A Mathematical Modeling and Statistical Analysis of Phycobiliprotein Fluorescence Decay under Exposure to Excitation Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Purification of C-Pc Complex
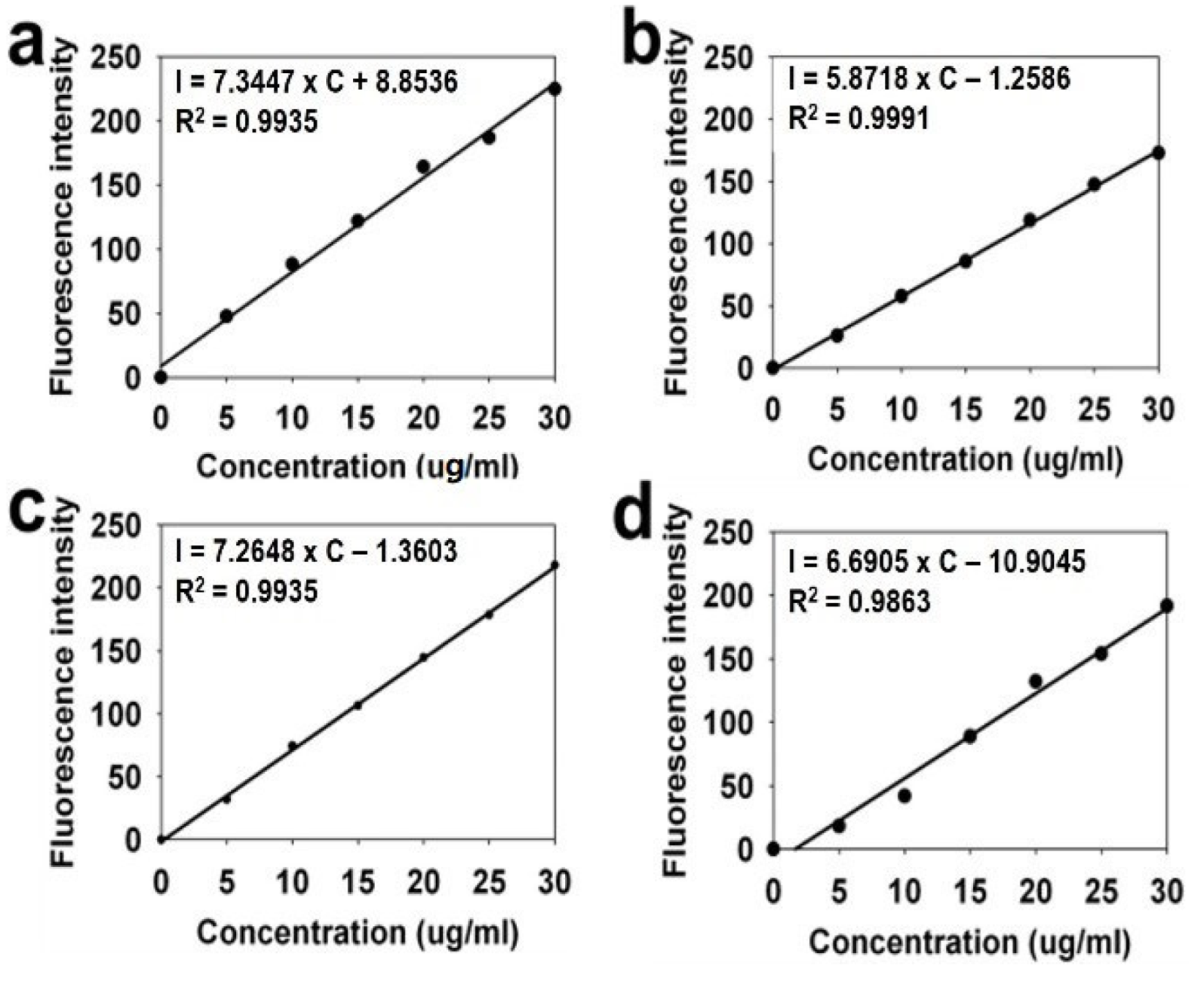
2.3. Fluorescence and Absorbance Spectroscopy
2.4. Statistical Analyses
3. Results
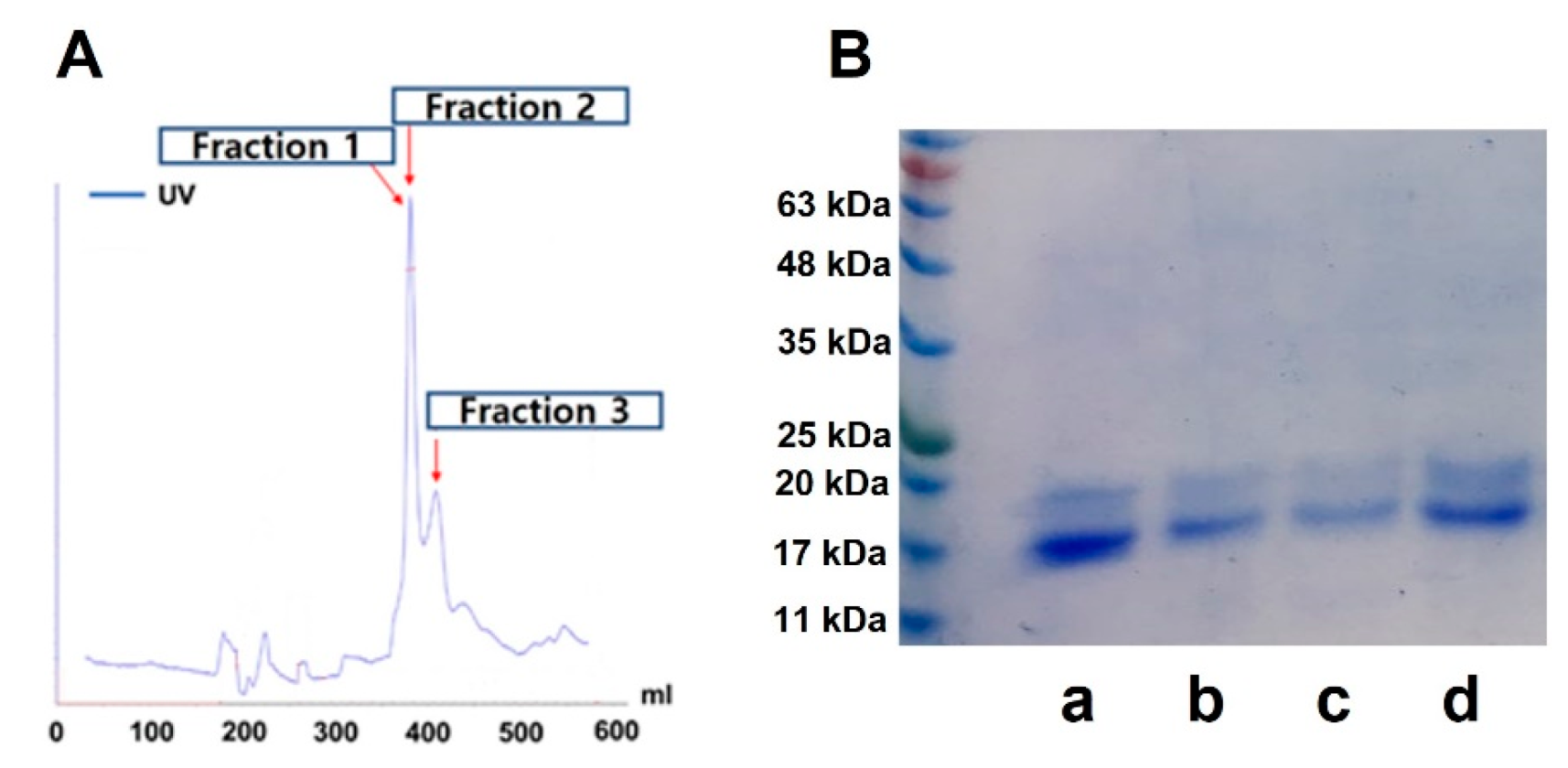
3.1. Purification of C-Pc from S. maxima
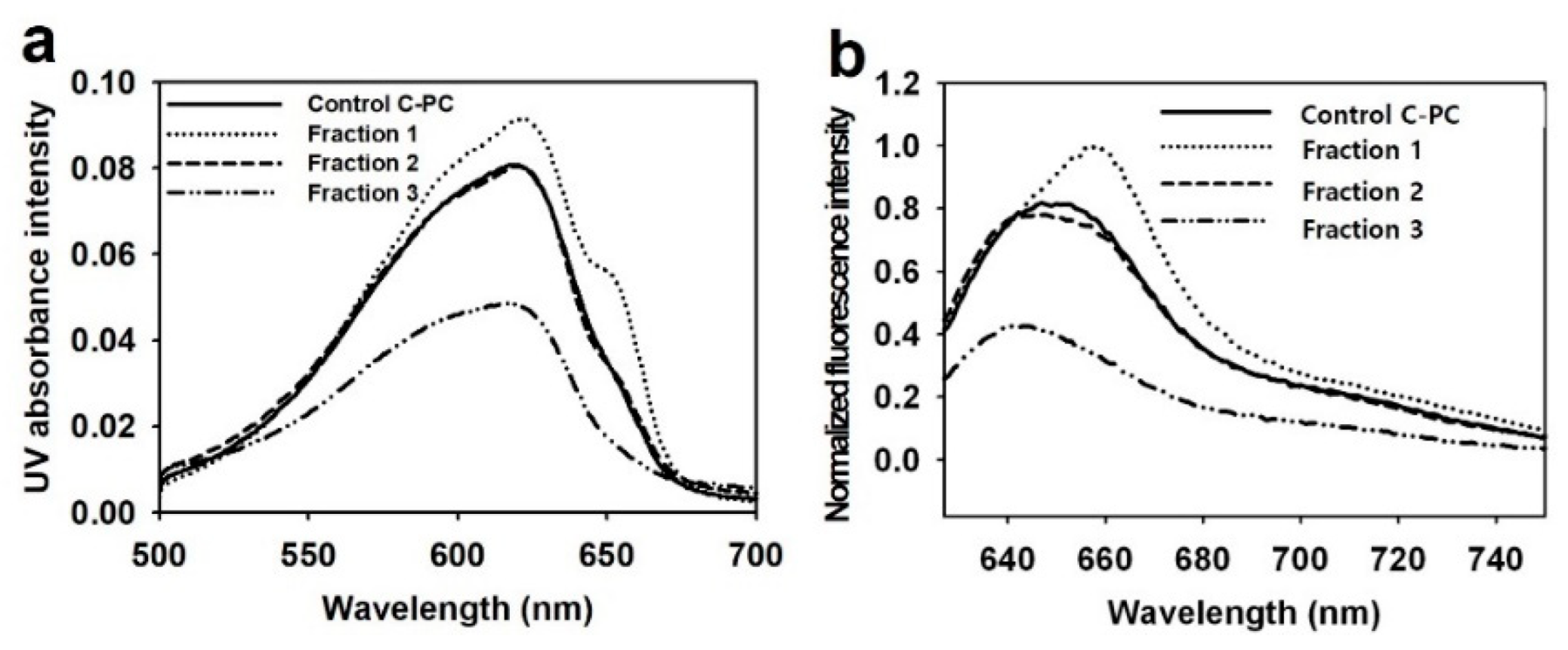
3.2. Time Serial Measurement of Fluorescence Intensity of C-Pc
3.3. Statistical Analysis of the Similarity of the Decay Trend among Fractions
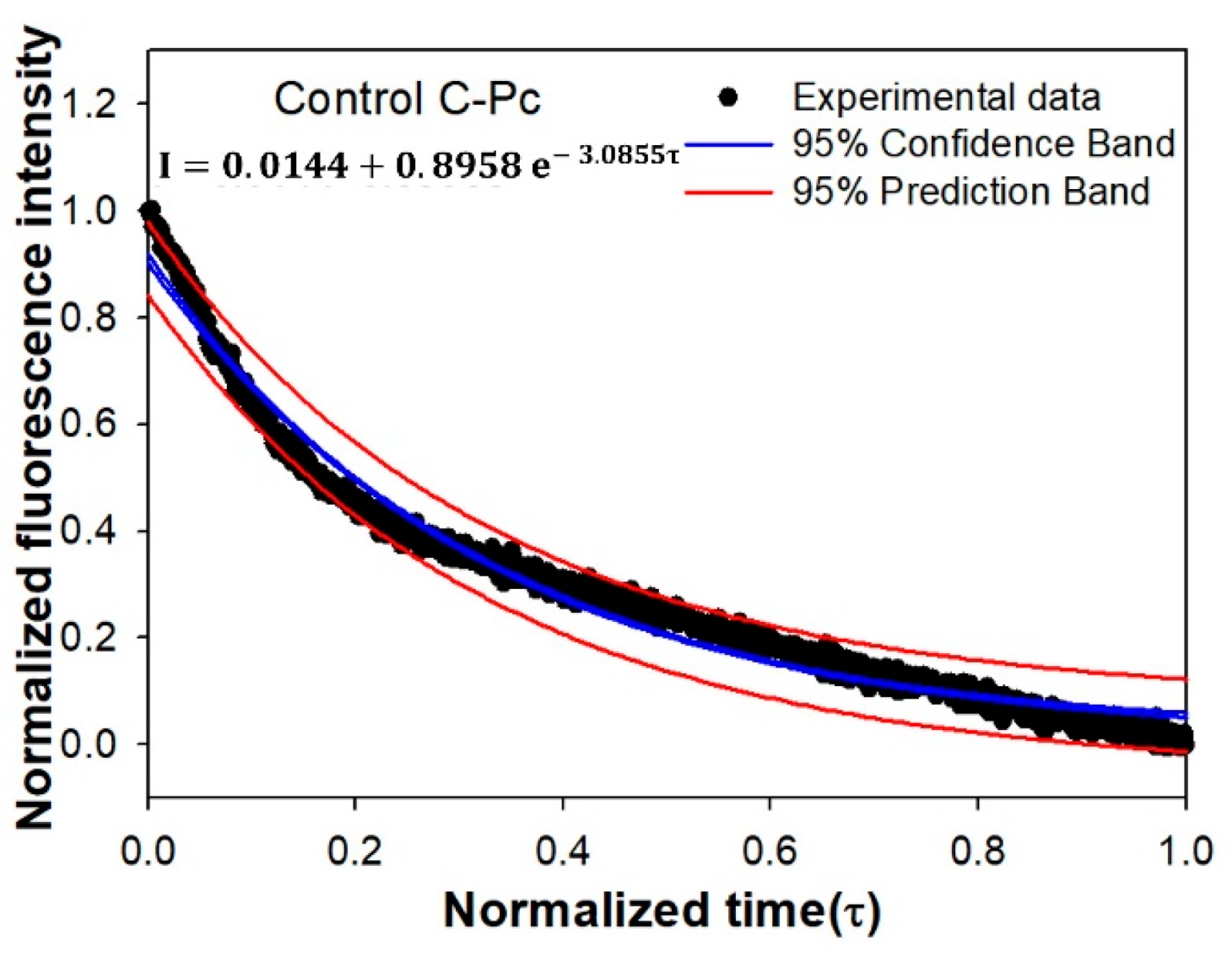
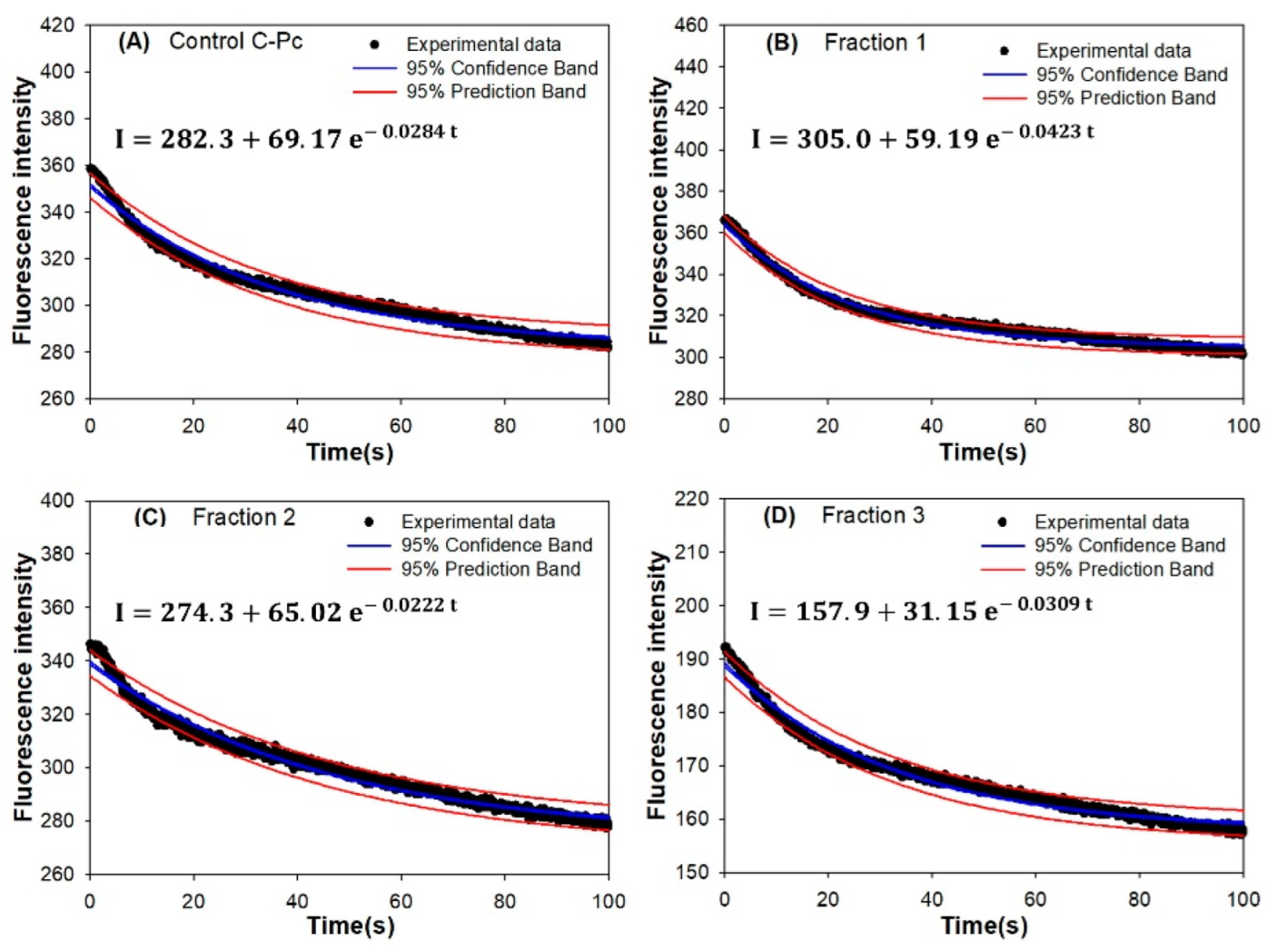
3.4. Mathematical Modeling of the Fluorescence Intensity of C-Pc
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C-Pc | C-Phycocyanin |
| APC | Allophycocyanin |
| PE | Phycoerythrin |
| Fluorescence intensity of first group | |
| Fluorescence intensity of second group | |
| Sample mean for the first group | |
| Standard deviation for the first group | |
| Sample mean for the second variable | |
| Standard deviation for the second group | |
| n | column length |
References
- Kathiravan, A.; Udayan, E.; Kumar, R.R. Bioprospecting of Spirulina biomass using novel extraction method for the production of C-Phycocyanin as effective food colourant. Vegetos 2022, 35, 484–492. [Google Scholar] [CrossRef]
