Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation for Experiments
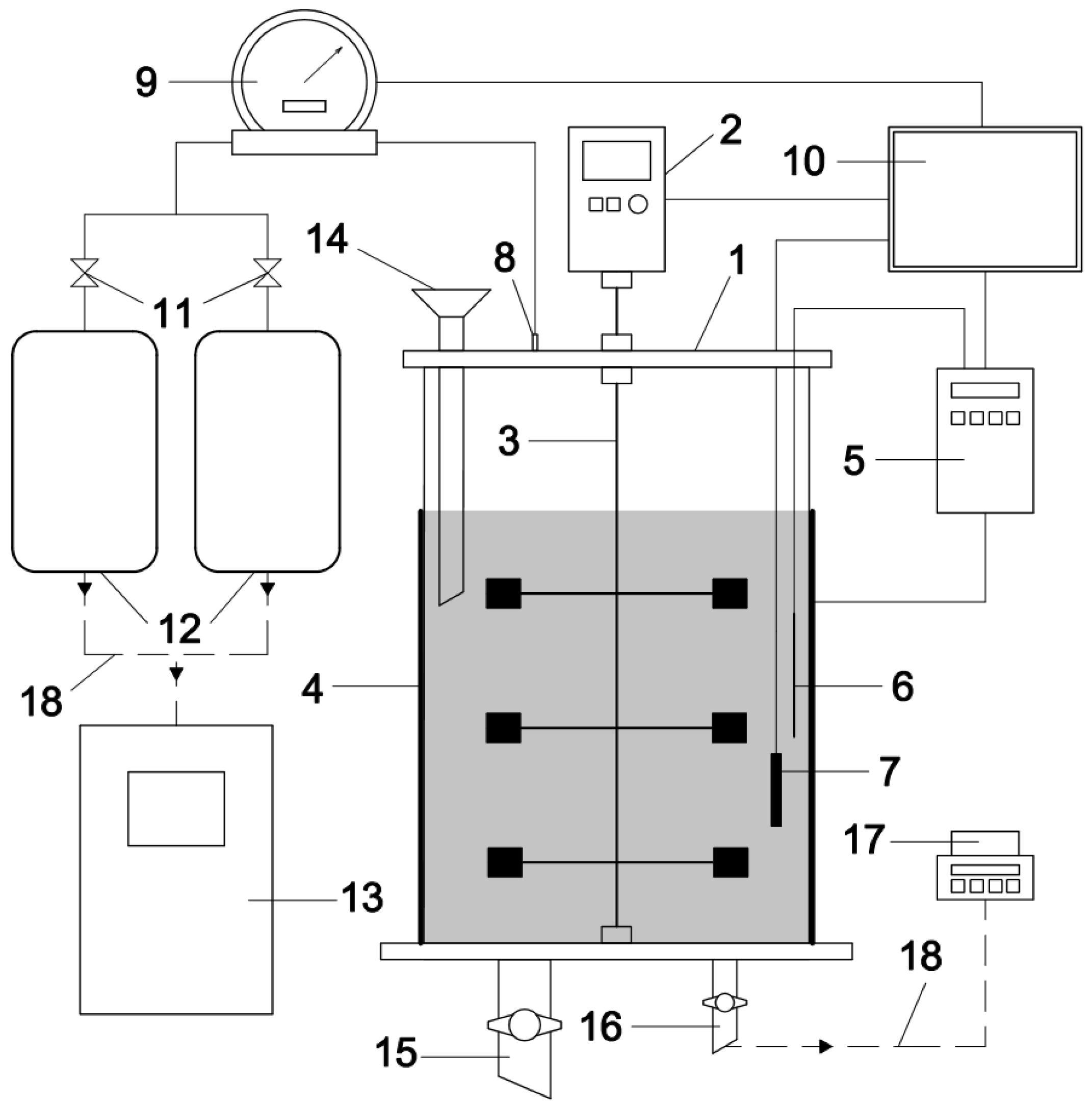
2.2. Laboratory Equipment and Instruments
2.3. Biogas Production at Different OLRs
2.4. Analytical Objects
3. Results and Discussion
3.1. Raw Materials’ Characteristics
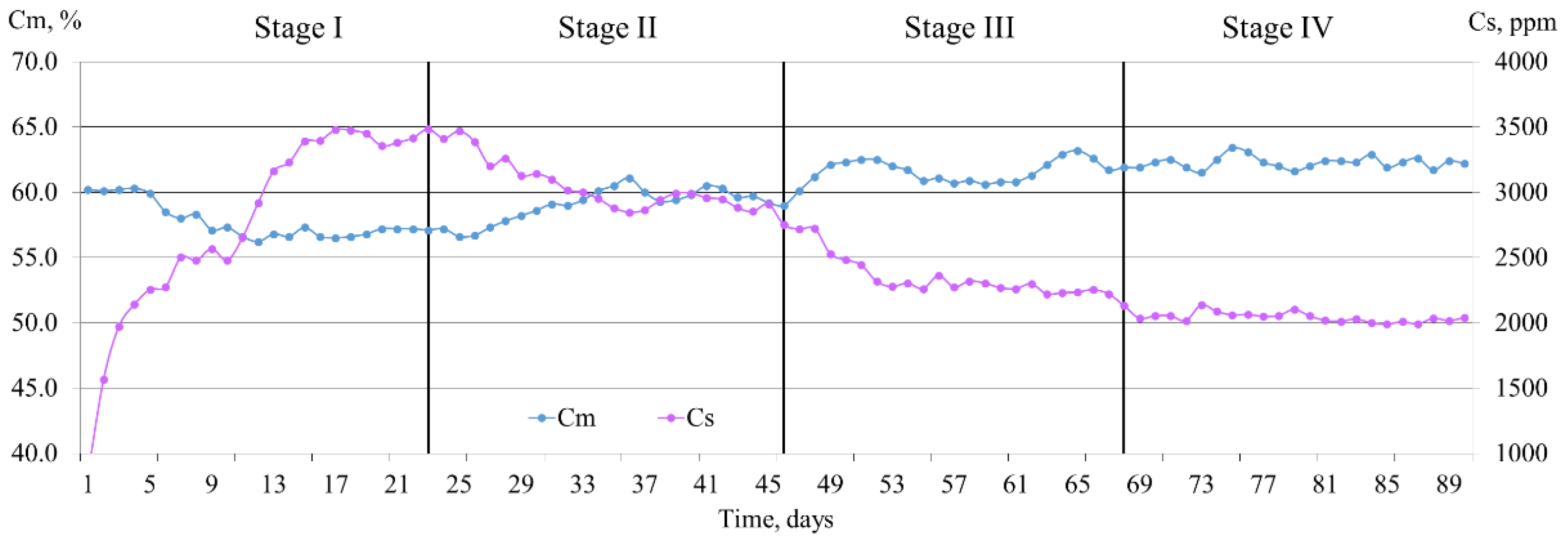
3.2. Biogas Production at Different OLRs
3.3. Main Changes in Biogas Composition
3.4. Biogas Production Indicators
3.5. Fat Influence on Digestated Substrate Changes
3.5.1. Digestate pH and TAN
3.5.2. TS Reduction and VS Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palacios-Lopez, D.; Bachofer, F.; Esch, T.; Heldens, W.; Hirner, A.; Marconcini, M.; Sorichetta, A.; Zeidler, J.; Kuenzer, C.; Dech, S.; et al. New Perspectives for Mapping Global Population Distribution Using World Settlement Footprint Products. Sustainability 2019, 11, 6056. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Andreev, K.E.; Dupre, M. Major Trends in Population Growth Around the World. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef]
