Abstract
Polyurethane composite foams were prepared by adding three different types of silica materials as a filler to improve the mechanical and thermal insulation properties. The first type of filler consists of silica aerogels with high-volume pores, with the expectation of improving the thermal insulation of PU foams because silica aerogel itself has superior thermal insulation properties. Silica nanoparticle is used for the second type that has a size very similar to the pore size of silica aerogels for comparison. The last type to produce polyurethane composite foam uses a sol–gel reaction to produce polysiloxane that reacts with polyols during the urethane reaction and forming process. In particular, in the case of silica aerogels and nanoparticles, their surfaces are modified with APTES and then polymeric methylene diphenylene diisocyanate (PMDI) to increase the interaction between the polymer matrix and inorganic fillers. The polyurethane foam structure was successfully produced in all cases of composite foams. As expected, the mechanical properties and the thermal insulation effect were enhanced by the addition of silica fillers, but found to be closely related to the cell structure of polyurethane foams. The addition of small amounts of inorganic fillers improves the mechanical and thermal properties, but the higher the amount of filler, the worse they are due to the agglomeration of fillers on the cell walls. The dispersion of added inorganic fillers within the foam cells should be controlled effectively. Surface-modified silica fillers exhibit better enhancement of mechanical and thermal insulation properties.
1. Introduction
Energy is vital for almost all human life and economic activities, and is recognized as an essential commodity in our life. Global energy consumption increases rapidly every year while energy resources are limited. Several types of renewable energy are being developed, but they are not yet meeting our needs [1]. In addition to resource depletion, the rising energy demand leads to the global concern for environmental pollution and economic problems. Hence it is very important not only to efficiently produce and consume energy but also to find a way to properly preserve and save energy. From this point of view, there is a significant interest in the use of an insulating material that prevents heat transfer and maintains a constant temperature by minimizing the loss of heat or the inflow of heat to the outside [2]. Insulation materials are widely used not only for building walls and doors, but also for home appliances, such as refrigerators and vending machines, and industrial applications, such as refrigerated vehicles and ships [3,4].
There are various types and shapes of insulation materials depending on the characteristics, structure, and purpose of the material used. Compared to other insulation materials, polyurethane (PU) foam with a pore structure has many advantages such as light weight, buffering properties, ease of processing, and diversity of properties and structures, as well as very excellent insulation properties [5,6,7,8]. Polyurethane is basically a polymer formed through urethane bonding between a polyol corresponding to a soft segment and a diisocyanate of a hard segment [9]. The hard-segment domains serve as physical crosslinks for the rubbery soft-segment matrix, imparting high elasticity of the material. The soft segment generally consists of polyether or polyester polyol, while the hard segment is formed by the extension of a diisocyanate with a chain extender of a low molecular weight polyol or amine. Polyurethane foams are formed by the reaction of urethane bonding in the presence of a blowing agent and an amine as a catalyst. Polyurethane foam is largely divided into soft and rigid foam, and rigid polyurethane foam is the most used as an insulator [10]. It is applied not only to buildings, but also to home appliances such as refrigerators, storage tanks for transport, and pipes. The insulation effect in foam structures can be divided into four major contributions: (1) heat conduction through the solid phase, (2) heat conduction through the gas-filled interior, (3) convection within the cell, and (4) radiation through the cell walls [11,12,13]. The individual contributions add up to give overall thermal insulation properties. If the cell size in the polymer foam is smaller than 4 mm, the contribution to convection becomes negligible, and the heat conduction of the polyurethane foam is ultimately determined by the foaming agent, the solid polymer, and the cell structure in the foam. Therefore, when the foaming agent and the solid polymer are fixed, the thermal insulation property of the polyurethane foam is determined by the cell structure, and the smaller and more uniform the cell size, the lower the thermal conductivity and the better the insulation effect.
