Effect of Methane Adsorption on Mechanical Performance of Coal
Abstract
:1. Introduction
2. Materials and Methods
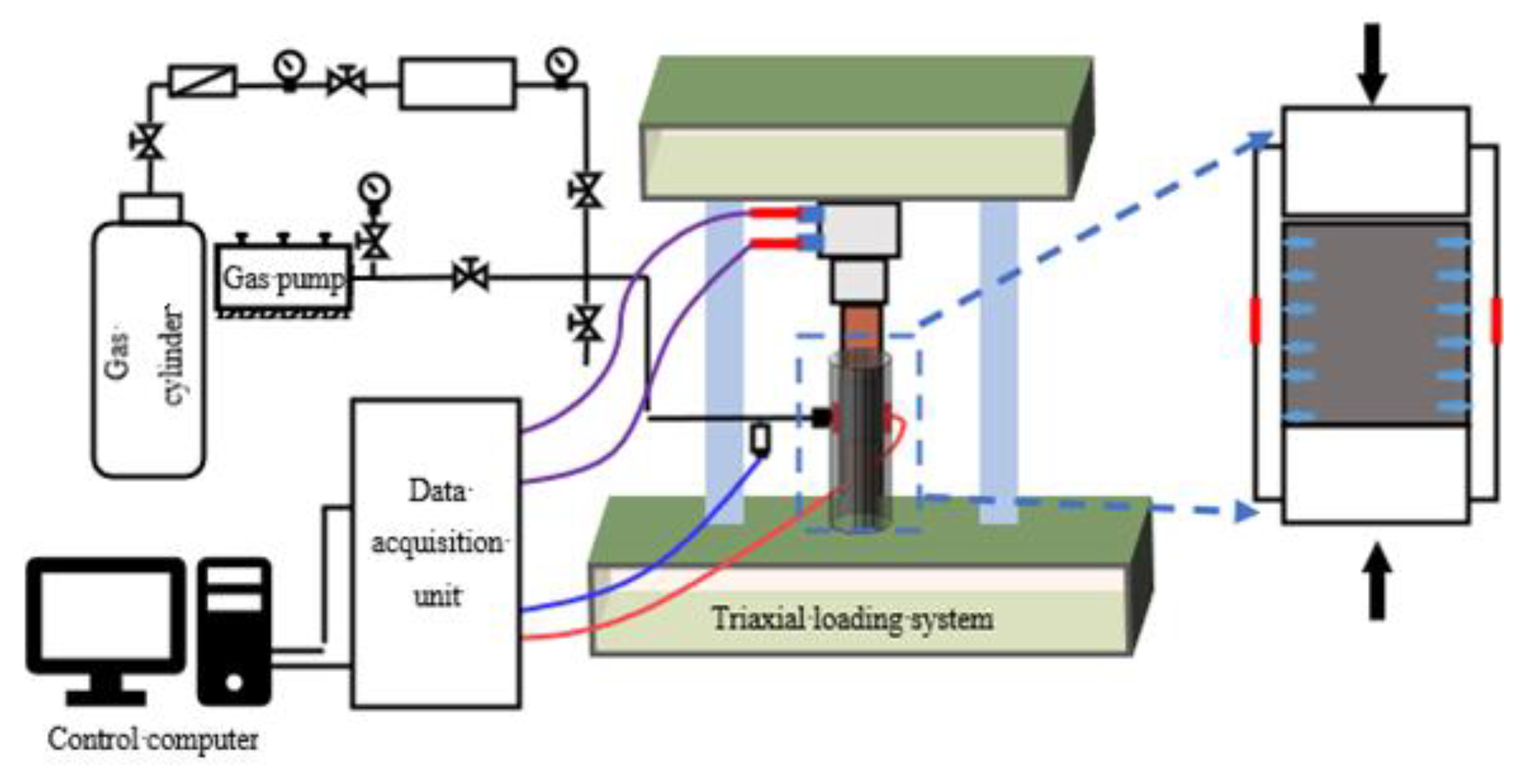
2.1. Instruments and Sample
- (1)
- The adsorption subsystem is comprised of a flow meter, positive pressure air pump, vacuum pump, pressure sensor, high-pressure gas steel cylinder, several pipelines, pipe valves, and pressure reducing valves, among others. This subsystem was used to inject gas into both ends of the installed coal sample at a constant temperature (the room temperature was controlled by air conditioning) and constant pressure (the gas pressure was controlled using metering pump controls) until adsorption equilibrium.
- (2)
- The mechanical testing subsystem includes a triaxial pressure chamber, pressure frame, deformation sensor, loading controller, and porous backing plate, among others. This subsystem was used to perform triaxial tests on the coal sample during gas adsorption equilibrium.
- (3)
- The data acquisition subsystem consists of an acquisition card and computer. This subsystem was used to collect sensor data.
