Use of Hydrogels to Regulate Orthodontic Tooth Movement in Animal Models: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
- (P)
- Population: animal models to which orthodontic forces were applied.
- (I)
- Interventions: the use of hydrogels to accelerate OTM or inhibit relapse.
- (C)
- Control: groups of animals to which hydrogel was not applied.
- (O)
- Outcome: acceleration of tooth movement or inhibition of relapse.
2.2. Search Protocol
2.3. Inclusion and Exclusion Criteria
2.3.1. Inclusion Criteria
- -
- All published animal studies were included.
- -
- Articles with the main objective of evaluate the use of hydrogels as vehicles to accelerate or inhibit the rate of orthodontic tooth movement.
- -
- All publications were considered except for those where the full-text article was not available, or the authors’ affiliation or the place of publication were not specified.
2.3.2. Exclusion Criteria
- -
- All those articles that, when talking about relapse, did so in the context of cancer or infectious process.
- -
- All those articles that, when talking about relapse, did so in the context of the mechanical properties of dental material.
- -
- Any article that did not have a control group.
2.4. Methods of Selection, Data Extraction, and Assessment of Risk of Bias
3. Results
3.1. Selection of Studies
3.2. General Characteristics of the Studies
3.3. Main Study Outcome Variables
3.4. Study Characteristics Relevant to Hydrogel Administration and Characterization
3.5. Relevant Characteristics for the Application of Orthodontic Force
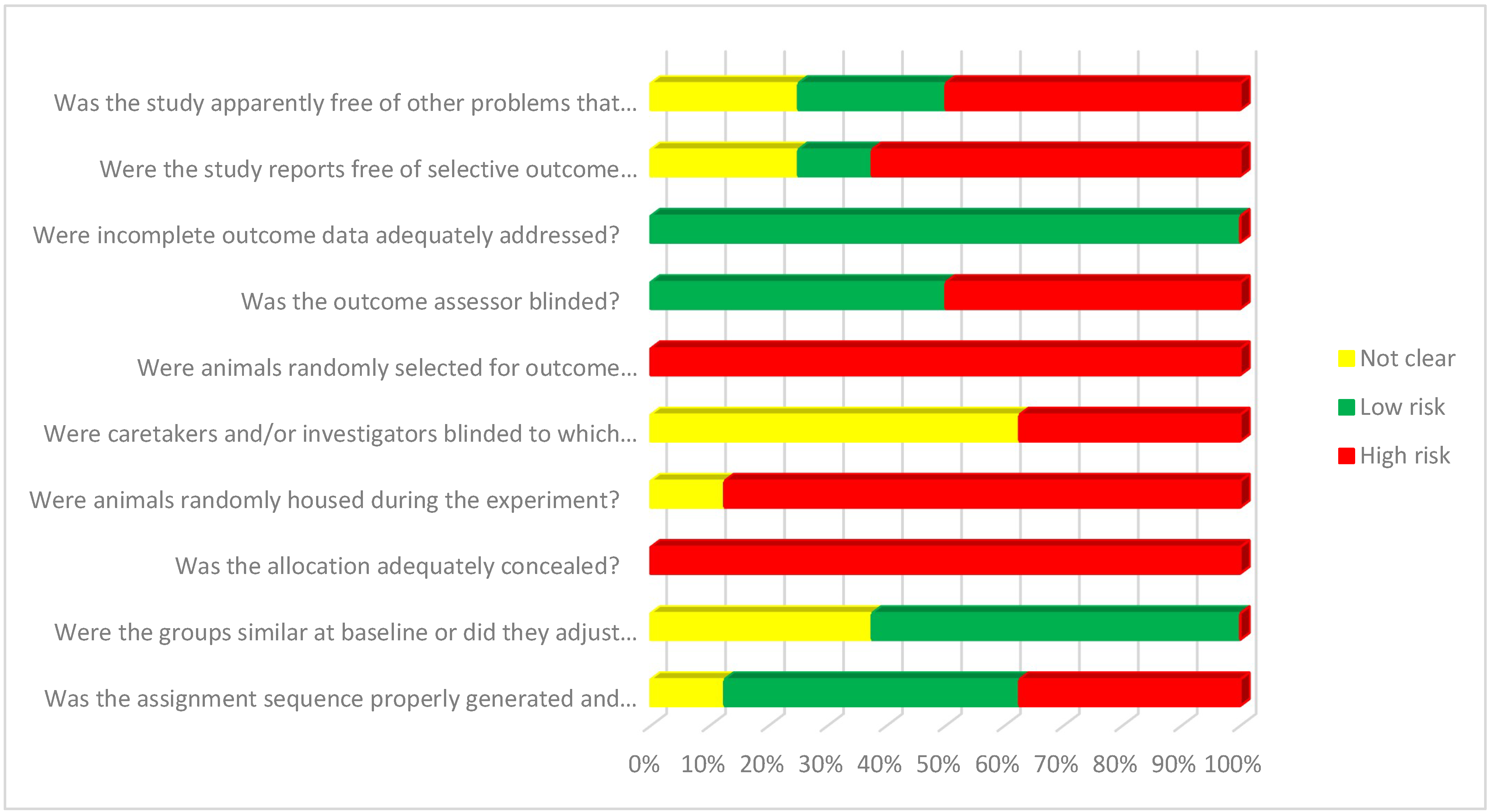
3.6. Evaluation of the Quality of the Included Studies
3.7. Risk of Bias Assessment
4. Discussion
4.1. Polymers
4.2. Biological Effects
4.2.1. Retention
4.2.2. Acceleration
4.3. Strengths and Limitations of This Review