Since the most important characteristic of polyurethane foam is the insulation effect, many studies are still being conducted to improve its insulation performance [14,15,16,17,18]. As mentioned above, since the insulation effect is determined by several factors, many efforts have been made to enhance the insulation property from various viewpoints and methods. In particular, an approach in the form of a composite material using minerals, ceramics, or metals as fillers is attracting much attention from many researchers [19,20,21]. Traditionally, polymer composite materials have been studied for a long time as a method to improve the mechanical and thermal properties of polymers themselves. The addition of a small amount of filler to the polymer matrix leads to the improvement of the physical properties of the polymer matrix due to the characteristics of the filler itself, and, in general, the mechanical and thermal properties are greatly improved [20]. This is because the mechanical/thermal properties of inorganic materials used as fillers are relatively superior to those of polymer materials. In the case of a polymer foam, similar effects such as the improvement of mechanical and thermal properties can be expected if a filler-added polymer foam composite is prepared. Since the added filler affects the cell structure in the foam, it can be expected to increase the properties of the foam, particularly the heat insulation effect, through changes in the cell structure as well as the properties of the filler itself. In fact, as a result of analyzing various physical properties of the polyurethane foam composite material produced in several previous studies, further improvement in the insulation effect and the mechanical properties was observed [22]. Therefore, in order to further improve the thermal insulation properties of the polyurethane foam, it is necessary to select and add an appropriate filler and control the cell structure of the foam to form a uniform cell structure of a small size.
Silica aerogel is an ideal porous material with a pearl necklace-like three-dimensional structure having interstitial mesopores. Such structural feature makes it possible to use it as an insulator because it shows low density and low thermal conductivity due to high porosity with 90% or more of air [23,24,25,26]. Silica aerogel as an insulating material has a thermal conductivity of 20 mW/mK or less, and has even smaller thermal conductivity than other conventional insulating materials. In addition, due to its high surface area, high porosity, and low density, research is being actively conducted to utilize it in various fields such as insulation or sound-absorbing materials for buildings, liquefied natural gas (LNG) carriers, and refrigeration equipment. However, silica aerogel is brittle and sensitive to humidity, which limits the use of silica aerogel alone as an insulator or sound absorbing material.
In this study, in order to improve the properties and performance of polyurethane foam, silica-based filler is added to prepare a polyurethane foam composite. As mentioned above, silica aerogel is a material with a very high porosity, and due to its superior thermal insulation effect, the addition of silica aerogels into polyurethane foams can be expected to improve their thermal insulation performance [27,28,29]. The surface of silica aerogels added into the composite foam is modified to interact with polyurethane matrix and improve the dispersion of the silica aerogel in the composite foam. In addition to silica aerogels, in this study, two different types of silica fillers were added to prepare polyurethane composite foam for comparison. First, silica nanoparticles of a size similar to that of silica aerogel are added as a filler. In this case, as in the case of aerogel, the surface of silica nanoparticles is treated to induce the improvement of interfacial adhesion between the polymer matrix and filler. The second method involves making a silica-polyurethane foam composite through a sol-gel method. Polysiloxane functional groups obtained through a sol–gel reaction with polyol is introduced into a polyurethane reaction and foaming process to prepare a silica–polyurethane composite foam [30,31]. Consequently, the polyurethane composite foams are prepared by introducing inorganic silica as a filler using the three methods described above, and their effects are compared to seek a better way for producing PU composite foams.
2. Experimental
2.1. Materials
Rigid polyols and polymeric methylene diphenyl diisocyanate (PMDI) supplied by Kumho Petrochemical (Korea) for refrigerator insulation were used for the synthesis of polyurethane foams. Silica aerogels was provided by Dongsung Finetech (South Korea), and silica nanoparticles with a size of 20 nm in the solid foam were purchased from US Research Nanomaterials. Tetraethyl orthosilicate (TEOS) from Aldrich was used as a silica precursor for polysiloxane production. Also from Aldrich, (3-aminopropyl)triethoxysilane (APTES) was purchased to modify the surfaces of silica aerogels and nanoparticles. All solvents used in this study were used as received without further purification.