2.2. Experimental Scheme
3. Results
4. Discussion
4.1. Quantification of Coal Mechanical Parameters
4.2. Effects of Methane Adsorption on Tensile Strength of Coal
4.3. Effects of Metamorphic Degree of Coal on Compressive Strength
5. Conclusions
- (1)
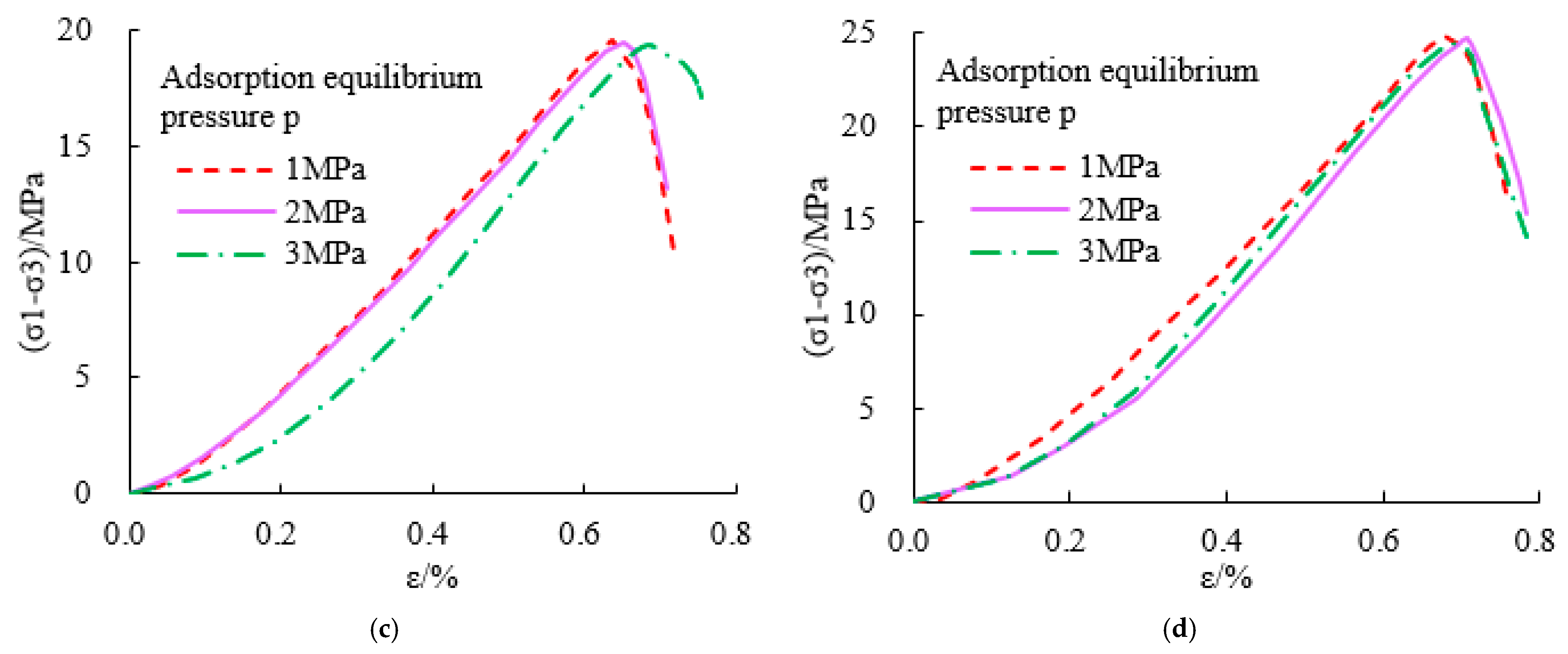
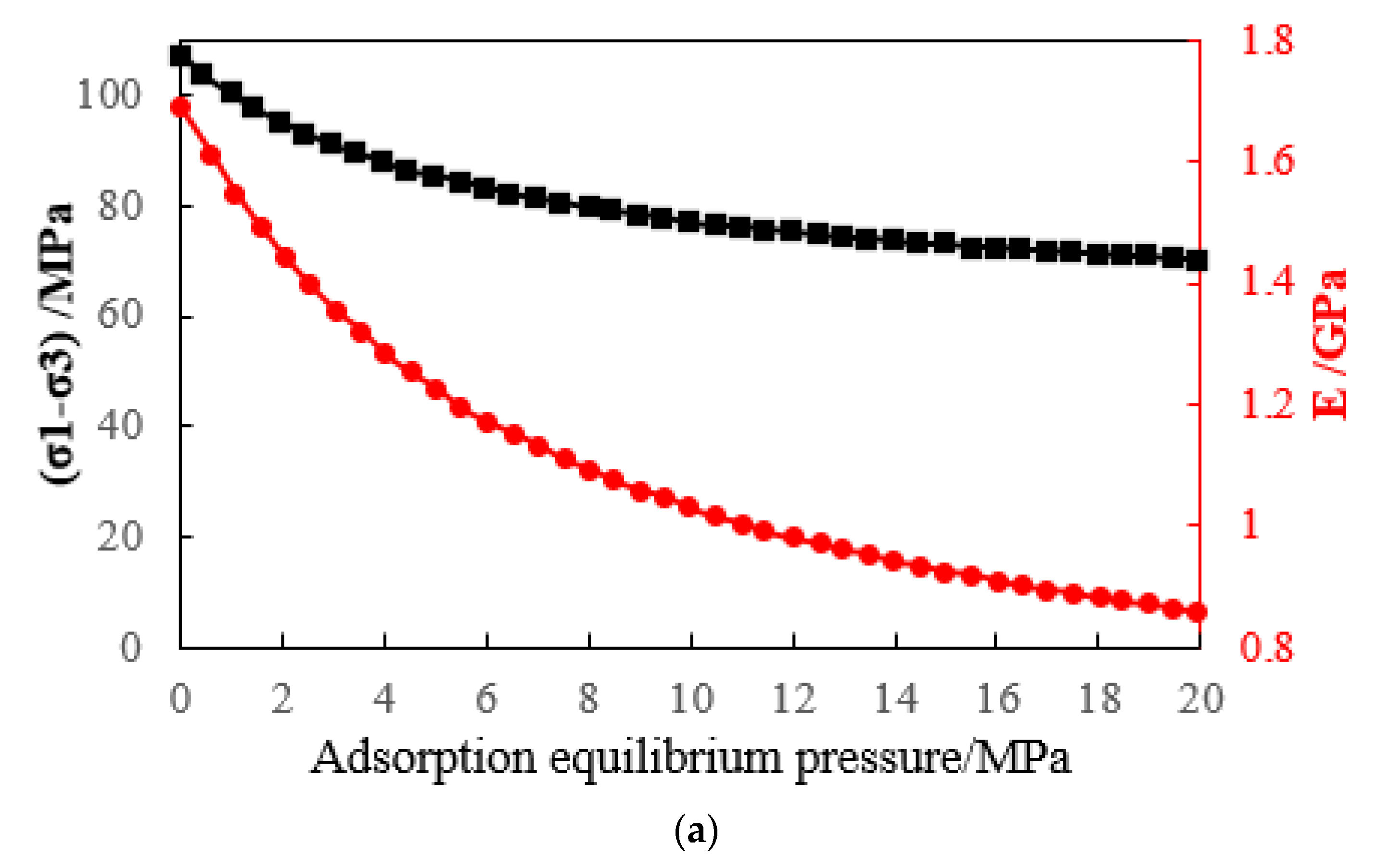
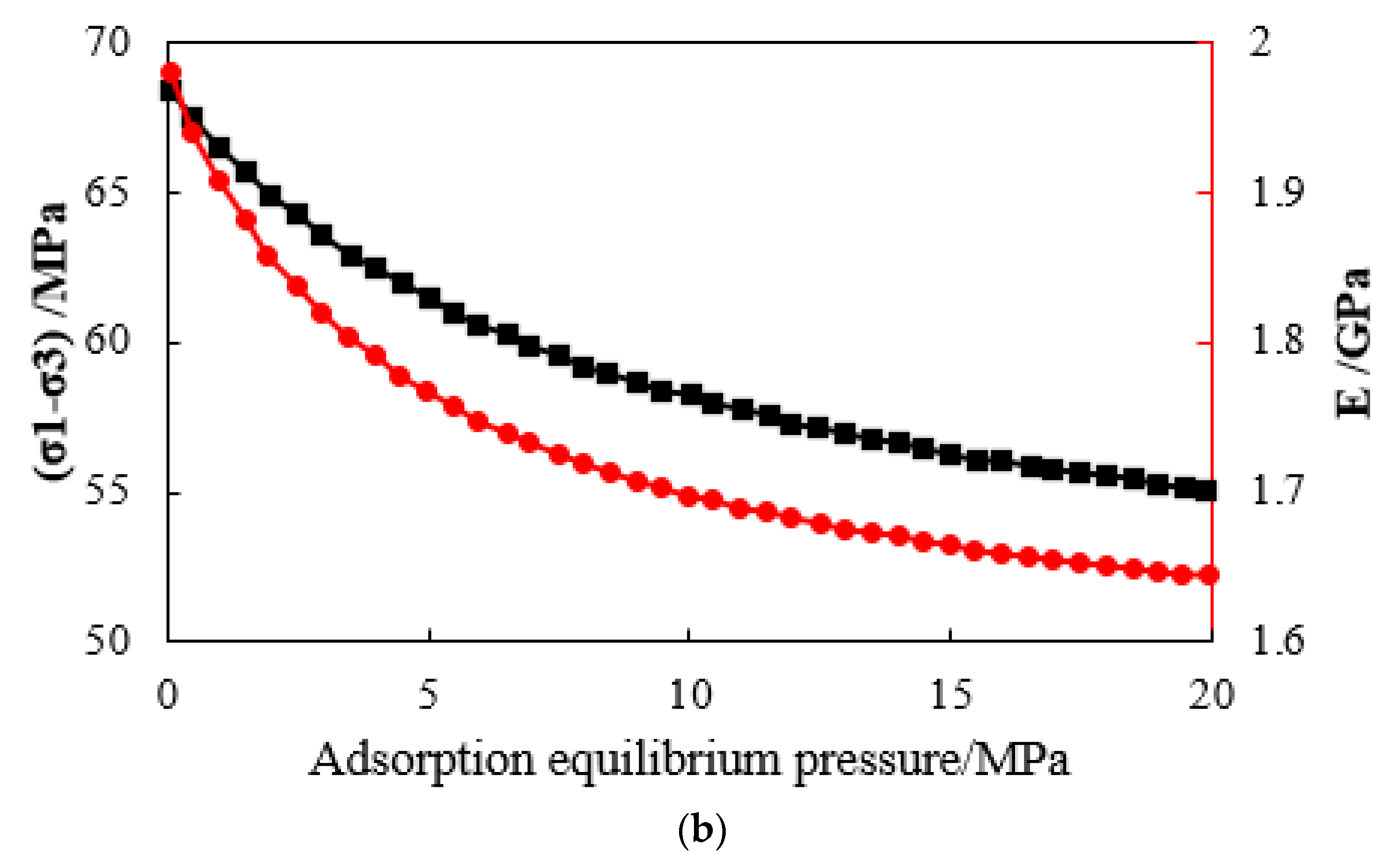
- The triaxial stress-strain curve of a adsorbed methane coal body is similar to that of a non-adsorbed coal body, that is, before reaching the peak strength, it has gone through four typical stages: microfracture compression and closure, elastic deformation, stable fracture expansion, and rapid fracture expansion stage. However, the compressive strength of coal decreases after methane adsorption. The greater the adsorption equilibrium pressure of methane, the smaller the compressive strength of coal.
- (2)
- When the adsorption equilibrium pressure is constant, the compressive strength, elastic modulus, and maximum strain of coal samples increase with the increase in the confining pressure; when the confining pressure is constant, the compressive strength, elastic modulus, and maximum strain of coal samples decrease with the increase in the confining pressure. The adsorbed methane reduces the surface energy of coal and then reduces the overall strength of coal. The adsorption of methane leads to the plastic effect of coal and the decrease in the elastic modulus of coal.