- Delabre, I.; Rodriguez, L.O.; Smallwood, J.M.; Scharlemann, J.P.W.; Alcamo, J.; Antonarakis, A.S.; Rowhani, P.; Hazell, R.J.; Aksnes, D.L.; Balvanera, P.; et al. Actions on Sustainable Food Production and Consumption for the Post-2020 Global Biodiversity Framework. Sci. Adv. 2021, 7, eabc8259. [Google Scholar] [CrossRef]
- Shahbandeh, M. Number of Chickens Worldwide from 1990 to 2020. Statista, Online Resource. 2022. Available online: https://www.statista.com/statistics/263962/number-of-chickens-worldwide-since-1990/ (accessed on 6 April 2022).
- Otero, A.; Mendoza, M.; Carreras, R.; Fernández, B. Biogas Production from Slaughterhouse Waste: Effect of Blood Content and Fat Saponification. Waste Manag. 2021, 133, 119–126. [Google Scholar] [CrossRef]
- European Commission. Best Available Techniques (BAT) Reference Document for the Slaughterhouses, Animal By-Products and Edible Co-Products Industries. Industrial Emissions Directive 2010/75/EU. 2021. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2021-06/SA-BREF-20210629.pdf (accessed on 6 April 2022).
- European Commission. Best Available Techniques in the Slaughterhouses and Animal By-Products Industries. Reference Document. 2005. Available online: https://eippcb.jrc.ec.europa.eu/reference/slaughterhouses-and-animals-products-industries (accessed on 6 April 2022).