2.2. Surface Modification of Silica Aerogels and Silica Nanoparticles
The surface of the silica aerogel was first modified with APTES. To this end, 1 g of silica aerogel was dispersed in toluene, and 0.5 g of APTES was added under a nitrogen atmosphere to react with the hydroxyl group of silica aerogel at 80 °C for 24 h. Then, the mixture was cooled down to room temperature, and APTES-modified silica aerogels were recovered through centrifugation and washed with ethanol to remove unreacted APTES. The obtained product was dried at 60 °C for 24 h. The silica aerogels modified with ATPES were finally reacted with PMDI. Next, 1 g of APTES-modified silica aerogels and 2.55 g of PMDI were mixed in DMF, and the reaction was carried out at 50 °C for 3 h. After centrifugation and washing with ethanol, silica aerogels modified with PMDI drying were finally obtained. Surface modification of silica nanoparticles was also performed in a similar manner to silica aerogels.
2.3. Preparation of Polyurethane Composite Foams
Polyurethane composite foam was prepared through the following process. First, silica aerogels or silica nanoparticles with surface modified with PMDI were mixed with PMDI corresponding to the hard segment of polyurethane. Then, an equal amount of PMDI containing silica aerogels or silica nanoparticles and a polyol were mixed, and foaming was allowed to proceed simultaneously with the urethane reaction. The amount of silica aerogels or silica nanoparticles added was set to be within 3% by weight. The reaction and foaming were carried out in the paper cup, and after mixing polyol and PMDI, the mixer was turned for about 20 s and the foam reaction was continued to finally prepare the polyurethane composite foams.
In order to make a polyurethane composite foam through a sol–gel reaction, a mixture of 48.6 wt% of TEOS, 43.0 wt% of ethanol, and 8.4 wt% of water in a 500 mL round flask was first reacted in HCl solution at room temperature for 24 h. TEOS forms siloxane adducts through hydrolysis and condensation reactions [30]. After removing the solvent from the reaction mixture using a rotary evaporator, the obtained adduct is added to the polyol in a predetermined amount. Thereafter, a polyurethane composite foam was prepared by mixing a polyol including a siloxane adduct and PMDI in the same manner as before.
2.4. Characterization
The shape and structure of the prepared polyurethane composite foams were observed with a scanning electron microscope (SEM, S-3400N, Hitachi). FT-IR measurements were performed using the VERTEX 90 V (Bruker). In order to measure the mechanical properties of the foam, the foam was cut to a size of 10 mm × 10 mm × 10 mm and a universal testing machine (UTM, 5569 Instron) was used. Thermal conductivity was measured using a Thermal Conductivity Measurement System (LFA467, Netzsch). The pore size and its distribution within the foams were analyzed with ImageJ software. To this end, several SEM images were converted to 8-bit binary images to obtain a clear cut-off between pores and foam matrix.
3. Results and Discussion
In this study, an effort was made to chemically bond the silica materials added as fillers to the polyurethane chains when preparing the polyurethane composite foam. This is expected to help the silica fillers to be well dispersed in the foam and to help improve the properties and performance of composite foams through synergetic effects of polymer matrix and filer. For this purpose, in the case of silica aerogels and nanoparticles, APTES, a silane coupling agent, is first introduced to the surface through a hydrolysis reaction, and then APTES attached to the surface of silica aerogels and silica nanoparticles is reacted with PMDI to produce silica aerogels and silica nanoparticles chemically modified with PMDI. These surface-modified silica aerogels and nanoparticles are mixed with PMDI and then reacted with a polyol to finally form a composite foam via foaming process.
Figure 1 shows the surface modification results of silica aerogels and silica nanoparticles. Figure 1a,b correspond to the FT-IR spectra before and after modification of silica aerogels and silica nanoparticles with APTES and PMDI. As a result of the modification with APTES, it can be seen that the #x2213;NH2 characteristic peak at the end of APTES is very weak yet clearly present. After reaction with PMDI, the corresponding characteristic peaks are clearly observed with ∓C=O peak at 1720 cm−1, characteristic peak of the benzene ring at 1660 cm−1, and -N-H stretching motion near 1540 cm−1. The same results are observed in the case of silica nanoparticles, and all these results indicate that PMDI was successfully bonded to the silica surface. This result also indirectly indicates that APTES was successfully attached to the silica surface by chemical bonding.
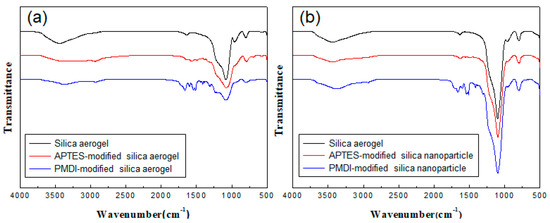
Figure 1.