- (3)
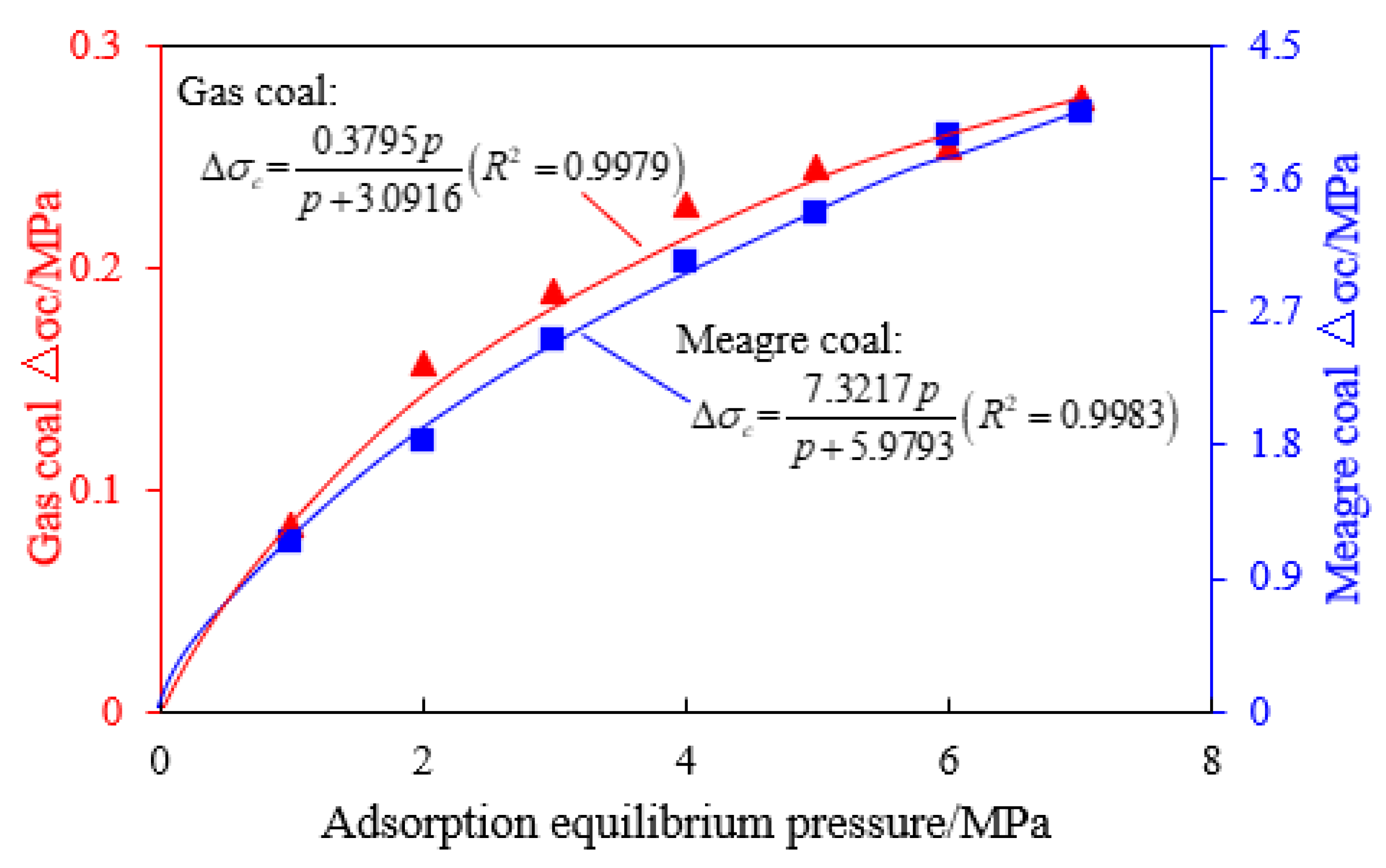
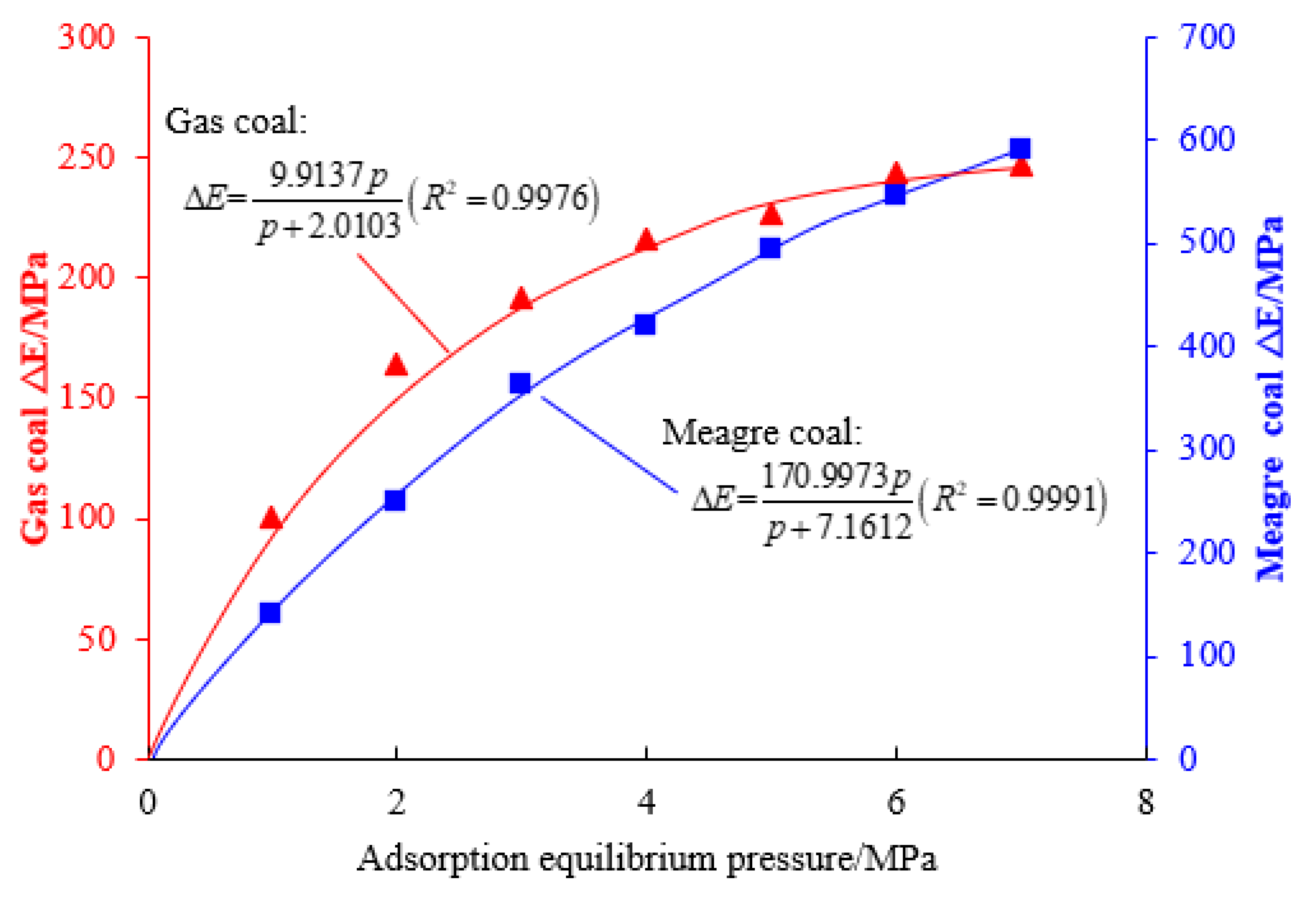
- The change in the mechanical properties (compressive strength and elastic modulus) of coal caused by methane adsorption can be described by the Langmuir curve and the correlation coefficient is more than 0.99. Under any stress environment, high-rank coal shows greater strength and lower elastic modulus than low-rank coal, which is mainly due to the existence of a developed cleat system in high-rank coal that provides more conditions for methane adsorption.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikoosokhan, S.; Vandamme, M.; Dangla, P. A poromechanical model for coal seams saturated with binary mixtures of CH4 and CO2. J. Mech. Phys. Solids 2014, 71, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; He, Y.; Zhao, Y.; Jiang, X.; Ren, S. Statistical analysis of acoustic emission in uniaxial compression of tectonic and non-tectonic coal. Appl. Sci. 2020, 10, 3555. [Google Scholar] [CrossRef]
- Masoudian, S.; Airey, D.W.; El-Zein, A. Experimental investigations on the effect of CO2 on mechanics of coal. Int. J. Coal Geol. 2014, 128, 12–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Cao, Z.; Gao, M.; Zhang, Y.; Xie, J. Mechanical behavior and permeability evolution of coal under different mining-induced stress conditions and gas pressure. Energies 2020, 13, 2677. [Google Scholar] [CrossRef]
