Abstract
Valorization of agri-food residues to produce bio-based platform chemicals will enhance the transition to the bio-economy era. To this end, a sustainable process has been developed for the overall valorization of grape stalks (GS) according to a circular approach, starting from the lignin fraction to further deal with the cellulose-rich residue. This non-conventional protocol fully adheres to green chemistry principles, exploiting the so-called enabling technologies—mainly ultrasound and microwaves—for energy-saving innovative processes. Firstly, ultrasound-assisted extraction (UAE, 40 kHz, 200 W) demonstrated to be an excellent technique for GS delignification combined with natural deep eutectic solvents (NaDESs). Delignification enables isolation of the pertinent lignin framework and the potential to obtain a polyphenol-rich liquid fraction, focusing on the valorization of GS as source of bioactive compounds (BACs). Among the NaDESs employed, the combination of choline chloride (ChCl) and levulinic acid (LevA) (ChLevA) presented noteworthy results, enabling a delignification higher than 70%. LevA is one of the top-value biobased platform chemicals. In this work, a flash microwave (MW)-assisted process was subsequently applied to the cellulose-rich fraction remained after delignification, yielding 85% LevA. The regeneration of this starting compound to produce ChLevA can lead to a further biomass delignification cycle, thus developing a new cascade protocol for a full valorization of GS.
1. Introduction
The growing need for fuels and high-added-value chemicals by the global community has boosted environmental issues, mainly because of the abusive consumption of non-renewable sources for these concerns. This fact not only leads to a notable decrease in fossil fuels, but also to increasing prices and greenhouse emissions. In this context, lignocellulosic biomass and agricultural waste have recently emerged as a renewable, sustainable, widely available alternative source for the development of fine chemicals [1,2,3]. The winemaking sector is one of the most productive worldwide, involving the generation of millions of tons of waste. As a result, several studies have focused their attention on the reuse of grape by-products in the food sector, as ingredients for making functional foods, since they are a good source of bioactive compounds [4,5,6].
Grapes are indeed one of the main agroeconomic crops around the world, with >77.8 million tons produced annually, with ca. 57% being utilized in the wine-making process [7,8]. The main vinification by-products are represented by grape stalks (GS) that account for 2.5–7.5 wt% of the overall grapes and are obtained after destemming process.
GS are not considered a hazardous waste, but their great content of organic matter and their seasonality contribute to potential pollution troubles. Therefore, GS valorization has become a challenging field of investigation for minimizing their environmental impact by the conversion of their main components (cellulose, hemicellulose, and lignin) into new value-added compounds. As reported by Atatoprak et al., several GS valorization processes—such as acid and enzymatic hydrolysis of polysaccharides and further fermentation—have been investigated and optimized in recent years [9]. However, utilizing this process exclusively to produce ethanol from GS sugars is unattractive in terms of cost balance. Therefore, other valued streams must be exploited for feasibility to use these wastes in an integrated manner, which increases profits and attracts stakeholders to discover these by-product resources.
In the last few years, biomass upgrading has been achieved using process intensification techniques, leading to higher efficiency and sustainability [1,2,3,4]. In this context, the cavitation phenomena in ultrasound (US)-assisted extraction (UAE) favors the matrix solvation and cell wall rupture [10]. The formation, growth, and collapse of gaseous microbubbles in a liquid phase may dramatically improve mass transfer [10]. The powerful local effects of these microbubbles provide harsh conditions in the reaction medium, as immense localized heating and pressure [11], because of the generation of microjet streams, shock waves, turbulences, or shear forces. This phenomenon generates mechanical effects, such as the breakdown of tissues in small fragments, and intensive mixing and heating [12], in contrast to conventional methods. Therefore, cavitation has a remarkable impact on accelerating chemical reactions, increasing conversions and yields, or enhancing selectivity in both homogeneous and heterogeneous systems [11]. UAE is a well-known process applied for the recovery of high-added-value metabolites from different lignocellulosic matrices [13,14,15].
Along with US, microwave (MW) heating shows a positive effect in terms of reaction control, residence times, and energy efficiency, thus being considered an environmentally friendly technology.
The interaction of MW with the matter originates by selective absorption of radiation by polar molecules (together with ionic conduction), leading to a volumetric heating in contrast to conventional treatments, where the heat is transferred by conduction from the flask wall [16,17]. These phenomena provide high efficiency for each process, minimizing side reactions and undesired by-products, as well as higher yields at shorter residence times (energy saving). In the last decade, the advantages of MW heating have been explored in many fields, ranging from the extraction of natural products (MAE), the hydrolysis of biopolymers to organic synthesis [18]. In particular, the increase in extraction yields of several active phytocompounds from plant biomass by using MAE could be mainly due to the degradation of plant tissues because of the water content in the cells, which is selectively heated by MW, leading to cell-wall expansion and rupture, and finally facilitating the release of the intracellular compounds and their further transformation [19].
Enabling technologies promote the delignification, which plays a crucial role in biomass valorization. The delignification can enhance the recovery of hemicelluloses, and other bioactive compounds (BACs) [14], such as antioxidants [20,21]. On the other hand, the accessibility of cellulose is also enhanced, thus favoring the further transformation of the remaining solid fraction into valuable products [22,23]. Among bio-derived platform chemicals, levulinic acid (LevA) has attracted attention in recent years [24,25] as a versatile chemical building block. This feature makes LevA a valuable compound to produce multiple high-end products with recognized application as solvents or fuels, as well as in coatings, electronics, or lubricants.