- Garcia, A.B.; Longo, E.; Bermejo, R. The application of a phycocyanin extract obtained from Arthrospira platensis as a blue natural colorant in beverages. J. Appl. Phycol. 2021, 33, 3059–3070. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Dinh, T.B.; Choi, S.; Saeger, J.D.; Depuydt, S.; Brown, M.T.; Han, T. Commercial potential of the cyanobacterium Arthrospira maxima: Physiological and biochemical traits and the purification of phycocyanin. Biology 2022, 11, 628. [Google Scholar] [CrossRef]
- Liu, Q.; Hyang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical application of Spirulina platensis derived C-Phycocyanin. Evid.-Based Complementary Altern. Med. 2016, 2016, 7803846. [Google Scholar]
- Zhang, W.; Kuang, C.; Chen, X.; Yang, X.; Guan, H. Enhanced photoelectron transfer from light-harvesting antenna of phycocyanin to Fe3O4 hierarchical structure. Appl. Surf. Sci. 2021, 566, 150652. [Google Scholar] [CrossRef]
- Schrantz, K.; Wyss, P.P.; Ihssen, J.; Toth, R.; Bora, D.K.; Vitol, E.A.; Rozhkova, E.; Pieles, U.; Thöny-Meyer, L.; Braun, A. Hematite Photoanode Co-Functionalized with Self-Assembling Melanin and C-Phycocyanin for Solar Water Splitting at Neutral pH. Catal. Today 2017, 284, 44–51. [Google Scholar] [CrossRef]