- Karacan, C.O. Heterogeneous sorption and swelling in a confined and stressed coal during CO2 injection. Energy Fuels 2003, 17, 1595–1608. [Google Scholar] [CrossRef]
- Potyondy, O.D. The bonded-particle model as a tool for rock mechanics research and application: Current trends and future directions. Geosyst. Eng. 2015, 18, 1–28. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, X.; Wang, W.; Wei, W.; Duan, M. Three-dimensional visualization of the evolution of pores and fractures in reservoir rocks under triaxial stress. Powder Technol. 2021, 378, 585–592. [Google Scholar] [CrossRef]
- Isaka, B.A.; Ranjith, P.G.; Rathnaweera, T.D.; Perera, M.S.A.; Kumari, W.G.P. Influence of long-term operation of supercritical carbon dioxide based enhanced geothermal system on mineralogical and microstructurally-induced mechanical alteration of surrounding rock mass. Renew. Energy 2019, 136, 428–441. [Google Scholar] [CrossRef]
- Xu, T.; Feng, G.; Shi, Y. On fluid-rock chemical interaction in CO2-based geothermal systems. J. Geochem. Explor. 2014, 144, 179–193. [Google Scholar] [CrossRef]
- Na, J.; Xu, T.; Yuan, Y.; Feng, B.; Tian, H.; Bao, X. An integrated study of fluid-rock interaction in a CO2-based enhanced geothermal system: A case study of Songliao Basin, China. Appl. Geochem. 2015, 59, 166–177. [Google Scholar] [CrossRef]