4.4. Recommendations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patterson, B.M.; Dalci, O.; Darendeliler, M.A.; Papadopoulou, A.K. Corticotomies and Orthodontic Tooth Movement: A Systematic Review. J. Oral Maxillofac. Surg. 2016, 74, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Shibata, Y.; Imai, S.; Tani, Y.; Shibasaki, Y.; Fukuhara, T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am. J. Orthod. 1984, 85, 508–518. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Briguglio, F.; Grassia, V.; Picciolo, G.; Fiorillo, L.; Matarese, G. Effectiveness of low-level laser therapy during tooth movement: A randomized clinical trial. Materials 2019, 12, 2187. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Xiao, J.; Li, X.; Li, Y.; Zhao, Z. The effectiveness of vibrational stimulus to accelerate orthodontic tooth movement: A systematic review. BMC Oral. Health 2017, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chou, M.Y.; Park, Y.G. Effect of low-level laser on the rate of tooth movement. Semin. Orthod. 2015, 21, 210–218. [Google Scholar] [CrossRef]
- Haliloglu Ozkan, T.; Arıcı, S.; Özkan, E. Acceleration of Orthodontic Tooth Movement: An Overview. Anadolu Klin. Tıp. Bilim. Derg. 2018, 23, 121–128. [Google Scholar] [CrossRef]
- Krishnan, V. Biological Mechanisms of Tooth Movement, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kopeček, J. Swell gels. Nature 2002, 417, 389–391. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Benítez, J.L.; De Astudillo, L.R.; De Gáscue, B.R. Materiales polimeros de tipo hidrogeles: Revisión sobre su caracterización mediante FTIR, DSC, MEB y MET. Rev. Lat. Metal. Y Mater. 2016, 36, 108–130. [Google Scholar]
- Fresneda, J.; Figueroa, C. Utilización de hidrogeles como liberadores de fármacos. In Proceedings of the 18 Convención Científica de Ingeniería y Arquitectura, La Habana, Cuba, 21–25 November 2016; pp. 1–12. Available online: https://www.researchgate.net/publication/311680954 (accessed on 5 February 2021).