These emerging technologies may assist a sustainable delignification in combination with green solvents, such as natural deep eutectic solvents (NaDESs), in contrast to the conventional alkaline conditions [26,27]. NaDESs are fluids composed of two or three components which are capable of self-association through hydrogen-bond interactions. NaDESs are prepared by combining hydrogen bonding donors (HBDs)—such as glycerol, lactic acid or urea, and hydrogen bonding acceptors (HBAs)—especially quaternary ammonium salts such as choline chloride (ChCl) [28,29]. NaDESs are non-toxic, biodegradable, inexpensive, and simply prepared with a full atom economic reaction. By contrast, their high viscosity may hinder the mass transfer in extraction and delignification leading to time-consuming processes [30]. This problem can be overcome by using NaDESs under enabling technologies, improving heat and mass transfer thanks to high-energy microenvironments [20,30].
In this work, GS delignification was carried out by combining UAE and NaDESs. Different choline chloride (ChCl)-based NaDESs were tested, mainly focusing on the bicomponent one ChLevA (ChCl + LevA). This US-assisted delignification afforded different routes for the exploitation of GS as a source of BACs. After delignification, the cellulose-rich fraction was subjected to acidic MW-assisted conversion to LevA, which can be further used to produce ChLevA, according to a circular approach.
2. Materials and Methods
2.1. Chemicals
All chemicals and solvents were purchased from commercial suppliers and used without further purification. ChLA and ChLAGly NaDESs were synthesized based on previous studies [20]. The further bicomponent ChLevA NaDES was obtained following the same procedure but adopting a 1:2 molar ratio between ChCl and LevA.
2.2. Feedstock Treatment and Characterization
Grape stalks (GSs) were provided by Alvinesa Natural Ingredients S.A. (Daimiel, Ciudad Real, Spain) at the beginning of the 2019 harvest season. The matrix was stored in a freezer and then treated and characterized according to previous studies (Table S1) [21].
2.3. Biomass Delignification under UAE
Delignification percentage was calculated considering the initial amount of GS for each experiment, and the weight of the cellulose-rich solid fraction obtained after this step, according to Equation (1).
UAE was carried out in a high-power US bath (Weber Ultrasonic AG, Karlsbad, Germany) composed of a 5 L stainless steel tank equipped with three probes screwed fixed into the bottom part. The US bath was fixed to operate at a frequency of 40 kHz and 200 W power.
For alkaline treatment, 5 g of GS were placed in a round-bottomed flask, along with 400 mg of NaOH (10 wt% respect to the dry matter). Deionized H2O (80 mL) was used as solvent (1:20 solid/liquid ratio). The crude was centrifuged (Rotofix 32, Hettich Zentrifugen, Tuttlingen, Germany) at 4000 rpm for 30 min, and the solid washed two times with a NaOH solution (0.125 M, 400 mg of NaOH in 80 mL deionized H2O). The solid fraction was separated and mildly dried in a heater at 45 °C, weighed to measure the delignification yield and stored for further conversion reactions. In parallel, the addition of HCl 2 N (20 mL) to the liquid fraction immediately promoted the precipitation of lignin at pH = 1–2. The resulting mixture was filtered under vacuum and the solid was washed with deionized H2O, while the filtrate was lyophilized.
The precipitated lignin was dried (45 °C) and then stored for further characterization.
For the pretreatment with NaDESs, 2.5 g of GS and 100 mL of solvent were employed for each extraction in a 1:50 solid:liquid ratio. For both systems, the reaction flask was immersed in the US bath, stabilized at 50 °C, and then put into rotation for 60 or 120 min. The crude was separated by centrifugation (4000 rpm, 45 min), washing the solid with the same NaDES solution. The residence time for centrifugation was longer, to solve the viscosity problems. The solid fraction was washed with approximately 500 mL deionized H2O to remove the NaDES. After that, it was dried (45 °C) and weighed to determine the delignification yield. Considering the liquid phase after centrifugation, approximately 2 L deionized H2O were added to the liquid fraction remaining after centrifugation, to remove the NaDES, thus promoting lignin precipitation after 12–16 h at 4 °C. This procedure was necessary for experiments with ChLA and ChLevA, enabling the recovery of a residual amount of lignin. The mixture was then filtered under vacuum, the filtrate lyophilized and then analyzed by DPPH assay. The precipitated lignin was dried at mild temperatures and stored for further FT-IR analysis.
2.4. Liquid Fraction Characterization
2.4.1. Antioxidant Activity
The antioxidant activity of the liquid fractions after delignification with NaOH and NaDESs was spectrophotometrically determined by means of the scavenging method with the DPPH radical, at 515 nm, in a Cary 60 UV–VIS spectrophotometer (Cary 60, Agilent Technologies, Santa Clara, CA, USA), according to previous studies [21]. Results were expressed in terms of IC50 values related to the initial concentration for each sample (Co), using a calibration curve by plotting the extract concentration vs. the corresponding scavenging effect (inhibition percentage).
2.4.2. Polyphenols Analysis
Regarding alkaline extracts, the TPC was also determined using the Folin–Ciocalteau method, according to the work of Salgado-Ramos et al. [1]. Experiments were carried out at 765 nm, in the UV–vis equipment previously described, and results expressed as mg gallic acid equivalents (mg GAE)/g dry matter (g DM).
For the characterization of the volatile compounds, the liquid fractions remaining after NaOH, ChLAGly, and ChLevA delignification at 120 min (50 mL) were firstly neutralized to approximately pH 5 and then extracted with ethyl ether (20 mL). The organic solvent was evaporated under vacuum, and the dried crude analyzed by GC-MS (Agilent Technologies 6850 Network GC System, Agilent Technologies, Santa Clara, CA, USA; 5973 Network Mass Selective Detector and 7683 B Automatic Sampler; HP-5MS capillary column (length: 30 m; i.d.: 0.25 mm; film thickness: 0.25 µm)) after solubilization with ethyl acetate (EtOAc) (1 mL).