FT-IR spectra of silica aerogels (a) and silica nanoparticles (b) with and without surface modification.
The TGA results in Figure 2a show the APTES modification result more directly. A greater weight loss can be observed in the silica aerogel modified with ATPES, which is due to the thermal decomposition of APTES adsorbed on the surface of the silica aerogel. Figure 2a also shows the effect of reaction temperature when APTES reacts on the silica aerogel surface, and it can be seen that a relatively large amount reacts on the silica surface when reacted at 80 °C. Based on these results, the ATPES–silica reaction was then performed at 80 °C. Figure 2b–d present the SEM micrographs showing the structure of silica aerogel before and after modification. It can be observed that there is little change in the appearance of the silica aerogel through the modification. Although not included here, the same results were obtained for silica nanoparticles.
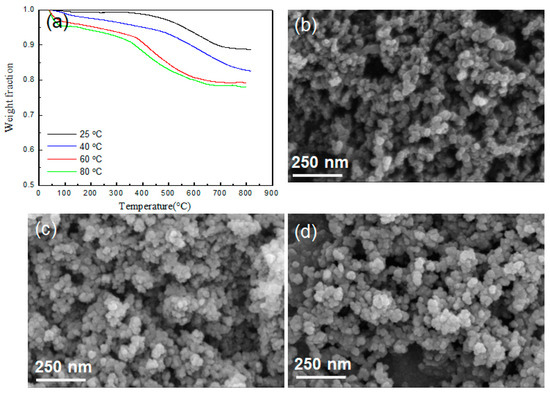
Figure 2.
(a) TGA curves of pure silica aerogel and silica aerogels modified with APTES at different temperature and SEM images of pure silica aerogel (b) and silica aerogels modified with APTES (c) and PMDI (d).
As described in the experimental section, silica aerogels and silica nanoparticles surface-modified with PMDI were mixed with PMDI and then reacted with polyol to prepare a polyurethane composite foam. Even with the addition of silica aerogels and nanoparticles, it was successfully foamed to produce a desired composite foam. Figure 3 presents the SEM micrographs showing the internal structure of a polyurethane composite foam to which silica aerogel is added. For comparison, polyurethane foam without filler and with unmodified silica aerogel were also prepared, and the results are included. As shown in Figure 3, a typical polyurethane foam structure is produced well in all cases. A foamed structure having pores of relatively uniform size was formed regardless of whether or not the surface of silica aerogels was modified. A closer examination reveals that when a small amount of silica aerogel is added, the pore size becomes smaller than when no silica aerogel is added. Furthermore, in the case of the modified silica aerogel, it can be observed that the pore size is slightly smaller than that of the unmodified silica aerogel. However, the excessive addition of silica aerogels was found to lead to an even worse result of the pore size becoming rather large and non-uniform, as shown in Figure 3c,g.
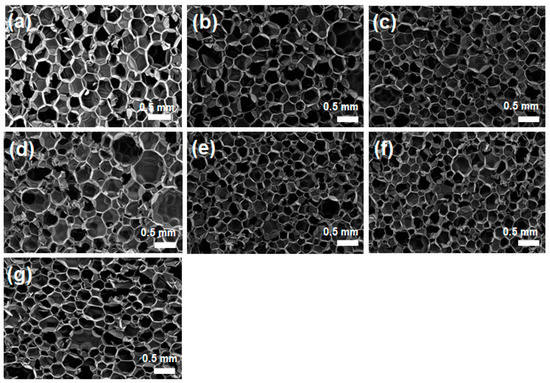
Figure 3.
SEM images of neat PU foam without additive (a) and PU composite foams with silica aerogels without surface modification at different aerogel compositions of (b) 1 wt%, (c) 3 wt%, and (d) 5 wt%, and with silica aerogels with surface-modified silica aerogels of (e) 1 wt%, (f) 3 wt%, and (g) 5 wt%.