- Stephansson, O.; Tsang, C.F.; Kautsky, F. Foreword. Special issue for thermos-hydro-mechanical coupling in rock mechanics. Int. J. Rock Mech. Min. Sci. 2001, 38, 1–4. [Google Scholar] [CrossRef]
- Cho, S.H.; Ogata, Y.J.; Kaneko, K. Strain-rate dependency of the dynamic tensile strength of rock. INT J. Rock Mech. Min. 2003, 40, 763–777. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranathunga, A.S.; Ranjith, P.G. Effect of coal rank on various fluid saturations creating mechanical property alterations using australian coals. Energies 2016, 9, 440. [Google Scholar] [CrossRef]
- Yongjia, W.; Mengtao, Z. The study of endochronic constitutive equations of coal effected by gas and the determining of parameters by experiment. Acta Mech. Solida Sin. 1996, 17, 229–234. [Google Scholar]
- Li, X.; Yin, G.; Zhao, H.; Wang, W.Z.; Jing, X.F. Experimental study of mechanical properties of outburst coal containing gas under triaxial compression. Chin. J. Rock Mech. Eng. 2010, 29, 3350–3358. [Google Scholar]
- Kang, X.-T.; Huang, G.; Song, Z.-I.; Deng, B.-Z.; Luo, J.-Y.; Zhang, X. Research on characteristics of energy dissipation and seepage of coal containing gas under triaxial compression. Rock Soil Mech. 2015, 36, 762–768. [Google Scholar]
- Qiu, Z.Y.; Pan, Y.S.; Luo, H. Study on influence of effective confining pressure on acoustic emission signal in coal fracture. J. Saf. Sci. Technol. 2015, 11, 47–53. [Google Scholar]