- Escobar, J.L.; García, D.M.; Zaldivar, D.; Katime, I. Hidrogeles. Principales Características en el diseño de sistemas de liberación controlada de fármacos. Rev. Iberoam Polímeros 2002, 3, 1–25. Available online: http://www.ehu.eus/reviberpol/pdf/Jul/escobar2.pdf (accessed on 5 February 2021).
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. pH- and ion-sensitive polymers for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Asefi, S.; Seifi, M.; Fard, G.H.; Lotfi, A. Innovative evaluation of local injective gel of curcumin on the orthodontic tooth movement in rats. Dent. Res. J. 2018, 15, 40–49. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, X.; Firth, F.; Mei, L.; Yi, J.; Gong, C.; Li, H.; Zheng, W.; Li, Y. Sclerostin injection enhances orthodontic tooth movement in rats. Arch. Oral. Biol. 2019, 99, 43–50. [Google Scholar] [CrossRef]
- Chang, J.H.; Chen, P.-J.; Arul, M.R.; Dutra, E.H.; Nanda, R.; Kumbar, S.G.; Yadav, S. Injectable RANKL sustained release formulations to accelerate orthodontic tooth movement. Eur. J. Orthod. 2020, 42, 317–325. [Google Scholar] [CrossRef]
- Soma, S.; Matsumoto, S.; Higuchi, Y.; Takano-Yamamoto, T.; Yamashita, K.; Kurisu, K.; Iwamoto, M.; Soma, S.; Matsumoto, S.; Higuchi, Y.; et al. Local and chronic application of PTH accelerates tooth movement in rats. J. Dent. Res. 2000, 79, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Alhasyimi, A.A.; Pudyani, P.P.; Asmara, W.; Ana, I.D. Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod. Craniofacial Res. 2018, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Alhasyimi, A.A.; Suparwitri, S.; Christnawati, C. Effect of Carbonate Apatite Hydrogel-Advanced Platelet-Rich Fibrin Injection on Osteoblastogenesis during Orthodontic Relapse in Rabbits. Eur. J. Dent. 2020, 15, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, Y.; Kanzaki, H.; Honda, Y.; Tanaka, T.; Yamaguchi, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; et al. Single local injection of epigallocatechin gallate-modified gelatin attenuates bone resorption and orthodontic tooth movement in mice. Polymers 2018, 10, 1384. [Google Scholar] [CrossRef]
- Utari, T.R.; Ana, I.D.; Pudyani, P.S.; Asmara, W. The intrasulcular application effect of bisphosphonate hydrogel toward osteoclast activity and relapse movement. Saudi Dent. J. 2021, 33, 292–298. [Google Scholar] [CrossRef]
- Botero, Y.L. Hidroxiapatita carbonatada, una opción como biomaterial para implantes: Una revisión del estado del arte. Rev. Colomb. Mater. 2016, 8, 79–97. [Google Scholar]
- Arredondo, A.; Londoño, M. Hidrogeles: Potenciales biomateriales para la liberación controlada de medicamentos. Rev. Ing. Bioméd. 2009, 3, 83–94. [Google Scholar]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Khan, S.A.; Schneider, M. Improvement of Nanoprecipitation Technique for Preparation of Gelatin Nanoparticles and Potential Macromolecular Drug Loading. Macromol. Biosci. 2013, 13, 455–463. [Google Scholar] [CrossRef]
- Simoni, R.C.; Lemes, G.F.; Fialho, S.; Gonçalves, O.H.; Gozzo, A.M.; Chiaradia, V.; Sayer, C.; Shirai, M.A.; Leimann, F.V. Effect of drying method on mechanical, thermal and water absorption properties of enzymatically crosslinked gelatin hydrogels. An. Da Acad. Bras. De Ciências 2017, 89, 745–755. [Google Scholar] [CrossRef]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Kan, B.; Gou, M.L.; Wang, X.H.; Li, X.Y.; Luo, F.; Zhao, X.; Wei, Y.Q.; et al. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int. J. Pharm. 2009, 365, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2021, 10, e2001341. [Google Scholar] [CrossRef]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Hellfritzsch, M.; Gutowski, T.; Scherließ, R.; Almeida, A.; Sarmento, B.; Škalko-Basnet, N.; Tho, I. Mucoadhesive buccal films based on a graft co-polymer—A mucin-retentive hydrogel scaffold. Eur. J. Pharm. Sci. 2020, 142, 105142. [Google Scholar] [CrossRef]