Figure 4 corresponds to the results of the addition of silica nanoparticles as a filler and exhibits results very similar to those found in the case of silica aerogels. Compared to the silica aerogels, however, it can be seen that not only the pore size is reduced, but also its distribution becomes more uniform. This change in the foam structure is expected to contribute to the improvement of the insulation effect. Likewise, the modified silica nanoparticles are more effective in changing the foam structure than unmodified silica nanoparticles. In the case of silica nanoparticles, however, their aggregates are clearly found in the cell wall at higher contents, as shown in Figure 4e,i, which cause the cell structure of polyurethane foam to become non-uniform, and this is expected to act as a defect in the mechanical and thermal insulation properties.
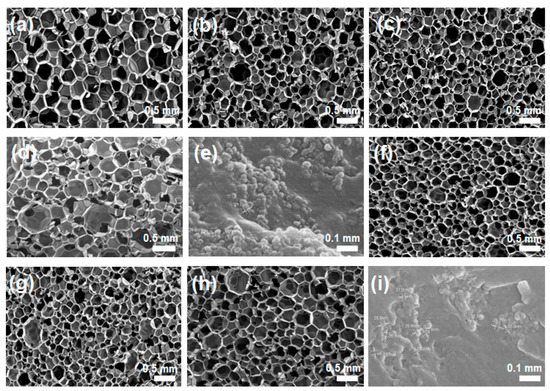
Figure 4.
SEM images of neat PU foam without additive (a) and PU composite foams with silica nanoparticles without surface modification at different aerogel compositions of (b) 1 wt%, (c) 3 wt%, and (d,e) 5 wt%, and with silica nanoparticles with surface-modified silica nanoparticles of (f)1 wt%, (g) 3 wt%, and (h,i) 5 wt%.
The results of adding silica particles through a sol–gel reaction are included in Figure 5, where SEM images of composite foams are shown. As in the previous cases, the silica particles added through the sol–gel reaction affect the polyurethane foam structure, and it can be seen that the pore size is generally reduced, compared to the case where the silica particles are not added. However, the pore size distribution is not uniform compared to the case where silica aerogels or silica nanoparticles are added. It showed definite effects on the change of cell structure, but the result was not as great as expected, which demonstrates that it is very difficult to control the cell structure and filler dispersion by the inclusion of polysiloxane domains in the polyurethane foam through sol–gel reaction and thereafter an in situ urethane reaction and foaming process.
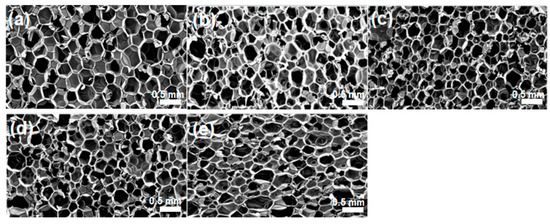
Figure 5.
SEM images of neat PU foam without additive (a) and PU composite foams with silica filler by sol-gel reaction at different composition of without surface modification at different aerogel compositions of (b) 0.5 wt%, (c) 1 wt%, (d) 1.5 wt%, and (e) 3 wt%.
Figure 6 compares the pore sizes of polyurethane composite foams fabricated by three different methods. Here, the foam pore size was measured using the Image J program. The pore size of all polyurethane composite foams was reduced compared to the polyurethane foam without filler, and this result occurs because the silica aerogels or nanoparticles added during foaming act as a nucleating agent. Generally, the added nanoparticles increase the number of cells and so decrease the size of cells in the foam [32]. Comparing the polyurethane composite foams, the pore size reduction effect is greater when the modifying additive is used. Therefore, in order to control the structure well, it is necessary to control the interfacial interaction between the polymer matrix and the additive even in the foam structure. When the additives are compared at the same concentration, the pore size becomes smaller when silica nanoparticle are added as a filler, but the difference is not very large considering the experimental error range.
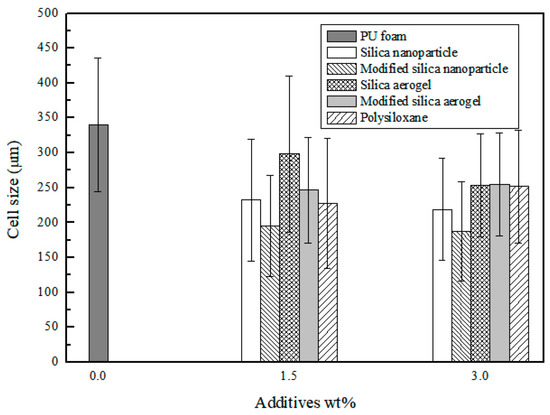
Figure 6.