The NaDES samples were also analyzed by HPLC-MS using a Waters 2525 pump linked to a Column Fluidics Organizer (Waters Corp., Singapore, Singapore) combined with a 2767 Sample Manager (Waters) coupled with a 2487 dual Abs detector and a Micromass ZQ. The used column was a Synergi Hydro-RP C18 column (250 mm, 4.6 mm, 4 μm; Phenomenex, Torrance, CA, USA), with 2% AcOH (A) and 2% MeCN (B) as the mobile phases (1 mL/min). The monitored wavelengths were 280 (for benzoic acids, catechins, and proanthocyanidins detection) and 340 nm (cinnamic derivatives and flavonoids detection). The elution was performed with a gradient program starting from 0% B (maintained for 6.5 min), up to 50% B in 30 min, and from 50% to 100% B from 30 to 36 min, followed by a 100% B step for a further 6 min.
2.5. Characterization of Isolated Lignins
The precipitated lignins after delignification were characterized by FT-IR and SEM.
FT-IR spectra were recorded using a Spectrum Two ATR (Perkin Elmer, Walthman, MA, USA). Samples were analyzed in transmittance mode with 16 scans and resolution of 2 cm−1, in a frequency range collected from 4000 to 500 cm−1.
SEM images were taken in a ZEISS Gemini500 high resolution scanning electron microscopy (HRSEM, ZEISS, Oberkochen, Germany), with an Oxford EDS 80 mm2 detector operating from 3 pA to 20 nA for electrical current intensity and 0.02–30 kV for voltage.
2.6. Microwave (MW)-Assisted Production of Levulinic Acid (LevA)
MW experiments were performed in a high-pressure resistant professional multimode MW reactor (300 °C, 199 bar) equipped with a multi rack position tool for simultaneous reactions (Synthwave Milestone, Srl, MLS Gmbh, Bergamo, Italy). Reactor frequency was 2.45 GHz and all samples were irradiated at 1500 W.
In a typical experiment, 300 mg of untreated (or delignified with ChLevA) GS (60 min) were charged in two different MW-vials (40 mL). HCl 1 M (3 mL) was added (1:10 solid–liquid ratio), according to previous studies [25]. The mixture was irradiated at 225 °C for 2 min under N2 (40 bar) pressure.
Upon completion of the reaction, the mixture was filtered, and solid was washed several times with deionized water to approximately obtain 25–30 mL of liquid fraction. The filtrate was extracted with EtOAC. The organic phase was then evaporated under vacuum, and the dried crude washed with CHCl3 (5 mL). This liquid fraction was passed through a nylon syringe filter and, after evaporation of the solvent, the remaining brownish viscous dried crude was characterized.
2.7. Characterization of LevA
For the NMR analysis, 600 µL of CDCl3 were added to the samples, that were further filtered with a nylon syringe filter. The sample was analyzed by 1H-NMR, with a Jeol JNM-ECZ600R spectrometer (Jeol, Tokyo, Japan) operating at a frequency of 600 MHz.
The GC-MS analysis of LevA was carried out after prior derivatization. In a typical experiment, the dried crudes were dissolved in 1.5 mL of CHCl3 and then 80 µL of BSTFA ((N,O-bis(trimethylsilyl)trifluoroacetamide) were added to the solution as derivatizing agent. The mixture was heated at 65 °C for 45 min under magnetic stirring.
2.8. Statistical Analysis
The results of delignification and antioxidant properties were statistically analyzed using a t-test in a two-way ANOVA by means of GraphPad Prism 8.0.2. software (GraphPad Software, San Diego, CA, USA). A value of p < 0.05 was considered as significant. All results were expressed as mean ± standard deviation (n = 3).
3. Results and Discussion
3.1. Delignification Step via Ultrasound-Assisted Extraction (UAE)
In this work, NaDESs were used as efficient, non-toxic solvents for GS delignification in combination with US. Based on the work of Calcio Gaudino et al. for wheat straw biomass delignification [20], and focusing on the development of a novel cascade protocol for biomass valorization, three different choline chloride (ChCl)-based NaDESs (Table 1) were prepared.

Table 1.
NaDESs employed.
Regarding ChLevA, and to the best of our knowledge, different ChCl–LevA molar ratios were preliminarily tested (1:1, 1:2, and 1:10) to assess which was the proper combination that would lead to the eutectic depression and best properties. Firstly, a white crystal at room temperature was found when an equimolar proportion was used, also according to the literature [31], thus this mixture was rejected for the experiments. The opposite behavior was observed for 1:10 molar ratio, where a yellowish fluid was easily formed. The reason for using this proportion was to compare the efficiency with LA-based NaDES at the same molar ratio. However, this eutectic mixture showed poor extraction capability, leading to a delignification yield below 10%. Therefore, according to Maugeri et al. [31], which reported the excellent physical properties, a 1:2 molar ratio was selected between ChCl and LevA for ChLevA.
Moreover, for the sake of comparison, an alkaline delignification (NaOH 0.125 M) was tested following the reported data on palm fronds or orange peel waste under US [32,33]. The operating conditions were chosen according to literature [34], fixing the proper temperature for cavitation at 50 °C. The range of US frequencies typically used for this kind of processes is 20–100 kHz, in which cell rupture and polymer degradation can be promoted [35]. This value was therefore set at 40 kHz, according to previous studies [20]. Results are shown in Figure 1.
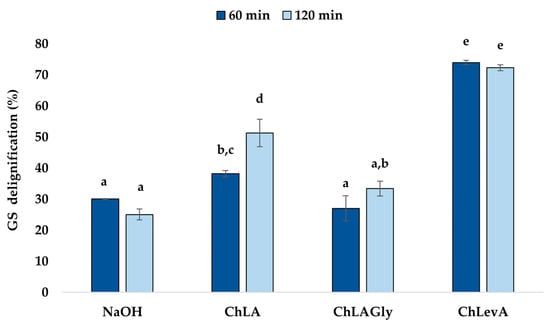
Figure 1.