- Riancho, J.A.; Delgado-Calle, J. Mecanismos de interacción osteoblasto-osteoclasto. Reumatol. Clin. 2011, 7, 1–4. [Google Scholar] [CrossRef]
- Graham, J.W. Bisphosphonates and Orthodontics: Clinical Implications. J. Clin. Orthod. 2006, XL, 425–428. [Google Scholar]
- Tyrovola, J.B.; Spyropoulos, M.N. Effects of drugs and systemic factors on orthodontic treatment. Ouintessence Int. 2001, 32, 365–371. [Google Scholar]
- Montes Angeles, C.D.; Llamosas Hernández, E.; García Hernández, A.L.; Perez Martínez, I.O. Curcumina, una alternativa terapéutica para la clínica dental (Parte I): Antiinflamatorio y analgésico. Revista ADM 2016, 73, 245–249. [Google Scholar]
- Delgado-Calle, J.; Pérez-Campo, F.M.; Riancho, J.A. Avances en el estudio de los mecanismos involucrados en la modulación de la expresión de esclerostina en células humanas. Rev. Osteoporos. Y Metab. Miner. Scieloes 2014, 6, 103–108. [Google Scholar] [CrossRef][Green Version]
- Farsai, P.S. Biology of Orthodontic Tooth Movement; Shroff, B., Ed.; Springer International Publishing: Cham, Switzerland, 2016. Available online: http://link.springer.com/10.1007/978-3-319-26609-1 (accessed on 5 February 2021).

| Study | Study Design | Subjects of Study | Average Age | Study Groups | Duration of the Study | Primary Evaluation Methods |
|---|---|---|---|---|---|---|
| Asefi 2020 (Iran) [20] | Experimental (Split mouth) | 40 male Wistar rats | 12 weeks | Group A: Negative control. Group B: Positive control, received 0.03 cc of saline solution and apparatus. Group C: Gelatin + Curcumin, received 0.03 cc of hydrogel + apparatus. Group D: Chitosan + Curcumin, received 0.03 cc of hydrogel + apparatus. | 21 days | Leaf gauge with 0.05 mm accuracy. |
| Lu 2019 (China) [21] | Experimental (Split mouth) | 48 male Wistar rats | 6 weeks | Group A: Sclerosin injection at 0.8 μg/kg. Group B: Sclerosin injection at 4 μg/kg. Group C: Sclerosin injection at 20 μg/kg. | 14 days | Micro computed tomography analysis. |
| Chang 2019 (USA) [22] | Experimental | 24 male Wistar rats | 15 weeks | Group A: Orthodontic spring without microparticle formula. Group B: Orthodontic spring with placebo microparticles. Group C: Orthodontic spring with microparticles with RANKL | 14 days | Micro computed tomography analysis. |
| Soma 2000 (Japan) [23] | Experimental | 56 male Wistar rats | 20 weeks | Group A: Control, treated with orthodontic force only. Group B: Orthodontic strength and local injection of vehicle dissolved in MC gel. Group C: Orthodontic force and local injection of 0.1 μg PTH dissolved in MC gel. Group D: Orthodontic force and local injection of 1 μg PTH dissolved in MC gel. Group E: Orthodontic force and local injection of 1 μg PTH dissolved in 0.9% saline. Group F: Orthodontic force and systemic injection of 1 μg PTH dissolved in MC gel. Group G: Local injection of 1 μg PTH dissolved in MC gel. | 12 days | Interproximal measuring tool. |
| Alhasyimi 2018 (Indonesia) [24] | Experimental | 45 male rabbits | 10–12 weeks | Group A: Control Group B: CHA Group C: CHA-aPRF | 21 days | Digital calibrator. TRAP staining |