Distribution of mean cell size of PU and PU composite foams with three different methods. The error bars correspond to the standard deviation of cell size in the foams.
The mechanical properties of polyurethane composite foams are presented in Figure 7. In the case of adding silica aerogel, the physical properties are improved when a small amount (0.5 wt%) of the aerogel is added. However, if the added amount is further increased, the compressive strength decreases and eventually shows properties similar to those of the original PU foam. The effects on the physical properties becomes more evident when silica nanoparticles are added into polyurethane foam (see Figure 7b). The compressive strength increased the most when 1 wt% of silica nanoparticles were added, and the compressive strength decreased when 3 wt% was added. On the other hand, when polysiloxane is added through sol–gel reaction to produce PU composite foam, the compressive strength increases at very small amount, but it is rather reduced at higher compositions. All these results are thought to be correlated with the distribution of additives in the PU cell wall. All types of fillers exhibit the effect of reducing the cell size, but the additives partially aggregate in the cell wall at higher content, eventually leading to a defect that reduces the mechanical properties.
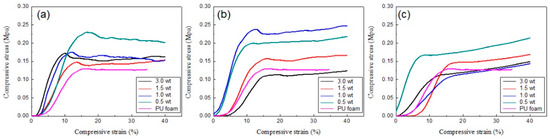
Figure 7.
Compressive stress of PU foam and PU composite foams with different composition of (a) silica aerogels, (b) silica nanoparticles, and (c) silica produced by sol-gel reaction.
The thermal conductivity of the PU composite foam containing silica aerogels and silica nanoparticles was measured and summarized in Figure 8. As expected, the PU composite foam to which silica aerogel is added exhibits the lowest thermal conductivity. Looking at the composition effect, the thermal conductivity initially decreases, but it rather increases with the composition by the amount of fillers further increasing. This behavior shows the same trend as the change in pore size. In the case of the same fillers added, the thermal conductivity is strongly affected by the cell structure, and it can be seen that the thermal conductivity decreases as the cell size decreases. The same results were observed for PU composites with silica nanoparticles added.
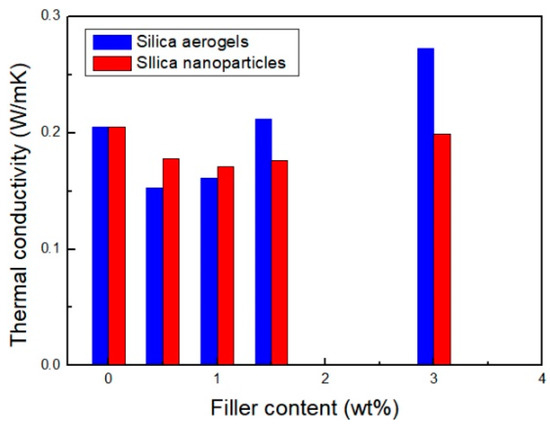
Figure 8.
Thermal conductivity of PU foam and PU composite foams with silica aerogels and silica nanoparticles of different compositions.
4. Conclusions
In this study, PU composite foams were prepared by adding silica to increase the mechanical properties and thermal insulation effect of PU foams. Three types of silica additives were used: silica aerogels with pores of several tens of nanometers, silica nanoparticles with a size of several tens of nanometers, and silica additives through a sol–gel reaction during the PU reaction and foaming. The structure, physical properties, and thermal conductivity of the PU composite foams thus obtained were analyzed according to the foam structure and the type and amount of the additives. Comparing the PU composite foams containing silica aerogels and nanoparticles, the case of adding silica nanoparticles showed better mechanical properties, and, in the case of thermal conductivity, PU composite foam with silica aerogel having pores used as a filler shows a lower value. However, overall thermal conductivity reduction or cell size reduction effect did not appear as expected, which is due to the dispersion of the added silica nanoparticles and silica aerogel. If the filler effect is to be further improved, the interaction between the matrix and the additive as well as the dispersion within the matrix must be effectively achieved.