- Harpalani, S. Gas Flow through Stressed Coal. Ph.D. Thesis, University of California Berkeley, Berkeley, CA, USA, 1985. [Google Scholar]
- Gawuga, J. Flow of Gas through Stressed Carboniferous Strata. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 1979. [Google Scholar]
- Xu, J.; Zhang, D.D.; Peng, S.J.; Liu, D.; Wang, L. Experimental research on influence of temperature on mechanical properties of coal containing methane. Chin. J. Rock Mech. Eng. 2011, 30, 2730–2735. [Google Scholar]
- Jiang, X.U.; Bobo, L.; Ting, Z.H.O.U. Experimental study of deformation and energy evolution law of coal under cyclic loading. Chin. J. Rock Mech. Eng. 2014, 33, 3563–3572. [Google Scholar]
- Yin, G.Z.; Wang, D.K.; Zhang, D.M.; Wang, W.Z. Test analysis of deformation characteristics and compressive strengths of two types of coal specimens containing gas. Chin. J. Rock Mech. Eng. 2009, 28, 410–417. [Google Scholar]
- Lei, Z.; Zhang, Y.; Zhang, S.; Fu, L.; Hu, Z.; Yu, Z.; Li, L.; Zhou, J. Electricity generation from a three-horizontal-well enhanced geothermal system in the Qiabuqia geothermal field, China: Slickwater fracturing treatments for different reservoir scenarios. Renew. Energy 2020, 145, 65–83. [Google Scholar] [CrossRef]
- Xia, M.; Zhou, K. Particle simulation of the failure process of brittle rock under triaxial compression. Int. J. Miner. Metall. Mater. 2010, 17, 507–513. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, Q.; Li, X.; Bai, B.; Hu, H. Effects of water and ScCO2 injection on the mechanical properties of granite at high temperatures. Adv. Civ. Eng. 2020, 2020, 8838989. [Google Scholar] [CrossRef]