Grape stalk (GS) delignification by alkaline and NaDESs treatment under UAE (40 kHz, 200 W). Results are expressed as mean ± SD. Values within each variable with different letters are significantly different (p < 0.05).
As observed, NaDESs showed a higher efficiency for US-assisted GS delignification in comparison with the alkaline system, representing a valuable improvement in terms of sustainability. This high extracting power could be attributed to the H-bonding interactions between NaDES components and the phenol groups in lignin structure [20].
Considering alkaline extracts (Figure 1), an increase in terms of sonication time (from 60 to 120 min) led to slightly lower delignification yields (30.05% vs. 25.05%, respectively). Maybe long times under alkaline conditions could promote an aldolic-type recondensation between the lignin monomers previously extracted. Utekar et al. [33] reported that a non-significant increase in terms of delignification was observed if the treatment time varies from 1 h to 2 h or 4 h at 50 °C. In our case, despite this decrease, the difference was not significant (p > 0.05).
Concerning NaDESs treatments, longer sonication times might be required to reduce their viscosity and enhance the mass transfer [20]. This fact was confirmed for ChLA and ChLAGly, being only significant for the former (p < 0.05). Particularly, in case of ChLAGly, the presence of glycerol (Gly)—denser than both lactic (LA) and levulinic acid (LevA)—as well as the higher amount of ChCl make this solvent even more viscous and dense, thus hindering the mass transfer also under US irradiation. This fact could explain the lower delignification yields achieved when a US-assisted ChLAGly pretreatment was adopted for GS and the absence of significant differences between 60- and 120-min treatment (p > 0.05), suggesting that even longer times should be required. Despite this limitation, ChLAGly was comparable in terms of delignification efficiency with the treatment using NaOH for 60 min (27.06% vs. 30.06%, respectively, p > 0.05). Moreover, when sonication time was prolonged up to 2 h, this NaDES proved even higher efficiency than NaOH (33.38% vs. 25.05%, p < 0.05).
Regarding ChLA, it should be noted that no significant difference was observed at 60 min compared to the tricomponent ChLAGly at 120 min (p > 0.05). Since sonication prompts mass transfer and cell rupture due to the solvent penetration into the solid matter, a less viscous fluid would favor the process and improve delignification yields even at shorter times. Moreover, at 120 min, delignification was significantly favored (p < 0.05), as expected, with a delignification yield higher than 50%.
Finally, the results achieved using ChLevA were noteworthy (Figure 1), showing an efficiency around 70% in terms of GS delignification, which was significantly higher compared to the other results (p < 0.05). Considering bicomponent NaDESs, despite the lower LevA acidity compared to LA, an excellent extraction capability for this NaDES at 1:2 molar ratio was unveiled. Furthermore, it was also worth noting that no statistical significance was found for 60- and 120-min UAE (73.99% vs. 72.31%, p > 0.05), thus highlighting the possibility of employing shorter times for a more sustainable valorization process.
Overall, the applicability of NaDESs for delignification purposes in combination with US was efficiently demonstrated for GS. Particularly, ChLevA proved an excellent candidate in this way. Moreover, it is worth noting the properties of LevA in terms of cost and availability [36], as well as its easier production from renewable sources as lignocellulosic biomass, making it available for NaDES production.
3.2. Cascade Protocol for Grape Stalk Valorization
US-assisted delignification process afforded some valorization pathways for GS, as represented in Figure 2.
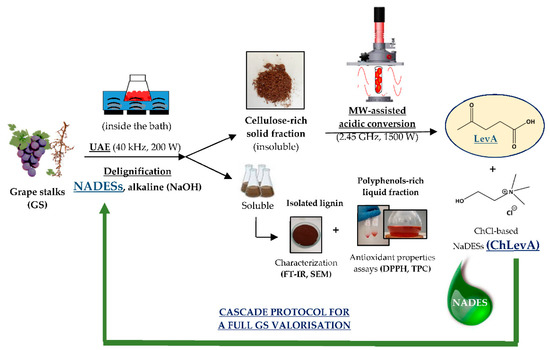
Figure 2.
Cascade protocol by applying enabling technologies (UAE and MW-assisted radiation) for the full valorization of grape stalks (GS).
Acid-insoluble lignin from GS was precipitated and isolated from the obtained liquid fraction after acidification at pH = 2. The analysis of its physical properties could be interesting in the frame of a complete further valorization; for instance, lignin contains a high number of phenolic groups, with remarkable antioxidant activity, useful in biomedical applications as anticancer, antiviral, or for diabetes treatment [37], among others; furthermore, by liquefaction or pyrolysis, a liquid bio-oil with 100 different kinds of phenolics can be obtained, useful for bioenergy purposes [38,39]. Finally, the oxidation of lignin affords bioaromatics (mainly vanillin and syringaldehyde [3]), endowed with many industrial applications in foods, drugs, or cosmetics, as well as long-chain fatty acids as biofuel precursors, as recently reported for GS as well [21].
In this work, a high amount of lignin has been removed by US-alkaline-assisted treatment, in agreement to other reported works [40]. After NaDES treatment, no solid was recovered upon delignification with ChLAGly, while a low amount of lignin was isolated when using both ChLA (30 mg) and ChLevA (10 mg). This finding could be explained by the formation of depolymerization products and acid soluble lignins due the synergy between cavitation effect and NaDESs. Moreover, the precipitation could be hindered by the successful interaction of NaDESs with the exposed phenol groups in lignin that could help keep lignin in the liquid phase by the formation of H-bonds.