| Utari 2020 (Indonesia) [27] | Experimental | 75 male guinea pigs | Group A: Control Group B: Bis-CR250 (250 mmol/L) Group C: Bis-CR500 (500 mmol/L) | 21 days | Histology and interproximal measuring tool. | |
| Alhasyimi 2020 (Indonesia) [25] | Experimental | 45 male rabbits | 10–12 weeks | Group A: Control Group B: CHA Group C: CHA-aPRF | 42 days | Histology |
| Katsumata 2018 (Japan) [26] | Experimental | 13 male BALB/C mice | 7 weeks | Group A: Control. Group B: Injected with a solution of 0.07 mg EGCG/10 mL Group C: Injected with a solution of 0.7 mg EGCG/10 mL | 21 days | Micro computed tomography analysis. TRAP staining |
| Study | Orthodontic Tooth Movement (OTM) Duration | Tooth Displacement Measurement | Influence on OTM Retention/Acceleration | Magnitude of OTM in the Control Groups | OTM Magnitude in the Experimental Group |
|---|---|---|---|---|---|
| Asefi 2020 (Iran) [20] | 21 days | The distance between maxillary first and second molars was measured three times. | Inhibition | Control group: 0.34 mm | Group G and CH: 0.26 mm |
| Lu 2019 (China) [21] | 14 days | It was measured between distal of the maxillary first molar to distal of the maxillary second molar. | Acceleration | Not reported | Group 4 μg/kg: 0.65 ± 0.06 mm Group 20 μg/kg: 0.72 ± 0.04 mm |
| Chang 2019 (USA) [22] | 14 days | Intermolar distance. | Acceleration | Control group: 0.24 mm ± 0.05 mm | Placebo formulation group: 0.32 ± 0.1 RANKL formulation group: 0.55 ± 0.25 |
| Soma 2000 (Japan) [23] | 12 days | Distance between first and second molar. | Acceleration | Control group: 0.54 + 0.08 mm | 1.6 times the control |
| Alhasyimi 2018 (Indonesia) [24] | 21 days | Distance between the mesial faces of the lower incisors. | Retention | Control group: 2.45 ± 0.09 mm | Group with CHA-Aprf: 0.91 ± 0.12 mm |
| Utari 2020 (Indonesia) [27] | 21 days | Distance between lower incisors. | Retention | Control group: 21 days: 2 mm | 21 days: 0.7 mm |
| Alhasyimi 2020 (Indonesia) [25] | 21 days | Distance between the mesial faces of the lower incisors. | Retention | Not measured | Not measured |
| Katsumata 2018 (Japan) [26] | 21 days | Distance of maxillary right and left first molars. | Retention | Not measured | Not measured |
| Author | Type of Therapy | Hydrogel | Active Agent | Dosage | Method of Administration | Frequency of Administration | Duration of Administration | OTM Result |
|---|---|---|---|---|---|---|---|---|
| Asefi 2020 (Iran) [20] | Retention | Chitosan and gelatin | Curcumin | 0.03 cc of curcumin | Local injection | Single application | 1 day | Significant decrease in bone and/or root resorption |
| Lu 2019 (China) [21] | Acceleration | Polyethylene glycol-polycaprolactone-poly-ethylene glycol (PECE) | Sclerosin | 0.1 mL | Local injection | Single application | 14 days | Improves tooth movement and osteoclastogenesis. |
| Chang 2019 (USA) [22] | Acceleration | Hydroxyethylcellulose | Formulation of RANKL | 1 μg RANKL: 1 mg microsphere in 3 μL 10% HEC gel | Local injection | Single application | Not reported | Accelerate OTM. |
| Soma 2000 (Japan) [23] | Acceleration | Methylcellulose | Parathyroid hormone | 0.1 and 1.0 µg/µL | Local injection | Every other day | 12 days | Accelerate OTM. |
| Alhasyimi 2018 (Indonesia) [24] | Retention | Gelatin | Carbonated Hydroxyapatite | 0.2 mL | Local injection | Every 7 days | Not reported | Reduces orthodontic relapse. |
| Utari 2020 (Indonesia) [27] | Retention | Gelatin | Risedronate Bisphosphonate | 1.00 and 1.92 mg in 5 mL PBS | Topic | Every 3 days | 14 days | Effectively reduces relapse |