- Saleem, M.; Blaisi, N.I.; Alshamrani, O.S.D.; Al-Barjis, A. Fundamental investigation of solid waste generation and disposal behaviour in higher education institute in the Kingdom of Saudi Arabia. Indoor Built Environ. 2018, 28, 1420326X1880485. [Google Scholar] [CrossRef]
- Jiang, L.; Cheng, Y.; Han, Z.; Li, Q.; Gao, Q.; Yan, C. Effect of frost heave on internal structure and mechanical behavior of rock mass at low temperature. J. Appl. Sci. Eng. 2018, 21, 527–539. [Google Scholar]
- Kumari, W.G.P.; Ranjith, P.G.; Perera, M.S.A.; Shao, S.; Chen, B.K.; Lashin, A.; Al Arifi, N.; Rathnaweera, T.D. Mechanical behaviour of Australian Strathbogie granite under in-situ stress and temperature conditions: An application to geothermal energy extraction. Geothermics 2017, 65, 44–59. [Google Scholar] [CrossRef]


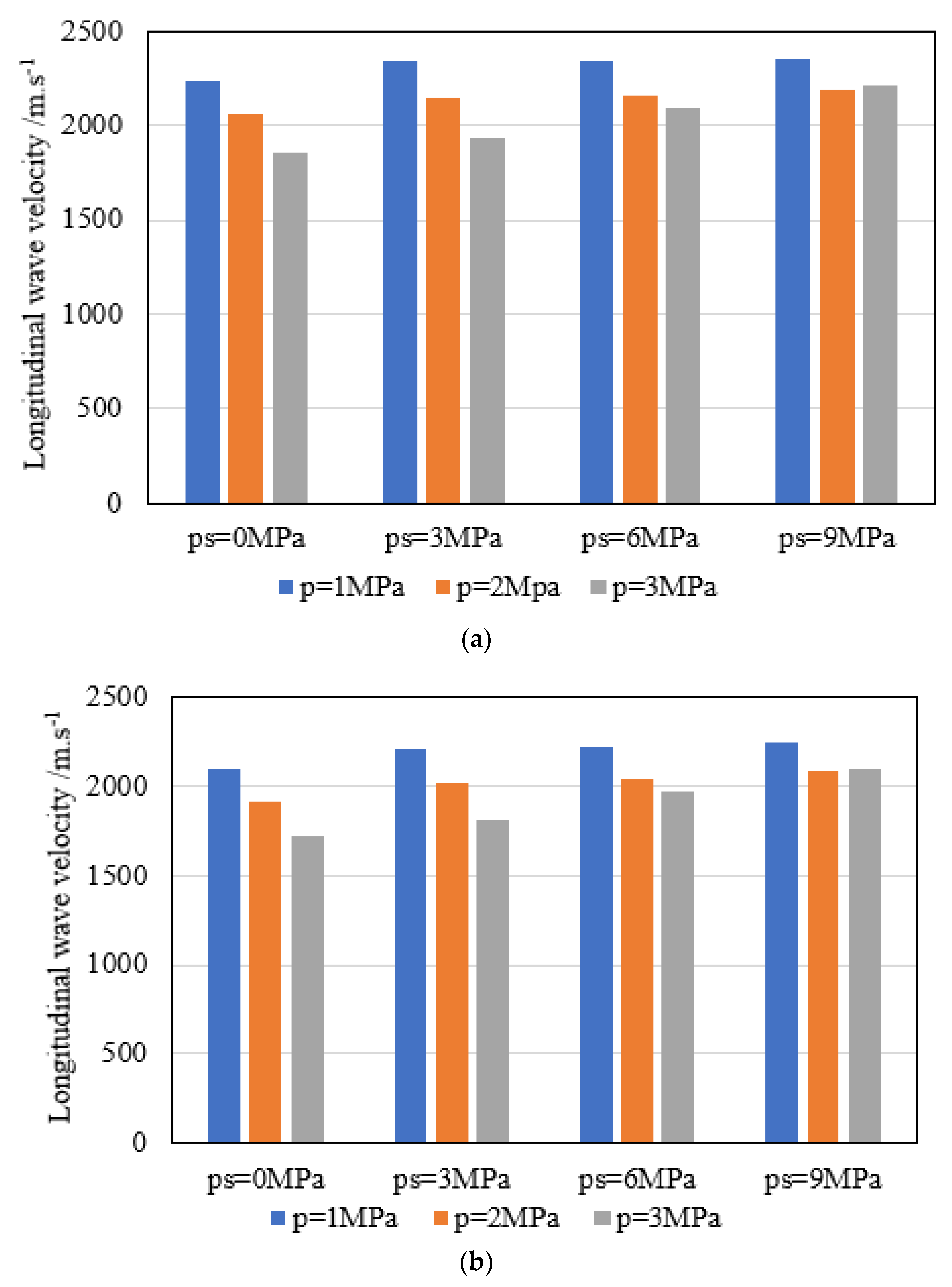
| Coal Quality | Adsorption Equilibrium Pressure p/MPa | Confining Pressure ps/MPa | Compressive Strength σc/MPa | Modulus of Elasticity E/GPa | Longitudinal Wave Velocity/m.s−1 |
|---|---|---|---|---|---|
| Meager coal | 1 | 0 | 9.17 | 1.98 | 2238.943 |
| 3 | 14.08 | 2.59 | 2338.796 | ||
| 6 | 22.38 | 3.87 | 2342.136 | ||
| 9 | 26.21 | 4.86 | 2350.827 | ||
| 2 | 0 | 8.89 | 1.69 | 2056.944 | |