The characterization by FT-IR and SEM (only for alkali lignin) of the isolated lignins after GS-delignification is shown in Figure 3.
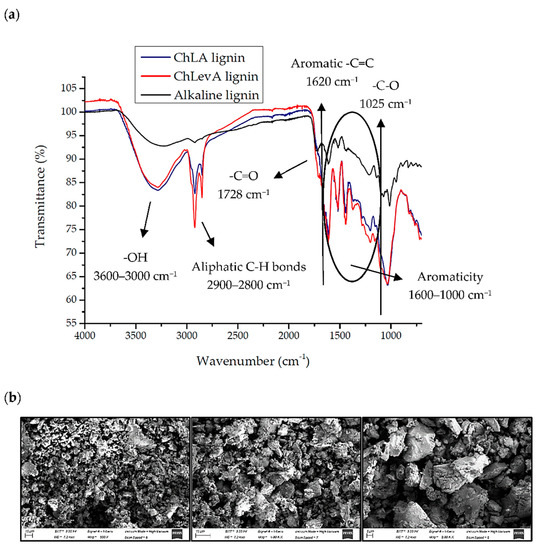
Figure 3.
FT-IR spectra for NaDES and alkali lignin (a); and SEM images at 500×, 1.00 K×, and 2.00 K× magnification for alkali lignin (b).
Alkaline treatment with NaOH lead to a less cross-linked structure, as it is possible to observe in the FT-IR spectrum (Figure 3a, black line). Indeed, the bands related to C=C and C-O stretching for aromatic rings—as well as the C-O and C-H vibration of aliphatic CH2- or phenol OH bonds (1600–1000 cm−1) [2,41,42]—were found to be less intense compared to NaDES lignins. This fact is in accordance with the literature, where Sun et al. reported that a high structural modification of the starting lignin occurs after alkaline treatment [43]. Moreover, both carbonyl and C-O bands (1728 and 1025 cm−1, respectively) are less intense in alkali lignin compared again to NaDES lignins, suggesting that the amount of ether linkages is considerably lower. By contrast, NaDESs delignification was useful to obtain a less-modified lignin with a cross-linked structure (Figure 3a, red and blue lines). This fact can be observed by both aromatic region (1600–1000 cm−1) and the sharp bands related to C=O and C-O linkages. As mentioned, these solvents establish hydrogen bonds with lignin monomers, thus avoiding deep structural modifications.
Furthermore, as a higher amount of lignin was recovered by US-alkaline-assisted treatment in contrast to NaDESs, it was possible to perform the SEM analysis for this solid (Figure 3b). The morphology showed plenty of irregular and hollow spheres according to the literature [44,45], which conglomerated and aggregated through intermolecular forces and adsorption during lignin precipitation [44]. All these spherical structures observed by SEM suggest an aromatic moiety, in agreement to previous work [2], which may be related to the monolignols involved in lignin structure.
In order to fully valorize each recovered fraction after US-assisted GS pretreatment, the liquid fraction recovered after lignin precipitation was considered.
After lignin isolation, an antioxidant-rich liquid fraction remained (see Figure 2), thus suggesting the exploitation of GS as source of bioactive compounds (BACs). The antioxidant activity of this crude was evaluated by using the free radical method with DPPH by comparison to the standard Trolox and ascorbic acid. Moreover, the TPC was evaluated by the Folin–Ciocalteau assay for the liquid fraction after alkaline treatment. It should be noted that TPC cannot be performed for NaDESs extracts because of the stratification of the end products of the Folin reaction, meaning that their phenolic compounds might not be detected by UV–vis spectrophotometry [20]. Results are detailed in Table 2.

Table 2.
Scavenging activity by DPPH assays and TPC for the liquid fractions obtained after grape stalks (GS) delignification processes via UAE (40 kHz, 200 W). Results are expressed as mean ± SD. Values within each variable (for DPPH) with different letters are significantly different (p < 0.05).
As mentioned, alkaline delignification led to a highly structurally modified lignin, which means that a deep depolymerization occurred. This fact could explain the high scavenging activity showed for experiments at 60 min (0.2616 ± 0.0015 IC50) (Table 2, entry 1). By contrast, for 120 min treatment (entry 2), the antioxidant power significantly decreases (p < 0.05), probably due to the occurrence of recondensation reactions that reduced the presence of free phenol groups, thus diminishing the scavenging activity.
Regarding TPC, the number of total polyphenols was similar for both 60 and 120 alkaline GS pretreatment (Table 2, entries 1–2). During delignification, some hemicellulose chains could be hydrolyzed to monosaccharides and give interference by reacting with the Folin-Ciocalteu reagent [46]. Furthermore, polyphenols can be degraded by exposition to US for too long, according to literature [47]. Therefore, the slight decrease at 120 min might be due to the formation of free hydroxyl radicals by the presence of high water content under UAE, which can negatively affect the antioxidant power. [48].
Considering the liquid fractions obtained after lignin extraction with NaDES, the antioxidant power was higher for longer pretreatments (120 min) compared to 60 min (Table 2, entries 3–8), with statistical significance for each system (p < 0.05). This fact agrees with the results previously reported (Figure 1) in terms of delignification, except for ChLevA, which showed the lowest activity, quite surprisingly. The results for ChLAGly at 120 min were worth noting (entry 6), being significantly higher compared to the others (p < 0.05), and comparable with the standard Trolox (p > 0.05). This fact could be attributed to a stronger depolymerization degree compared to the bicomponent NaDESs, which could also explain the absence of precipitated lignin with this solvent.