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Trends in Biodiesel Production from Animal Fat Waste. Appl. Sci. 2020, 10, 3644. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T.; Ahmad, N.; Sher, F.; Khan, S.U.-D.; Ahmad, A.; Khan, R.; Wazeer, I. Valorisation and Emerging Perspective of Biomass Based Waste-to-Energy Technologies and Their Socio-Environmental Impact: A Review. J. Environ. Manag. 2021, 287, 112257. [Google Scholar] [CrossRef] [PubMed]
- Esteves, E.M.M.; Herrera, A.M.N.; Esteves, V.P.P.; Morgado, C.d.R.V. Life Cycle Assessment of Manure Biogas Production: A Review. J. Clean. Prod. 2019, 219, 411–423. [Google Scholar] [CrossRef]
- Baral, K.R.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.H.; Olesen, J.E.; Petersen, S.O. Greenhouse Gas Emissions during Storage of Manure and Digestates: Key Role of Methane for Prediction and Mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Ali, J.; Rasheed, T.; Afreen, M.; Anwar, M.T.; Nawaz, Z.; Anwar, H.; Rizwan, K. Modalities for Conversion of Waste to Energy—Challenges and Perspectives. Sci. Total Environ. 2020, 727, 138610. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Paris Agreement. Document. 2015. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 6 April 2022).
- Anaya Menacho, W.; Mazid, A.M.; Das, N. Modelling and Analysis for Biogas Production Process Simulation of Food Waste Using Aspen Plus. Fuel 2022, 309, 122058. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Gerbens-Leenes, W. The Water Footprint of Global Food Production. Water 2020, 12, 2696. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Gołasa, P.; Wysokiński, M.; Bieńkowska-Gołasa, W.; Gradziuk, P.; Golonko, M.; Gradziuk, B.; Siedlecka, A.; Gromada, A. Sources of Greenhouse Gas Emissions in Agriculture, with Particular Emphasis on Emissions from Energy Used. Energies 2021, 14, 3784. [Google Scholar] [CrossRef]
- European Commission. Proposal for Amending Directive (EU) 2018/2001 of the European Parliament and of the Council, Regulation (EU) 2018/1999 of the European Parliament and of the Council and Directive 98/70/EC of the European Parliament and of the Council as Regards the Promotion of Energy from Renewable Sources, and Repealing Council Directive (EU) 2015/652. Document. 2021. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:dbb7eb9c-e575-11eb-a1a5-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 6 April 2022).
- Cheng, D.; Liu, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Bui, X.T. Sustainable Enzymatic Technologies in Waste Animal Fat and Protein Management. J. Environ. Manag. 2021, 284, 112040. [Google Scholar] [CrossRef]
- Hafid, H.S.; Omar, F.N.; Abdul Rahman, N.; Wakisaka, M. Innovative Conversion of Food Waste into Biofuel in Integrated Waste Management System. Crit. Rev. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Zieniuk, B.; Mazurczak-Zieniuk, P.; Fabiszewska, A. Exploring the Impact of Lipid-Rich Food Industry Waste Carbon Sources on the Growth of Candida Cylindracea DSM 2031. Fermentation 2020, 6, 122. [Google Scholar] [CrossRef]
- Diamantis, V.; Eftaxias, A.; Stamatelatou, K.; Noutsopoulos, C.; Vlachokostas, C.; Aivasidis, A. Bioenergy in the Era of Circular Economy: Anaerobic Digestion Technological Solutions to Produce Biogas from Lipid-Rich Wastes. Renew. Energy 2021, 168, 438–447. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving Biogas Production Using Additives in Anaerobic Digestion: A Review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Rubežius, M.; Venslauskas, K.; Navickas, K.; Bleizgys, R. Influence of Aerobic Pretreatment of Poultry Manure on the Biogas Production Process. Processes 2020, 8, 1109. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between Carbon/Nitrogen Ratio, Synergy and Microbial Characteristics of Long-Term Semi-Continuous Anaerobic Co-Digestion of Food Waste, Cattle Manure and Corn Straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef]
- Nkuna, R.; Roopnarain, A.; Rashama, C.; Adeleke, R. Insights into Organic Loading Rates of Anaerobic Digestion for Biogas Production: A Review. Crit. Rev. Biotechnol. 2021, 1–21. [Google Scholar] [CrossRef]
- Ao, T.; Chen, L.; Chen, Y.; Liu, X.; Wan, L.; Li, D. The Screening of Early Warning Indicators and Microbial Community of Chicken Manure Thermophilic Digestion at High Organic Loading Rate. Energy 2021, 224, 120201. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Li, P.; Yin, L.; Liu, D.; Zhang, Y.; Li, W.; Zheng, G. A Novel Alternate Feeding Mode for Semi-Continuous Anaerobic Co-Digestion of Food Waste with Chicken Manure. Bioresour. Technol. 2014, 164, 309–314. [Google Scholar] [CrossRef]
- Arriagada, C.B.; Sanhueza, P.F.; Guzmán-Fierro, V.G.; Medina, T.I.; Fernández, K.F.; Roeckel, M.D. Efficient Poultry Manure Management: Anaerobic Digestion with Short Hydraulic Retention Time to Achieve High Methane Production. Poult. Sci. 2019, 98, 6636–6643. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, H.; Shi, C.; Kobayashi, T.; Xu, K.-Q. Nutrient Augmentation Enhances Biogas Production from Sorghum Mono-Digestion. Waste Manag. 2021, 119, 63–71. [Google Scholar] [CrossRef]
- Wu, D.; Peng, X.; Li, L.; Yang, P.; Peng, Y.; Liu, H.; Wang, X. Commercial Biogas Plants: Review on Operational Parameters and Guide for Performance Optimization. Fuel 2021, 303, 121282. [Google Scholar] [CrossRef]
- International Organization for Standardization. Natural Gas—Calculation of Calorific Values, Density, Relative Density and Wobbe Indices from Composition. Standard ISO 6976:2016. 2016. Available online: https://www.iso.org/standard/55842.html (accessed on 6 April 2022).