- Sekar, N.; Umasankar, Y.; Ramasamy, R.P. Photocurrent Generation by Immobilized Cyanobacteria Via Direct Electron Transport in Photo-Bioelectrochemical Cells. Phys. Chem. Chem. Phys. 2014, 16, 7862–7871. [Google Scholar] [CrossRef]
- Fabre, J.F.; Niangoran, N.U.F.; Gaignard, C.; Buso, D.; Mouloungui, Z.; Valentin, R. Extraction, purification and stability of C-phycocyanin from Arthrospira platensis. Eur. Food Res. Technol. 2022, 248, 1583–1599. [Google Scholar] [CrossRef]
- Edwards, M.R.; Hauer, C.; Stack, R.F.; Eisele, L.E.; MacColl, R. Thermophilic C-Phycocyanin: Effect of Temperature, Monomer Stability, and Structure. Biochim. Biophys. Acta–Bioenerg. 1997, 1321, 157–164. [Google Scholar] [CrossRef]
- Patel, A.; Pawar, R.; Mishra, S.; Sonawane, S.; Ghosh, P.K. Kinetic Studies on Thermal Denaturation of C-Phycocyanin. Indian J. Biochem. Biophys. 2004, 41, 254–257. [Google Scholar]
- Faieta, M.; Toong, C.; Corradini, M.G.; Ludescher, R.D.; Pittia, P. Degradation kinetics of C-Phycocyanin under isothermal and dynamic thermal treatments. Food Chem. 2022, 382, 132266. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of Phycocyanin Extracted from Spirulina Sp.: Influence of Temperature, pH and Preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Liu, L.N.; Chen, X.L.; Zhang, Y.Z.; Zhou, B.C. Characterization, Structure and Function of Linker Polypeptides in Phycobilisomes of Cyanobacteria and Red Algae: An Overview. Biochim. Biophys. Acta-Bioenerg. 2005, 1708, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, R.; Ortiz-Butrón, R.; Blas-Valdivia, V.; Hernández-García, A.; Cano-Europa, E. Phycobiliproteins Or C-Phycocyanin of Arthrospira (Spirulina) Maxima Protect Against HgCl2-Caused Oxidative Stress and Renal Damage. Food Chem. 2012, 135, 2359–2365. [Google Scholar] [CrossRef]
- Gómez-Lojero, C.; Pérez-Gómez, B.; Krogmann, D.W.; Peña-Diaz, A. The Tricylindrical Core of the Phycobilisome of the Cyanobacterium Arthrospira (Spirulina) Maxima. Int. J. Biochem. Cell Biol. 1997, 29, 959–970. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Mullineaux, C.W.; Sarcina, M. Probing the Dynamics of Photosynthetic Membranes with Fluorescence Recovery After Photobleaching. Trends Plant Sci. 2002, 7, 237–240. [Google Scholar] [CrossRef]
- Joshua, S.; Bailey, S.; Mann, N.H.; Mullineaux, C.W. Involvement of Phycobilisome Diffusion in Energy Quenching in Cyanobacteria. Plant Physiol. 2005, 138, 1577–1585. [Google Scholar] [CrossRef][Green Version]
