervaporative Desulfurization Performance of Pebax-Ag+ @MOFs Hybrid Membranes by Integrating Multiple Transport Mechanisms. Ind. Eng. Chem. Res. 2019, 58, 16911–16921. [Google Scholar] [CrossRef]
- Haque, E.; Lee, J.E.; Jang, I.T.; Hwang, Y.K.; Chang, J.-S.; Jegal, J.; Jhung, S.H. Adsorptive Removal of Methyl Orange from Aqueous Solution with Metal-Organic Frameworks, Porous Chromium-Benzenedicarboxylates. J. Hazard. Mater. 2010, 181, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Yang, J.-M. A Facile Approach to Fabricate an Immobilized-Phosphate Zirconium-Based Metal-Organic Framework Composite (UiO-66-P) and Its Activity in the Adsorption and Separation of Organic Dyes. J. Colloid Interface Sci. 2017, 505, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, Q.; Lv, M.; Xu, Y.; Yang, H.; Liu, X.; Wei, F. Selective Adsorption of Cationic Dyes by UiO-66-NH2. Appl. Surf. Sci. 2015, 327, 77–85. [Google Scholar] [CrossRef]
- Shahriyari Far, H.; Hasanzadeh, M.; Najafi, M.; Rahimi, R. In-Situ Self-Assembly of Mono- and Bi-Metal Organic Frameworks onto Clay Mineral for Highly Efficient Adsorption of Pollutants from Wastewater. Chem. Phys. Lett. 2022, 799, 139626. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Li, Y.; Zhang, R.; Lu, G. Large-Scale Synthesis of Monodisperse UiO-66 Crystals with Tunable Sizes and Missing Linker Defects via Acid/Base Co-Modulation. ACS Appl. Mater. Interfaces 2017, 9, 15079–15085. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Ma, H.; Wang, Z.-H.; Li, C.-Y.; Zhao, N.; Wang, S. Simultaneous Adsorption of Methyl Orange and Methylene Blue from Aqueous Solution Using Amino Functionalized Zr-Based MOFs. Microporous Mesoporous Mater. 2019, 282, 179–187. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River Flow Forecasting through Conceptual Models Part I—A Discussion of Principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Giustetto, R.; Compagnoni, R. An Unusual Occurrence of Palygorskite from Montestrutto, Sesia-Lanzo Zone, Internal Western Alps (Italy). Clay Miner. 2011, 46, 371–385. [Google Scholar] [CrossRef]
- Giustetto, R.; Chiari, G. Crystal Structure Refinement of Palygorskite from Neutron Powder Diffraction. Eur. J. Mineral. 2004, 16, 521–532. [Google Scholar] [CrossRef]
- Giustetto, R.; Seenivasan, K.; Pellerej, D.; Ricchiardi, G.; Bordiga, S. Spectroscopic Characterization and Photo/Thermal Resistance of a Hybrid Palygorskite/Methyl Red Mayan Pigment. Microporous Mesoporous Mater. 2012, 155, 167–176. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of Acid Activation of Palygorskite on Their Toluene Adsorption Behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Tang, J.; Dong, W.; Wang, G.; Yao, Y.; Cai, L.; Liu, Y.; Zhao, X.; Xu, J.; Tan, L. Efficient Molybdenum(VI) Modified Zr-MOF Catalysts for Epoxidation of Olefins. RSC Adv. 2014, 4, 42977–42982. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Liu, X.; Yue, C.; Ao, L.; Deng, T.; Zhang, Y. Heteropoly Acid-Encapsulated Metal–Organic Framework as a Stable and Highly Efficient Nanocatalyst for Esterification Reaction. RSC Adv. 2019, 9, 16357–16365. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile Synthesis of Morphology and Size-Controlled Zirconium Metal–Organic Framework UiO-66: The Role of Hydrofluoric Acid in Crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Engineering the Surface of a New Class of Adsorbents: Metal–Organic Framework/Graphite Oxide Composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef]
- Muschi, M.; Devautour-Vinot, S.; Aureau, D.; Heymans, N.; Sene, S.; Emmerich, R.; Ploumistos, A.; Geneste, A.; Steunou, N.; Patriarche, G.; et al. Metal–Organic Framework/Graphene Oxide Composites for CO2 Capture by Microwave Swing Adsorption. J. Mater. Chem. A 2021, 9, 13135–13142. [Google Scholar] [CrossRef]
- Middea, A.; Fernandes, T.L.A.P.; Neumann, R.; Gomes, F.M.O.; Spinelli, L.S. Evaluation of Fe(III) Adsorption onto Palygorskite Surfaces. Appl. Surf. Sci. 2013, 282, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Nanthamathee, C.; Chantarangkul, C.; Jakkrawhad, C.; Payaka, A.; Dechatiwongse, P. Fine-Tuning the Dye Adsorption Capacity of UiO-66 by a Mixed-Ligand Approach. Heliyon 2022, 8, e08961. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliadou, I.A.; Ioannidou, T.; Anagnostopoulou, M.; Polyzotou, A.; Papoulis, D.; Christoforidis, K.C. Adsorption of Methyl Orange on a Novel Palygorskite/UiO-66 Nanocomposite. Appl. Sci. 2022, 12, 7468. https://doi.org/10.3390/app12157468
Vasiliadou IA, Ioannidou T, Anagnostopoulou M, Polyzotou A, Papoulis D, Christoforidis KC. Adsorption of Methyl Orange on a Novel Palygorskite/UiO-66 Nanocomposite. Applied Sciences. 2022; 12(15):7468. https://doi.org/10.3390/app12157468
Chicago/Turabian StyleVasiliadou, Ioanna A., Thaleia Ioannidou, Maria Anagnostopoulou, Antonios Polyzotou, Dimitrios Papoulis, and Konstantinos C. Christoforidis. 2022. "Adsorption of Methyl Orange on a Novel Palygorskite/UiO-66 Nanocomposite" Applied Sciences 12, no. 15: 7468. https://doi.org/10.3390/app12157468
APA StyleVasiliadou, I. A., Ioannidou, T., Anagnostopoulou, M., Polyzotou, A., Papoulis, D., & Christoforidis, K. C. (2022). Adsorption of Methyl Orange on a Novel Palygorskite/UiO-66 Nanocomposite. Applied Sciences, 12(15), 7468. https://doi.org/10.3390/app12157468