Overall, liquid fractions after alkaline delignification at 60 min and with ChLAGly at 120 min could be considered as the richest in antioxidant compounds. Therefore, these two liquid fractions were more deeply characterized by GC-MS to investigate the presence of volatile phenolic compounds. Furthermore, despite the lower DPPH results, ChLevA liquid fraction after 120 min treatment was also analyzed because of the engaging delignification observed. Chromatograms mainly showed the selective extraction of 2,6-di-tert-butyl-4-methylphenol for all the systems (Figure S1), endowed with antioxidant properties and useful in some biological in vitro and in vivo assays, as reported [49]. The alkaline extract contained also other minor volatile phenolic compounds. The two liquid fractions remaining after delignification with NaDESs (ChLAGly and ChLevA) were also further analyzed by HPLC-DAD. As shown in Figure S2, the former contained more phenolic compounds than the extract from ChLevA treatment. This result could explain the lower antioxidant activity of ChLevA liquid fraction.
Finally, the production of LevA via MW-assisted flash conversion of the cellulose-rich solid fraction remaining after delignification was accomplished (see Figure 2), on the basis of the good results achieved in a previous work [25].
Due to the highest delignification, and considering the shorter residence times, the cellulose-rich fraction obtained after ChLevA treatment at 60 min was selected for this further valorization step. Moreover, the obtained LevA could be reused to prepare ChLevA again, which can efficiently extract lignin from GS (Figure 1), paving the way for a circular and sustainable protocol towards full-biomass valorization. Results are summarized in Table 3.

Table 3.
Conversion of grape stalks (GS) to levulinic acid (LevA). Reaction conditions (considering previous work [25]): 225 °C, 2 min, 40 bar N2. MW-assisted radiation (2.45 GHz, 1500 W).
As observed, delignification notably improves LevA yields compared to the raw GS (Table 3, entries 1–2), as expected. Some literature works reported that after delignification treatment the accessibility of cellulose is enhanced by removing both lignin and hemicellulose, which favors the further conversion of the remaining fraction into valuable products [22,23]. Therefore, 85% LevA in terms of molar yield was achieved in a 2 min MW treatment at 225 °C, which corresponds to 60% in terms of ponderal yield and 68% conversion. These results were similar to those obtained from post-harvest tomato plant (PHTP) in our previous work (Table 3, entry 3) [25], demonstrating these optimized conditions being efficient also for other types of biomasses. Furthermore, it should be noted that LevA was obtained in a 100%-selective transformation, since it was the only product detected in the organic phase, as observed by GC-MS and NMR analyses (Figure S3).
Overall, delignification was demonstrated as a valuable tool in terms of valorization, not only for antioxidants and lignin recovery, but also for enhancing the yields achieved by the conversion of cellulose-rich fraction. In parallel, the employment of process intensification technologies could improve the efficiency and sustainability of the cascade protocol.
4. Conclusions
In this work, the development of green circular protocol for the valorization of grape stalks has been carried out by applying process intensification technologies. Firstly, US-assisted delignification with NaDESs was performed. ChLevA afforded yields higher than 70% in a 60 min treatment, in contrast to alkaline conditions and the others ChCl-based NaDES. This step also led to the recovery of the pertinent lignin framework, as well as the liquid fractions rich in antioxidants, thus enabling the consideration of exploiting GS as a source of biologically active compounds (BACs). In this context, the obtained crude fraction from the delignification with ChLAGly (120 min treatment) showed the best scavenging power. Finally, the remaining cellulose-rich fraction was converted under MW to obtain LevA, with molar yields around 85% in a 100% selective transformation. This LevA could be used to regenerate ChLevA, which can efficiently delignify GS in a circular protocol towards the full biomass valorization. This work paves the way to a bio-refinery based on winemaking wastes by means of green protocols with enabling technologies, which supposes a valuable achievement in terms of sustainability due to the large number of by-products generated during the winemaking process. The present novel cascade protocol is well-suited for the full valorization of agricultural lignocellulosic waste.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12157417/s1, Table S1: Fiber analysis for grape stalks (GS) according to the National Renewable Energy Laboratory (NREL) procedure. Figure S1: GC-MS analyses of the liquid fraction obtained after lignin isolation from GS delignification via UAE (40 kHz, 200 W): alkaline delignification (a), ChLAGly (b) and ChLevA (c) at 120 min treatment. Figure S2: HPLC chromatograms of the liquid fraction obtained after lignin isolation from GS delignification via UAE (40 kHz, 200 W): ChLAGly (a) and ChLevA (b) at 120 min treatment; Figure S3: GC-MS (a) and NMR (CDCl3, 600 MHz) (b) analyses for the transformation to levulinic acid (LevA) from GS. Reaction conditions detailed in Table 3. References [50,51,52] are cited in the supplementary materials.