- Yamanaka, G.; Glazer, A.N. Dynamic Aspects of Phycobilisome Structure. Archi. Microbiol. 1980, 124, 39–47. [Google Scholar] [CrossRef]
- Barbara, A.; Zilinskas, B.A.; Greenwald, L.S. Phycobilisome Structure and Function. Photosynth. Res. 1986, 10, 7–35. [Google Scholar]
- Gutfreund, H. Kinetics for the Life Sciences: Receptors, Transmitters and Catalysts; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Sun, B.; Lim, D.S.W.; Kuo, J.S.; Kuyper, C.L.; Chiu, D.T. Fast Initiation of Chemical Reactions with Laser-Induced Breakdown of a Nanoscale Partition. Langmuir 2004, 20, 9437–9440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Errede, B.; Elston, T.C. Mathematical Analysis and Quantification of Fluorescent Proteins as Transcriptional Reporters. Biophys. J. 2008, 94, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, K.V.; Selvaraju, M.; Thirugnanamoorthy, K. Enrichment of Chlorophyll and Phycobiliproteins in Spirulina Platensis by the use of Reflector Light and Nitrogen Sources: An in-Vitro Study. Biomass Bioenergy 2012, 47, 436–441. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and Characterization of C-Phycocyanin from Cyanobacterial Species of Marine and Freshwater Habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Bhaskar, S.U.; Gopalaswamy, G.; Raghu, R. A Simple Method for Efficient Extraction and Purification of C-Phycocyanin from Spirulina Platensis Geitler. Indian J. Exp. Biol. 2005, 43, 277–279. [Google Scholar]