- Almeida, P.V.; Rodrigues, R.P.; Teixeira, L.M.; Santos, A.F.; Martins, R.C.; Quina, M.J. Bioenergy Production through Mono and Co-Digestion of Tomato Residues. Energies 2021, 14, 5563. [Google Scholar] [CrossRef]
- Rubežius, M.; Bleizgys, R.; Venslauskas, K.; Navickas, K. Influence of Biological Pretreatment of Poultry Manure on Biochemical Methane Potential and Ammonia Emission. Biomass Bioenergy 2020, 142, 105815. [Google Scholar] [CrossRef]
- Song, L.; Li, D.; Cao, X.; Tang, Y.; Liu, R.; Niu, Q.; Li, Y.-Y. Optimizing Biomethane Production of Mesophilic Chicken Manure and Sheep Manure Digestion: Mono-Digestion and Co-Digestion Kinetic Investigation, Autofluorescence Analysis and Microbial Community Assessment. J. Environ. Manag. 2019, 237, 103–113. [Google Scholar] [CrossRef]
- Rizzo, P.F.; Bres, P.A.; Young, B.J.; Zubillaga, M.S.; Riera, N.I.; Beily, M.E.; Argüello, A.; Crespo, D.C.; Sánchez, A.; Komilis, D. Temporal Variation of Physico-Chemical, Microbiological, and Parasitological Properties of Poultry Manure from Two Egg Production Systems. J. Mater Cycles Waste Manag. 2020, 22, 1140–1151. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, C.; Liu, R.; Yellezuome, D.; Zhu, X.; Bai, R.; Liu, M.; Sun, M. Anaerobic Co-Digestion of Corn Stover and Chicken Manure Using Continuous Stirred Tank Reactor: The Effect of Biochar Addition and Urea Pretreatment. Bioresour. Technol. 2021, 319, 124197. [Google Scholar] [CrossRef] [PubMed]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic Digestion of Chicken Manure: Mitigating Process Inhibition at High Ammonia Concentrations by Selenium Supplementation. Biomass Bioenergy 2018, 108, 439–446. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Mahdy, A.; Wandera, S.M.; Yin, D.; Dong, R. Improved High Solid Anaerobic Digestion of Chicken Manure by Moderate in Situ Ammonia Stripping and Its Relation to Metabolic Pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Teleken, J.G.; Weiser Meier, T.R.; Alves, H.J. Two-Stage Anaerobic Digestion in Agroindustrial Waste Treatment: A Review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef] [PubMed]