| Alhasyimi 2020 (Indonesia) [25] | Retention | Gelatin | Carbonated Hydroxyapatite | 0.2 mL of CHA | Intrasulcular injection | 3 times | Day 0, 7 and 14 | Helps osteoblastogenesis |
| Katsumata 2018 (Japan) [26] | Retention | Gelatin | Epigallocatechin | 5 μL | Local injection | Single application | Not reported | Inhibits Osteoclastogenesis |
| Asefi 2020 [20] | Lu 2019 [21] | Chang 2019 [22] | Soma 2000 [23] | Alhasyimi 2018 [24] | Utari 2020 [27] | Alhasyimi 2020 [25] | Katsumata 2018 [26] | Total | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Title | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 2. Summary | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 50% |
| INTRODUCTION | |||||||||
| 3. Background | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 62.5% |
| 4. Objectives | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| METHODS | |||||||||
| 5. Statement of Ethics | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 87.5% |
| 6. Procedures | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 7. Experimental animals | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 8. Housing and agriculture | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 50% |
| 9. Sample Size | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 10. Assignment of animals to experimental groups | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 11. Experimental results | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 12. Statistical methods | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| RESULTS | |||||||||
| 13. Reference data | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 14. Numbers analyzed | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 15. Results and estimation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 16. Adverse events | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 37.5% |
| DISCUSSION | |||||||||
| 17. Interpretation/scientific implications | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 18. Generalization/Translation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% |
| 19. Financing | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero Jiménez, O.G.; Dib Kanán, A.; Dipp Velázquez, F.A.; Aristizábal Pérez, J.F.; Moyaho Bernal, M.d.l.Á.; Salas Orozco, M.F.; Casillas Santana, M.A. Use of Hydrogels to Regulate Orthodontic Tooth Movement in Animal Models: A Systematic Review. Appl. Sci. 2022, 12, 6683. https://doi.org/10.3390/app12136683
Montero Jiménez OG, Dib Kanán A, Dipp Velázquez FA, Aristizábal Pérez JF, Moyaho Bernal MdlÁ, Salas Orozco MF, Casillas Santana MA. Use of Hydrogels to Regulate Orthodontic Tooth Movement in Animal Models: A Systematic Review. Applied Sciences. 2022; 12(13):6683. https://doi.org/10.3390/app12136683
Chicago/Turabian StyleMontero Jiménez, Olin Guadalupe, Alejandro Dib Kanán, Farid Alfonso Dipp Velázquez, Juan Fernando Aristizábal Pérez, María de los Ángeles Moyaho Bernal, Marco Felipe Salas Orozco, and Miguel Angel Casillas Santana. 2022. "Use of Hydrogels to Regulate Orthodontic Tooth Movement in Animal Models: A Systematic Review" Applied Sciences 12, no. 13: 6683. https://doi.org/10.3390/app12136683
APA StyleMontero Jiménez, O. G., Dib Kanán, A., Dipp Velázquez, F. A., Aristizábal Pérez, J. F., Moyaho Bernal, M. d. l. Á., Salas Orozco, M. F., & Casillas Santana, M. A. (2022). Use of Hydrogels to Regulate Orthodontic Tooth Movement in Animal Models: A Systematic Review. Applied Sciences, 12(13), 6683. https://doi.org/10.3390/app12136683