Author Contributions
Conceptualization, S.T. and M.S.-R.; Methodology, M.S.-R. and F.M.; Formal analysis, F.M. and M.S.-R.; Investigation, E.C.G.; Resources, A.M. and G.C.; Data curation, M.S.-R.; Writing—original draft preparation, M.S.-R. and E.C.G.; Writing—review and editing, S.T. and G.C.; Supervision, S.T. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
This work was supported by the University of Turin (Ricerca locale 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Barba, F.J.; Moreno, A. Almond Hull Biomass: Preliminary Characterization and Development of Two Alternative Valorisation Routes by Applying Innovative and Sustainable Technologies. Ind. Crops Prod. 2022, 179, 114697. [Google Scholar] [CrossRef]
- Lorente, A.; Remón, J.; Salgado, M.; Huertas-Alonso, A.J.; Sánchez-Verdú, P.; Moreno, A.; Clark, J.H. Sustainable Production of Solid Biofuels and Biomaterials by Microwave-Assisted, Hydrothermal Carbonization (MA-HTC) of Brewers’ Spent Grain (BSG). ACS Sustain. Chem. Eng. 2020, 8, 18982–18991. [Google Scholar] [CrossRef]
- Tabasso, S.; Mariatti, F.; Grillo, G.; Boffa, L.; Tibaldi, P.S.; Cravotto, G. Sustainable Microwave-Assisted Aerobic Oxidation of Tomato Plant Waste into Bioaromatics and Organic Acids. Ind. Eng. Chem. Res. 2019, 58, 8578–8584. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Holeckova, D.; Tremlova, B.; Kulawik, P. Effect of Grape Seed Flour on the Antioxidant Profile, Textural and Sensory Properties of Waffles. Processes 2021, 9, 131. [Google Scholar] [CrossRef]
- Barbanera, M.; Cardarelli, A.; Carota, E.; Castellini, M.; Giannoni, T.; Ubertini, S. Valorisation of Winery and Distillery By-Products by Hydrothermal Carbonization. Sci. Rep. 2021, 11, 23973. [Google Scholar] [CrossRef] [PubMed]
- Portilla Rivera, O.M.; Saavedra Leos, M.D.; Solis, V.E.; Domínguez, J.M. Recent Trends on the Valorisation of Winemaking Industry Wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100415. [Google Scholar] [CrossRef]
- Zhu, Z.; Hao, M.; Zhang, N. Influence of Contents of Chemical Compositions on the Mechanical Property of Sisal Fibers and Sisal Fibers Reinforced PLA Composites. J. Nat. Fibers 2020, 17, 101–112. [Google Scholar] [CrossRef]
- The International Organization of Vine and Wine (OIV). Statistical Report on World Vitiviniculture; International Organization of Vine and Wine: Paris, France, 2021. [Google Scholar]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape Stalk Valorisation for Fermentation Purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Verdini, F.; Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Cravotto, G. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Appl. Sci. 2021, 11, 4693. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono and Mechanochemical Technologies in the Catalytic Conversion of Biomass. Chem. Soc. Rev. 2021, 50, 1785–1812. [Google Scholar] [CrossRef]
- Wu, Z.; Ferreira, D.F.; Crudo, D.; Bosco, V.; Stevanato, L.; Costale, A.; Cravotto, G. Plant and Biomass Extraction and Valorisation under Hydrodynamic Cavitation. Processes 2019, 7, 965. [Google Scholar] [CrossRef] [Green Version]
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L.; Grillo, G.; et al. Lignin—Derived Antioxidants as Value-Added Products Obtained under Cavitation Treatments of the Wheat Straw Processing for Sugar Production. J. Clean. Prod. 2021, 303, 126369. [Google Scholar] [CrossRef]
- al Khawli, F.; Martí-Quijal, F.J.; Pallarés, N.; Barba, F.J.; Ferrer, E. Ultrasound Extraction Mediated Recovery of Nutrients and Antioxidant Bioactive Compounds from Phaeodactylum Tricornutum Microalgae. Appl. Sci. 2021, 11, 1701. [Google Scholar] [CrossRef]
- Lucas-Torres, C.; Lorente, A.; Cabañas, B.; Moreno, A. Microwave Heating for the Catalytic Conversion of Melon Rind Waste into Biofuel Precursors. J. Clean. Prod. 2016, 138, 59–69. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, À.; Moreno, A. Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Cravotto, G.; Carnaroglio, D. (Eds.) Microwave Chemistry; De Gruyter: Berlin, Germany, 2017; ISBN 9783110479935. [Google Scholar]
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process Intensification Technologies for the Recovery of Valuable Compounds from Cocoa By-Products. Innov. Food Sci. Emerg. Technol. 2021, 68, 102601. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tabasso, S.; Grillo, G.; Cravotto, G.; Dreyer, T.; Schories, G.; Altenberg, S.; Jashina, L.; Telysheva, G. Wheat Straw Lignin Extraction with Bio-Based Solvents Using Enabling Technologies. Comptes Rendus Chim. 2018, 21, 563–571. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Mariatti, F.; Tabasso, S.; Prado Sánchez-Verdú, M.; Moreno, A.; Cravotto, G. Sustainable and non-conventional protocols for the three-way valorisation of lignin from grape stalks. Chem. Eng. Process. Process Intensif. 2022, 178, 109027. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Song, C.; Liu, S.; Li, X.; Long, J. Intensified Levulinic Acid/Ester Production from Cassava by One-Pot Cascade Prehydrolysis and Delignification. Appl. Energy 2017, 204, 1094–1100. [Google Scholar] [CrossRef]
- Rapado, P.; Faba, L.; Ordóñez, S. Influence of Delignification and Reaction Conditions in the Aqueous Phase Transformation of Lignocellulosic Biomass to Platform Molecules. Bioresour. Technol. 2021, 321, 124500. [Google Scholar] [CrossRef] [PubMed]
- Satira, A.; Paone, E.; Bressi, V.; Iannazzo, D.; Marra, F.; Calabrò, P.S.; Mauriello, F.; Espro, C. Hydrothermal Carbonization as Sustainable Process for the Complete Upgrading of Orange Peel Waste into Value-Added Chemicals and Bio-Carbon Materials. Appl. Sci. 2021, 11, 10983. [Google Scholar] [CrossRef]