- Slaný, O.; Klempová, T.; Shapaval, V.; Zimmermann, B.; Kohler, A.; Čertík, M. Biotransformation of Animal Fat-By Products into ARA-Enriched Fermented Bioproducts by Solid-State Fermentation of Mortierella Alpina. JoF 2020, 6, 236. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.-V.N.; Subramanian, S. Sequential Production of Hydrogen and Methane by Anaerobic Digestion of Organic Wastes: A Review. Environ. Chem. Lett. 2021, 19, 1043–1063. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Liang, D.; Li, N. Conductive Materials in Anaerobic Digestion: From Mechanism to Application. Bioresour. Technol. 2020, 298, 122403. [Google Scholar] [CrossRef]
- Liu, C.; Ren, L.; Yan, B.; Luo, L.; Zhang, J.; Awasthi, M.K. Electron Transfer and Mechanism of Energy Production among Syntrophic Bacteria during Acidogenic Fermentation: A Review. Bioresour. Technol. 2021, 323, 124637. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Wang, X. Obstacles Faced by Methanogenic Archaea Originating from Substrate-Driven Toxicants in Anaerobic Digestion. J. Hazard. Mater. 2021, 403, 123938. [Google Scholar] [CrossRef]
- Srivastava, S.K. Advancement in Biogas Production from the Solid Waste by Optimizing the Anaerobic Digestion. Waste Dispos. Sustain. Energy 2020, 2, 85–103. [Google Scholar] [CrossRef] [Green Version]
- Hegde, S.; Trabold, T.A. Anaerobic Digestion of Food Waste with Unconventional Co-Substrates for Stable Biogas Production at High Organic Loading Rates. Sustainability 2019, 11, 3875. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; He, S.; Kang, X.; Sun, Y.; Yuan, Z.; Xing, T.; Guo, Y.; Li, L. Effect of Organic Loading Rate and Temperature on the Anaerobic Digestion of Municipal Solid Waste: Process Performance and Energy Recovery. Front. Energy Res. 2020, 8, 89. [Google Scholar] [CrossRef]
- Usman, M.; Salama, E.-S.; Arif, M.; Jeon, B.-H.; Li, X. Determination of the Inhibitory Concentration Level of Fat, Oil, and Grease (FOG) towards Bacterial and Archaeal Communities in Anaerobic Digestion. Renew. Sustain. Energy Rev. 2020, 131, 110032. [Google Scholar] [CrossRef]
- Rahman, M.A.; Shahazi, R.; Nova, S.N.B.; Uddin, M.R.; Hossain, M.S.; Yousuf, A. Biogas Production from Anaerobic Co-Digestion Using Kitchen Waste and Poultry Manure as Substrate—Part 1: Substrate Ratio and Effect of Temperature. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Jurgutis, L.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas Production from Chicken Manure at Different Organic Loading Rates in a Mesophilic Full Scale Anaerobic Digestion Plant. Biomass Bioenergy 2020, 141, 105693. [Google Scholar] [CrossRef]