| 3 | 13.87 | 2.41 | 2151.145 | ||
| 6 | 22.19 | 3.73 | 2154.296 | ||
| 9 | 26.13 | 4.79 | 2190.796 | ||
| 3 | 0 | 8.68 | 1.43 | 1858.014 | |
| 3 | 13.61 | 2.29 | 1933.194 | ||
| 6 | 22.01 | 3.59 | 2090.706 | ||
| 9 | 25.97 | 4.67 | 2210.848 | ||
| Gas coal | 1 | 0 | 8.36 | 1.69 | 2100.566 |
| 3 | 12.65 | 2.18 | 2210.477 | ||
| 6 | 19.62 | 3.78 | 2223.801 | ||
| 9 | 24.85 | 4.39 | 2243.496 | ||
| 2 | 0 | 8.03 | 1.33 | 1919.577 | |
| 3 | 12.41 | 1.97 | 2021.629 | ||
| 6 | 19.50 | 3.58 | 2043.039 | ||
| 9 | 24.71 | 4.26 | 2089.539 | ||
| 3 | 0 | 7.69 | 1.19 | 1721.695 | |
| 3 | 12.17 | 1.71 | 1806.884 | ||
| 6 | 19.38 | 3.35 | 1972.387 | ||
| 9 | 24.56 | 4.13 | 2102.529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, F.; Yin, J.; Feng, J. Effect of Methane Adsorption on Mechanical Performance of Coal. Appl. Sci. 2022, 12, 6597. https://doi.org/10.3390/app12136597
Cai F, Yin J, Feng J. Effect of Methane Adsorption on Mechanical Performance of Coal. Applied Sciences. 2022; 12(13):6597. https://doi.org/10.3390/app12136597
Chicago/Turabian StyleCai, Feng, Jingwen Yin, and Juqiang Feng. 2022. "Effect of Methane Adsorption on Mechanical Performance of Coal" Applied Sciences 12, no. 13: 6597. https://doi.org/10.3390/app12136597
APA StyleCai, F., Yin, J., & Feng, J. (2022). Effect of Methane Adsorption on Mechanical Performance of Coal. Applied Sciences, 12(13), 6597. https://doi.org/10.3390/app12136597