- Tabasso, S.; Montoneri, E.; Carnaroglio, D.; Caporaso, M.; Cravotto, G. Microwave-Assisted Flash Conversion of Non-Edible Polysaccharides and Post-Harvest Tomato Plant Waste to Levulinic Acid. Green Chem. 2014, 16, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Harahap, A.F.P.; Rahman, A.A.; Sadrina, I.N.; Gozan, M. Optimization of Pretreatment Conditions for Microwave-Assisted Alkaline Delignification of Empty Fruit Bunch by Response Surface Methodology. Optimization 2019, 10, 1479. [Google Scholar] [CrossRef] [Green Version]
- Gazliya, N.; Gazliya, N.; Aparna, K. Microwave-Assisted Alkaline Delignification of Banana Peduncle. J. Nat. Fibers 2021, 18, 664–673. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural Deep Eutectic Solvents for Lignocellulosic Biomass Pretreatment: Recent Developments, Challenges and Novel Opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A. New Deep Eutectic Solvent Assisted Extraction of Highly Pure Lignin from Maritime Pine Sawdust (Pinus Pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef]
- Grillo, G.; Gaudino, E.C.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef]
- Maugeri, Z.; Domínguez De María, P. Novel Choline-Chloride-Based Deep-Eutectic-Solvents with Renewable Hydrogen Bond Donors: Levulinic Acid and Sugar-Based Polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Ong, V.Z.; Wu, T.Y.; Chu, K.K.L.; Sun, W.Y.; Shak, K.P.Y. A Combined Pretreatment with Ultrasound-Assisted Alkaline Solution and Aqueous Deep Eutectic Solvent for Enhancing Delignification and Enzymatic Hydrolysis from Oil Palm Fronds. Ind. Crops Prod. 2021, 160, 112974. [Google Scholar] [CrossRef]
- Utekar, P.G.; Kininge, M.M.; Gogate, P.R. Intensification of Delignification and Enzymatic Hydrolysis of Orange Peel Waste Using Ultrasound for Enhanced Fermentable Sugar Production. Chem. Eng. Process. Process Intensif 2021, 168, 108556. [Google Scholar] [CrossRef]
- Yachmenev, V.; Condon, B.; Klasson, T.; Lambert, A. Acceleration of the Enzymatic Hydrolysis of Corn Stover and Sugar Cane Bagasse Celluloses by Low Intensity Uniform Ultrasound. J. Biobased Mater. Bioenergy 2009, 3, 25–31. [Google Scholar] [CrossRef]
- Gogate, P.R.; Sutkar, V.S.; Pandit, A.B. Sonochemical Reactors: Important Design and Scale up Considerations with a Special Emphasis on Heterogeneous Systems. Chem. Eng. J. 2011, 166, 1066–1082. [Google Scholar] [CrossRef]
- Huber, V.; Muller, L.; Hio, J.; Degot, P.; Touraud, D.; Kunz, W. Improvement of the Solubilization and Extraction of Curcumin in an Edible Ternary Solvent Mixture. Molecules 2021, 26, 7702. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I. Extraction of Lignin and Therapeutic Applications of Lignin-Derived Compounds. A Review. Environ. Chem. Lett. 2020, 18, 771–785. [Google Scholar] [CrossRef]
- Lorente, A.; Remón, J.; Budarin, V.L.; Sánchez-Verdú, P.; Moreno, A.; Clark, J.H. Analysis and Optimisation of a Novel “Bio-Brewery” Approach: Production of Bio-Fuels and Bio-Chemicals by Microwave-Assisted, Hydrothermal Liquefaction of Brewers’ Spent Grains. Energy Convers. Manag. 2019, 185, 410–430. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Lin, F.; Zhang, H.; Xiao, R. Structural Elucidation of Industrial Bioethanol Residual Lignin from Corn Stalk: A Potential Source of Vinyl Phenolics. Fuel Process. Technol. 2018, 169, 50–57. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Alkaline and Ultrasound Assisted Alkaline Pretreatment for Intensification of Delignification Process from Sustainable Raw-Material. Ultrason. Sonochem. 2014, 21, 216–225. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal Carbonization of Biomass as a Route for the Sequestration of CO2: Chemical and Structural Properties of the Carbonized Products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Chen, B.; Zhu, L. Transformation, Morphology, and Dissolution of Silicon and Carbon in Rice Straw-Derived Biochars under Different Pyrolytic Temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazimudheen, G.; Sekhar, N.C.; Sunny, A.; Kallingal, A.; Hasanath, B. Physiochemical Characterization and Thermal Kinetics of Lignin Recovered from Sustainable Agrowaste for Bioenergy Applications. Int. J. Hydrog. Energy 2021, 46, 4798–4807. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.; Jiang, J.; Xu, H.; Zhang, N.; Xie, J.; Wei, M. Isolation and Characterization of Bacillus Sp. Capable of Degradating Alkali Lignin. Front. Energy Res. 2021, 9, 807286. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough Study of Reactivity of Various Compound Classes toward the Folin− Ciocalteu Reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikucka, W.; Zielinska, M.; Bulkowska, K.; Witonska, I. Recovery of Polyphenols from Distillery Stillage by Microwave-Assisted, Ultrasound-Assisted and Conventional Solid–Liquid Extraction. Sci. Rep. 2022, 12, 3232. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Santhakumari, S.; Jayakumar, R.; Logalakshmi, R.; Prabhu, N.M.; Abdul Nazar, A.K.; Karutha Pandian, S.; Veera Ravi, A. In Vitro and in Vivo Effect of 2,6-Di-Tert-Butyl-4-Methylphenol as an Antibiofilm Agent against Quorum Sensing Mediated Biofilm Formation of Vibrio Spp. Int. J. Food Microbiol. 2018, 281, 60–71. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Álvarez, A.; Cachero, S.; González-Sánchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J.L. Novel method for holocellulose analysis of non-woody biomass wastes. Carbohydr. Polym. 2018, 189, 250–256. [Google Scholar] [CrossRef]
- Genevini, P.; Adani, F.; Villa, C. Rice hull degradation by co-composting with dairy cattle slurry. Soil Sci. Plant Nutr. 1997, 43, 135–147. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).