- Tao, Y.; Ersahin, M.E.; Ghasimi, D.S.M.; Ozgun, H.; Wang, H.; Zhang, X.; Guo, M.; Yang, Y.; Stuckey, D.C.; van Lier, J.B. Biogas Productivity of Anaerobic Digestion Process Is Governed by a Core Bacterial Microbiota. Chem. Eng. J. 2020, 380, 122425. [Google Scholar] [CrossRef] [Green Version]
- Abd Allah, W.E.; Tawfik, M.A.; Sagade, A.A.; Gorjian, S.; Metwally, K.A.; El-Shal, H. Methane Production Enhancement of a Family-Scale Biogas Digester Using Cattle Manure and Corn Stover under Cold Climates. Sustain. Energy Technol. Assess. 2021, 45, 101163. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef] [Green Version]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of Methane Fermentation as a Valorisation Method for Food Waste Products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in Biogas Production: Pretreatment and Codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Schwan, B.; Abendroth, C.; Latorre-Pérez, A.; Porcar, M.; Vilanova, C.; Dornack, C. Chemically Stressed Bacterial Communities in Anaerobic Digesters Exhibit Resilience and Ecological Flexibility. Front. Microbiol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Stiborova, H.; Strejcek, M.; Musilova, L.; Demnerova, K.; Uhlik, O. Diversity and Phylogenetic Composition of Bacterial Communities and Their Association with Anthropogenic Pollutants in Sewage Sludge. Chemosphere 2020, 238, 124629. [Google Scholar] [CrossRef]
- Forouzanmehr, F.; Solon, K.; Maisonnave, V.; Daniel, O.; Volcke, E.I.P.; Gillot, S.; Buffiere, P. Sulfur Transformations during Two-Stage Anaerobic Digestion and Intermediate Thermal Hydrolysis. Sci. Total Environ. 2022, 810, 151247. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Qaramaleki, S.V.; Coronella, C.J.; Mohedano, A.F.; de la Rubia, M.A. Energy Valorization of Cow Manure by Hydrothermal Carbonization and Anaerobic Digestion. Renew. Energy 2020, 160, 623–632. [Google Scholar] [CrossRef]
- Mahdy, A.; Song, Y.; Salama, A.; Qiao, W.; Dong, R. Simultaneous H2S Mitigation and Methanization Enhancement of Chicken Manure through the Introduction of the Micro-Aeration Approach. Chemosphere 2020, 253, 126687. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane Yields during Anaerobic Co-Digestion of Animal Manure with Other Feedstocks: A Meta-Analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, S.; Xia, D.; Zhao, W.; Li, Q.; Liu, X.; Lv, J. The Biochemical Mechanism of Enhancing the Conversion of Chicken Manure to Biogenic Methane Using Coal Slime as Additive. Bioresour. Technol. 