- Mishra, S.K.; Shrivastav, A.; Mishra, S. Effect of Preservatives for Food Grade C-PC from Spirulina Platensis. Process Biochem. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Pancha, I.; Jain, D.; Mishra, S. Effect of Preservatives for Food Grade C-Phycoerythrin, Isolated from Marine Cyanobacteria Pseudanabaena sp. Int. J. Biol. Macromol. 2010, 47, 597–602. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and Purification of C-Phycocyanin from Spirulina Platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef]
- Riter, R.E.; Edington, M.D.; Beck, W.F. Isolated-Chromophore and Exciton-State Photophysics in C-Phycocyanin Trimers. J. Phys. Chem. B 1997, 101, 2366–2371. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of Phycocyanin Extraction from Spirulina Platensis using Factorial Design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Yekta, A.; Xu, B.; Duhamel, J.; Adiwidjaja, H.; Winnik, M.A. Fluorescence Studies of Associating Polymers in Water: Determination of the Chain End Aggregation Number and a Model for the Association Process. Macromolecules 1995, 28, 956–966. [Google Scholar] [CrossRef]
- Delon, A.; Usson, Y.; Derouard, J.; Biben, T.; Souchier, C. Photobleaching, Mobility, and Compartmentalisation: Inferences in Fluorescence Correlation Spectroscopy. J. Fluoresc. 2004, 14, 255–267. [Google Scholar] [CrossRef] [PubMed]


| Fraction | Pearson Correlation Coefficient | p-Value |
|---|---|---|
| 1 vs. 2 | 0.982 | p < 0.001 |
| 1 vs. 3 | 0.993 | p < 0.001 |
| 2 vs. 3 | 0.993 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Shin, A.H. A Mathematical Modeling and Statistical Analysis of Phycobiliprotein Fluorescence Decay under Exposure to Excitation Light. Appl. Sci. 2022, 12, 7469. https://doi.org/10.3390/app12157469
Hwang J, Shin AH. A Mathematical Modeling and Statistical Analysis of Phycobiliprotein Fluorescence Decay under Exposure to Excitation Light. Applied Sciences. 2022; 12(15):7469. https://doi.org/10.3390/app12157469
Chicago/Turabian StyleHwang, Jinha, and Alyssa H. Shin. 2022. "A Mathematical Modeling and Statistical Analysis of Phycobiliprotein Fluorescence Decay under Exposure to Excitation Light" Applied Sciences 12, no. 15: 7469. https://doi.org/10.3390/app12157469
APA StyleHwang, J., & Shin, A. H. (2022). A Mathematical Modeling and Statistical Analysis of Phycobiliprotein Fluorescence Decay under Exposure to Excitation Light. Applied Sciences, 12(15), 7469. https://doi.org/10.3390/app12157469