Author Contributions
Conceptualization, H.J. and S.H.K.; methodology, D.I.L. and Y.H.H.; validation, Y.H.H. and H.J.; formal analysis, D.I.L. and S.H.K.; investigation, D.I.L. and Y.H.H.; data curation, D.I.L.; writing—original draft preparation, D.I.L. and S.H.K.; writing—review and editing, H.J. and S.H.K.; supervision, H.J. and S.H.K.; project administration, S.H.K.; funding acquisition, S.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program (Grant No. 10077584) through the Korea Evaluation Institute of Industry Technology (KEIT), funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bogdanov, D.; Ram, M.; Aghahosseini, A.; Gulagi, A.; Oyewo, A.S.; Child, M.; Caldera, U.; Sadovskaia, K.; Farfan, J.; de Souza Noel Simas Barbosa, L.; et al. Low-Cost Renewable Electricity as the Key Driver of the Global Energy Transition towards Sustainability. Energy 2021, 227, 120467. [Google Scholar] [CrossRef]
- Naldzhiev, D.; Mumovic, D.; Strlic, M. Polyurethane Insulation and Household Products—A Systematic Review of Their Impact on Indoor Environmental Quality. Build. Environ. 2020, 169, 106559. [Google Scholar] [CrossRef]
- Hung Anh, L.D.; Pásztory, Z. An Overview of Factors Influencing Thermal Conductivity of Building Insulation Materials. J. Build. Eng. 2021, 44, 102604. [Google Scholar] [CrossRef]
- Villasmil, W.; Fischer, L.J.; Worlitschek, J. A Review and Evaluation of Thermal Insulation Materials and Methods for Thermal Energy Storage Systems. Renew. Sustain. Energy Rev. 2019, 103, 71–84. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R.S. PU Foam Derived from Renewable Sources: Perspective on Properties Enhancement: An Overview. Eur. Polym. J. 2017, 95, 255–274. [Google Scholar] [CrossRef]
- Wu, J.W.; Sung, W.F.; Chu, H.S. Thermal Conductivity of Polyurethane Foams. Int. J. Heat Mass Transf. 1999, 42, 2211–2217. [Google Scholar] [CrossRef]
- Hu, F.; Wu, S.; Sun, Y. Hollow-Structured Materials for Thermal Insulation. Adv. Mater. 2019, 31, 1801001. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Engels, H.-W.; Pirkl, H.-G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Hawkins, M.C.; O’Toole, B.; Jackovich, D. Cell Morphology and Mechanical Properties of Rigid Polyurethane Foam. J. Cell. Plast. 2005, 41, 267–285. [Google Scholar] [CrossRef]
- Tseng, C.J.; Yamaguchi, M.; Ohmori, T. Thermal Conductivity of Polyurethane Foams from Room Temperature to 20 K. Cryogenics 1997, 37, 305–312. [Google Scholar] [CrossRef]
- Collishaw, P.G.; Evans, J.R.G. An Assessment of Expressions for the Apparent Thermal Conductivity of Cellular Materials. J. Mater. Sci. 1994, 29, 486–498. [Google Scholar] [CrossRef]
- Alvarez-Lainez, M.; Rodriguez-Perez, M.A.; de Saja, J.A. Thermal Conductivity of Open-Cell Polyolefin Foams. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 212–221. [Google Scholar] [CrossRef]
- Do, T.V.V.; Le, V.H.V.; Thai, N.U.N.; Dai, H.N.; Grillet, A.; Thuc, C.N.H. The Influence of Nano-silica on the Thermal Conductivity of Polyurethane Foam. J. Appl. Polym. Sci. 2021, 138, 50715. [Google Scholar] [CrossRef]
- Singh, I.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Advancement in Plant Oil Derived Polyol-Based Polyurethane Foam for Future Perspective: A Review. Eur. J. Lipid Sci. Technol. 2020, 122, 1900225. [Google Scholar] [CrossRef]