2022, 344, 126226. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Rasapoor, M.; Young, B.; Brar, R.; Sarmah, A.; Zhuang, W.-Q.; Baroutian, S. Recognizing the Challenges of Anaerobic Digestion: Critical Steps toward Improving Biogas Generation. Fuel 2020, 261, 116497. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Korzeniewska, E.; Harnisz, M.; Czatzkowska, M. Intensification of Biogas Production Using Various Technologies: A Review. Int. J. Energy Res. 2020, 44, 6240–6258. [Google Scholar] [CrossRef]
- Muratçobanoğlu, H.; Gökçek, Ö.B.; Mert, R.A.; Zan, R.; Demirel, S. Simultaneous Synergistic Effects of Graphite Addition and Co-Digestion of Food Waste and Cow Manure: Biogas Production and Microbial Community. Bioresour. Technol. 2020, 309, 123365. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.S.; Jalalah, M.; Li, X.; Salama, E.-S. Synergistic Ammonia and Fatty Acids Inhibition of Microbial Communities during Slaughterhouse Waste Digestion for Biogas Production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Huang, W.; Jiang, M.; Xu, C.; Fan, G.; Yan, J.; Chai, B.; Zhang, Y.; Zhang, Y.; Zhang, S.; et al. Challenges of Anaerobic Digestion in China. Int. J. Environ. Sci. Technol. 2021, 18, 3685–3696. [Google Scholar] [CrossRef]
- Mahdy, A.; Bi, S.; Song, Y.; Qiao, W.; Dong, R. Overcome Inhibition of Anaerobic Digestion of Chicken Manure under Ammonia-Stressed Condition by Lowering the Organic Loading Rate. Bioresour. Technol. Rep. 2020, 9, 100359. [Google Scholar] [CrossRef]
- Yan, M.; Fotidis, I.A.; Tian, H.; Khoshnevisan, B.; Treu, L.; Tsapekos, P.; Angelidaki, I. Acclimatization Contributes to Stable Anaerobic Digestion of Organic Fraction of Municipal Solid Waste under Extreme Ammonia Levels: Focusing on Microbial Community Dynamics. Bioresour. Technol. 2019, 286, 121376. [Google Scholar] [CrossRef]
- Musa, M.; Idrus, S.; Hasfalina, C.; Daud, N. Effect of Organic Loading Rate on Anaerobic Digestion Performance of Mesophilic (UASB) Reactor Using Cattle Slaughterhouse Wastewater as Substrate. IJERPH 2018, 15, 2220. [Google Scholar] [CrossRef] [Green Version]