- Kirpluks, M.; Kalnbunde, D.; Benes, H.; Cabulis, U. Natural Oil Based Highly Functional Polyols as Feedstock for Rigid Polyurethane Foam Thermal Insulation. Ind. Crops Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Li, M.E.; Wang, S.X.; Han, L.X.; Yuan, W.J.; Cheng, J.B.; Zhang, A.N.; Zhao, H.B.; Wang, Y.Z. Hierarchically Porous SiO2/Polyurethane Foam Composites towards Excellent Thermal Insulating, Flame-Retardant and Smoke-Suppressant Performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Suppes, G.J.; Ghosh, T.K. Simulation of the Optimized Thermal Conductivity of a Rigid Polyurethane Foam during Its Foaming Process. Fibers Polym. 2019, 20, 358–374. [Google Scholar] [CrossRef]
- Saha, M.C.; Kabir, M.E.; Jeelani, S. Enhancement in Thermal and Mechanical Properties of Polyurethane Foam Infused with Nanoparticles. Mater. Sci. Eng. A 2008, 479, 213–222. [Google Scholar] [CrossRef]
- Chen, L.; Rende, D.; Schadler, L.S.; Ozisik, R. Polymer Nanocomposite Foams. J. Mater. Chem. A 2013, 1, 3837–3850. [Google Scholar] [CrossRef]
- Sung, G.; Kim, J.H. Influence of Filler Surface Characteristics on Morphological, Physical, Acoustic Properties of Polyurethane Composite Foams Filled with Inorganic Fillers. Compos. Sci. Technol. 2017, 146, 147–154. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym. Plast. Technol. Eng. 2018, 57, 346–369. [Google Scholar] [CrossRef]
- Parvathy Rao, A.; Venkateswara Rao, A. Microstructural and Physical Properties of the Ambient Pressure Dried Hydrophobic Silica Aerogels with Various Solvent Mixtures. J. Non-Cryst. Solids 2008, 354, 10–18. [Google Scholar] [CrossRef]
- Pajonk, G.M. Transparent Silica Aerogels. J. Non-Cryst. Solids 1998, 225, 307–3148. [Google Scholar] [CrossRef]
- Kocon, L.; Despetis, F.; Phalippou, J. Ultralow Density Silica Aerogels by Alcohol Supercritical Drying. J. Non-Cryst. Solids 1998, 225, 96–100. [Google Scholar] [CrossRef]
- Yoo, J.K.; Wagle, R.; Lee, C.W.; Lee, E.Y. Synthesis of Silica Aerogel Thin Sheets and Evaluation of Its Thermal, Electrical, and Mechanical Properties. Int. J. Appl. Ceram. Technol. 2019, 16, 832–842. [Google Scholar] [CrossRef]
- Chang, K.J.; Wang, Y.Z.; Peng, K.C.; Tsai, H.S.; Chen, J.R.; Huang, C.T.; Ho, K.S.; Lien, W.F. Preparation of Silica Aerogel/Polyurethane Composites for the Application of Thermal Insulation. J. Polym. Res. 2014, 21, 338. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and Characterization of Silica Aerogel Reinforced Rigid Polyurethane Foam for Thermal Insulation Application. J. Non-Cryst. Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, J.-H.; Kim, J.-D.; Lee, D.-H.; Kim, S.-K.; Lee, J.-M.; Kim, C.; Ahn, J.-H.; Kim, J.-H.; Lee, J.-D.; et al. Influence of Silica-Aerogel on Mechanical Characteristics of Polyurethane-Based Composites: Thermal Conductivity and Strength. Materials 2021, 14, 1790. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Lamanna, R.; di Maio, E.; Iannace, S. Polyurethane-Silica Hybrid Foam by Sol-Gel Approach: Chemical and Functional Properties. Polymer 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Song, E.H.; Jeong, S.H.; Park, J.U.; Kim, S.; Kim, H.E.; Song, J. Polyurethane-Silica Hybrid Foams from a One-Step Foaming Reaction, Coupled with a Sol-Gel Process, for Enhanced Wound Healing. Mater. Sci. Eng. C 2017, 79, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Merillas, B.; Villafañe, F.; Rodríguez-Pérez, M.Á. Nanoparticles Addition in PU Foams: The Dramatic Effect of Trapped-Air on Nucleation. Polymers 2021, 13, 2952. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).