- Ghofrani-Isfahani, P.; Valverde-Pérez, B.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Supervisory Control of an Anaerobic Digester Subject to Drastic Substrate Changes. Chem. Eng. J. 2020, 391, 123502. [Google Scholar] [CrossRef]

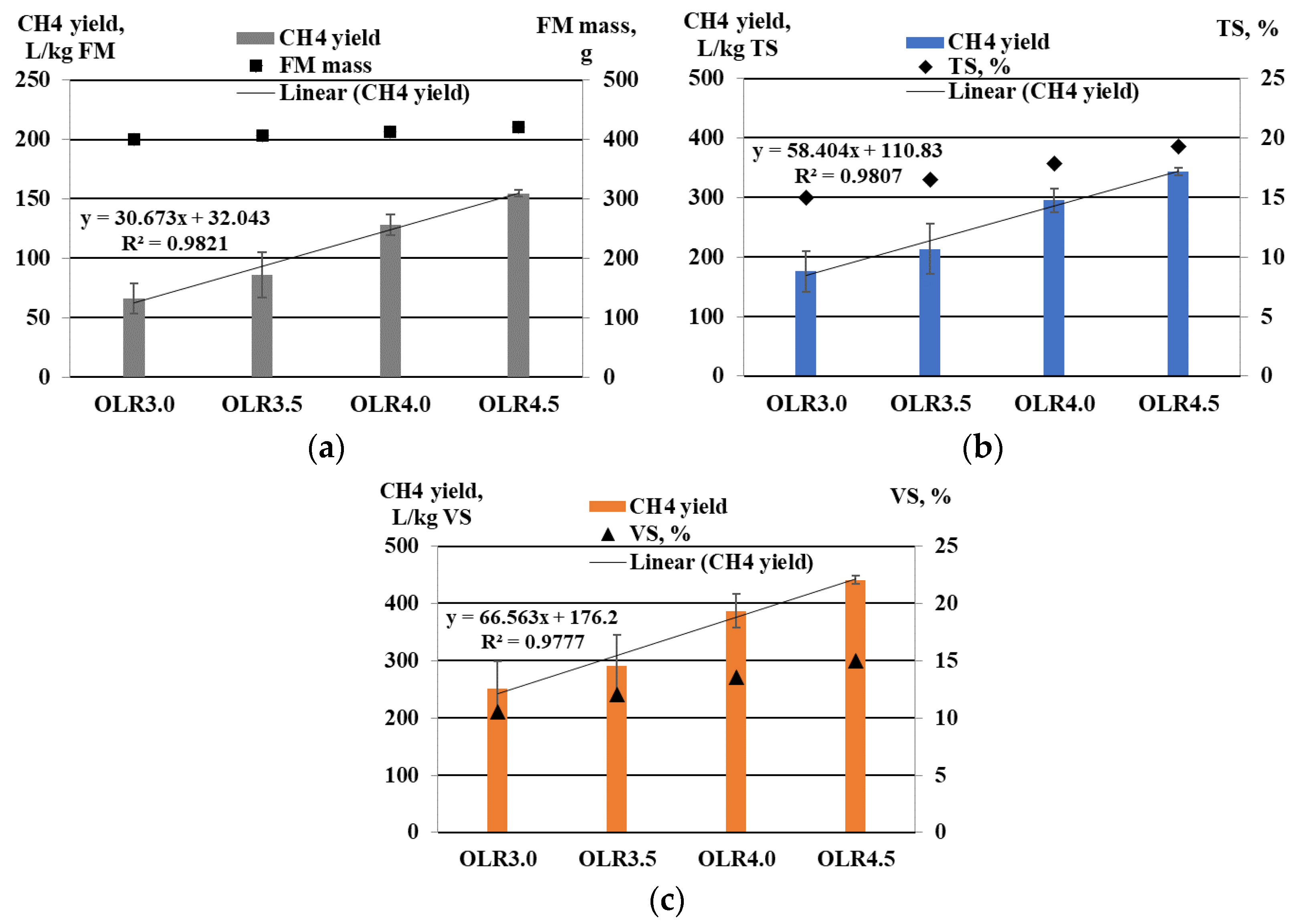
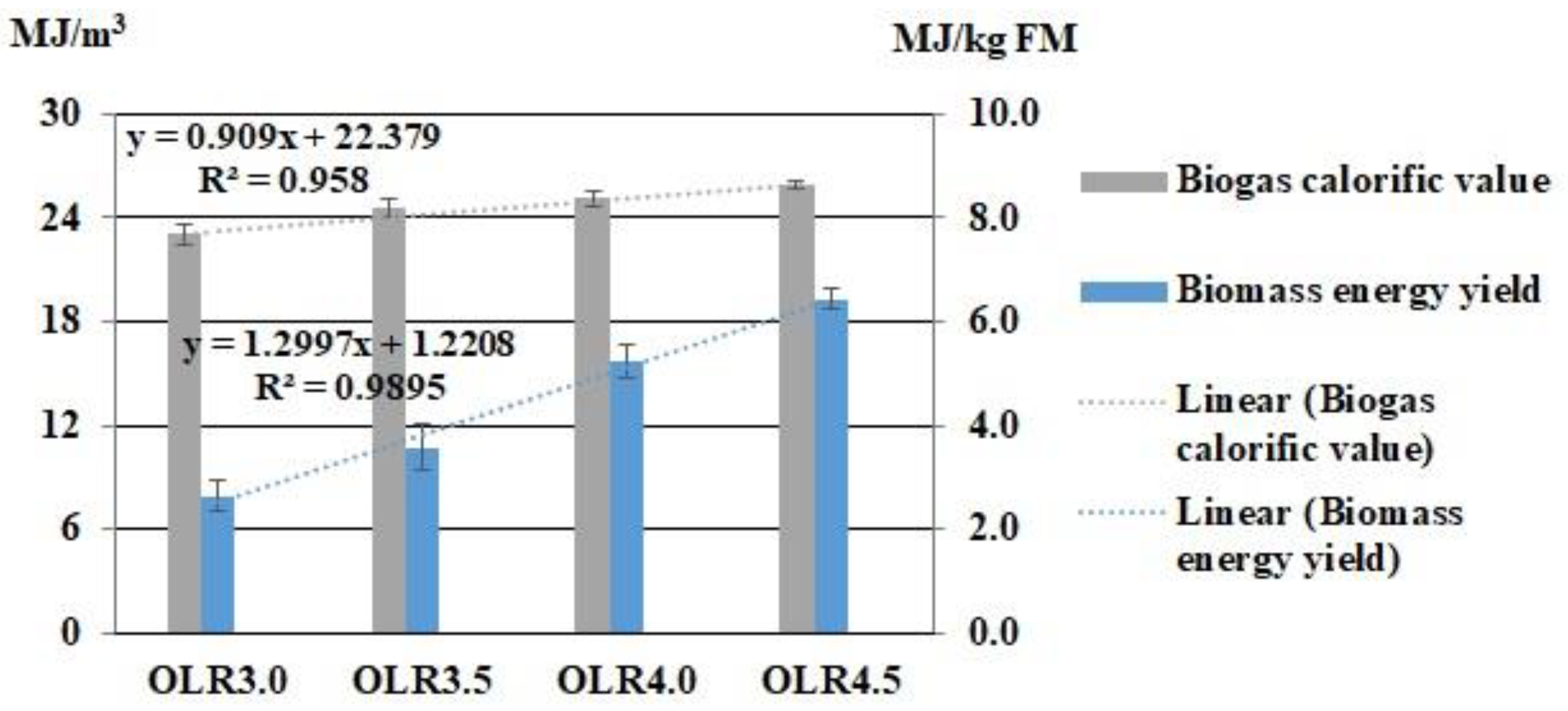

| Experiment | FM Description | OLR, kg VS·(m3·Day)−1 | FM Mass, g |
|---|---|---|---|
| OLR3.0 | RM + water | 3.00 | 399.4 |
| OLR3.5 | RM + water + fat 7 g | 3.50 | 406.4 |
| OLR4.0 | RM + water + fat 14 g | 4.00 | 413.4 |
| OLR4.5 | RM + water + fat 21 g | 4.50 | 420.4 |
| Parameter Description | Material Analysed | |
|---|---|---|
| RM | FAT | |
| Total solids (TS), % | 37.76 ± 0.62 | 99.98 ± 0.01 |
| Volatile solids (VS), % | 26.45 ± 0.27 | 99.96 ± 0.02 |
| Total carbon (C), % | 26.8 ± 0.3 | 86.0 ± 0.1 |
| Total nitrogen (N), % | 2.36 ± 0.12 | 0.93 ± 0.10 |
| C/N ratio | 11:1 | 93:1 |
| Total sulphur (S), % | 0.39 ± 0.28 | 3.16 ± 0.15 |
| Ammoniacal nitrogen (TAN), % | 0.55 ± 0.15 | n/d |
| pH | 7.80 ± 0.03 | n/d |
| Parameter | Experiment | |||
|---|---|---|---|---|
| OLR3.0 | OLR3.5 | OLR4.0 | OLR4.5 | |
| OLR increase, % | n/a | 16.7 | 28.6 | 37.5 |
| FM increase, % | n/a | 1.75 | 3.51 | 5.26 |
| Cm increase, % | n/a | 5.20 | 7.96 | 9.57 |
| Mvs increase, % | n/a | 25.1 | 55.8 | 77.3 |
| Parameter | Experiment | |||
|---|---|---|---|---|
| OLR3.0 | OLR3.5 | OLR4.0 | OLR4.5 | |
| TS, % | 5.28 ± 0.46 | 5.68 ± 0.31 | 5.32 ± 0.21 | 7.11 ± 0.59 |
| VS, % | 3.95 ± 0.28 | 3.72 ± 0.19 | 3.42 ± 0.12 | 5.03 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buivydas, E.; Navickas, K.; Venslauskas, K.; Žalys, B.; Župerka, V.; Rubežius, M. Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat. Appl. Sci. 2022, 12, 4652. https://doi.org/10.3390/app12094652
Buivydas E, Navickas K, Venslauskas K, Žalys B, Župerka V, Rubežius M. Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat. Applied Sciences. 2022; 12(9):4652. https://doi.org/10.3390/app12094652
Chicago/Turabian StyleBuivydas, Egidijus, Kęstutis Navickas, Kęstutis Venslauskas, Bronius Žalys, Vidmantas Župerka, and Mantas Rubežius. 2022. "Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat" Applied Sciences 12, no. 9: 4652. https://doi.org/10.3390/app12094652
APA StyleBuivydas, E., Navickas, K., Venslauskas, K., Žalys, B., Župerka, V., & Rubežius, M. (2022). Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat. Applied Sciences, 12(9), 4652. https://doi.org/10.3390/app12094652






