Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms and Growth Conditions
2.3. Batch Cultivation in Synthetic Media
2.4. Batch Cultivations on AD Effluent
2.5. Analytical Methods
2.5.1. Estimation of Substrate Consumption during Cultivation
2.5.2. Cell Dry Weight and Lipids Extraction
2.5.3. GC-MS Analysis of Intracellular Lipids
2.5.4. Fluorescence Microscopy
2.6. Statistical Analysis
3. Results and Discussion
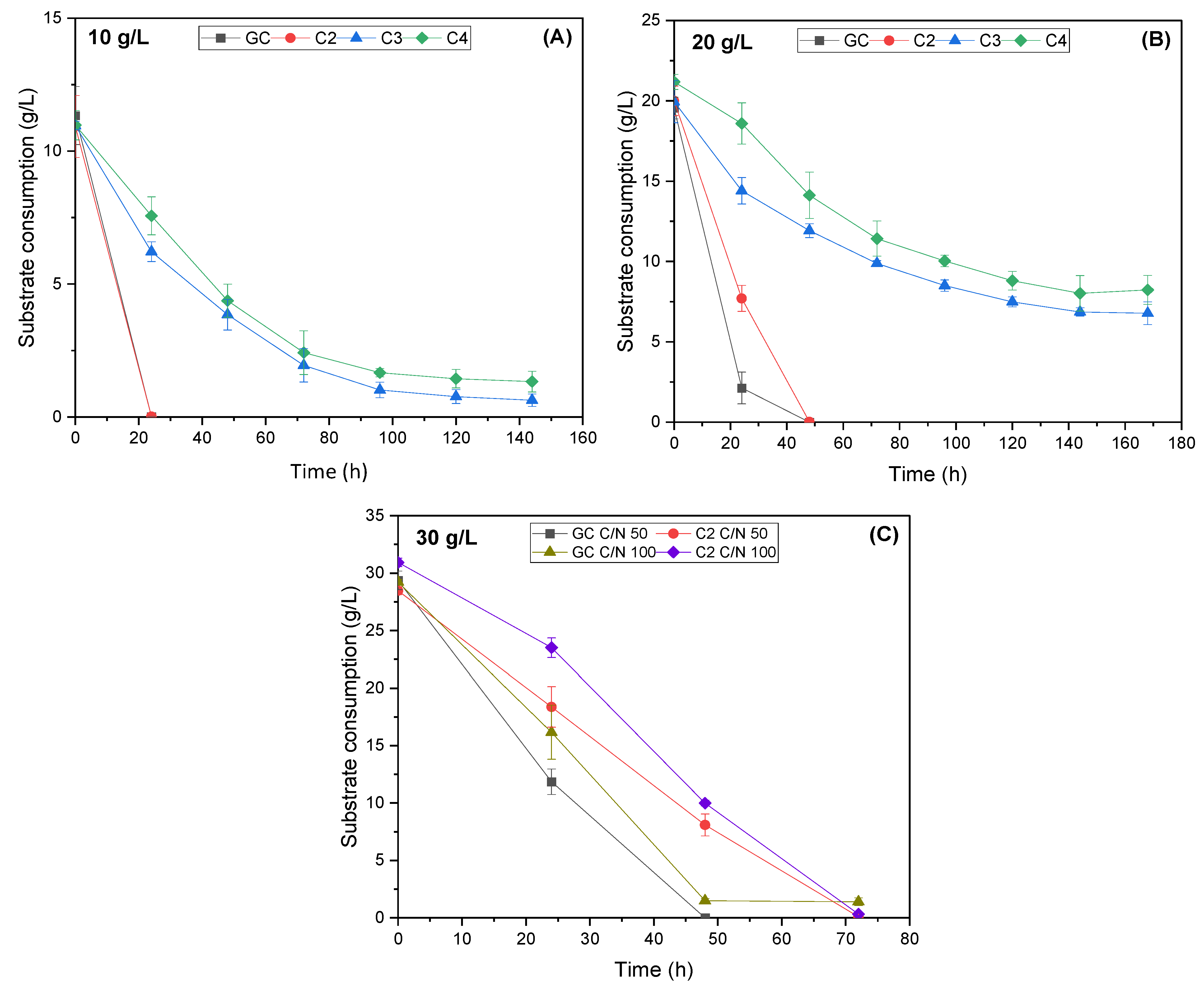
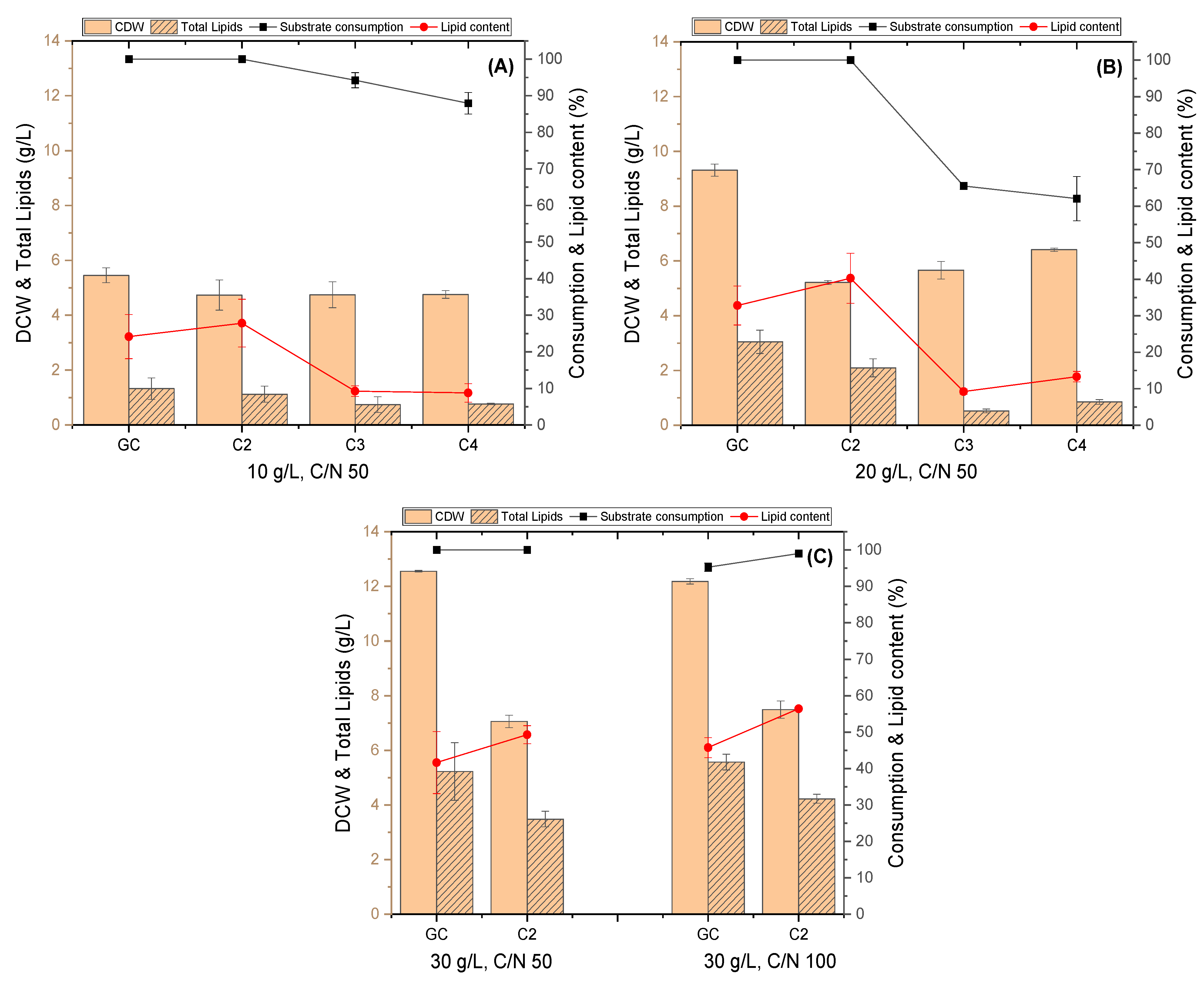
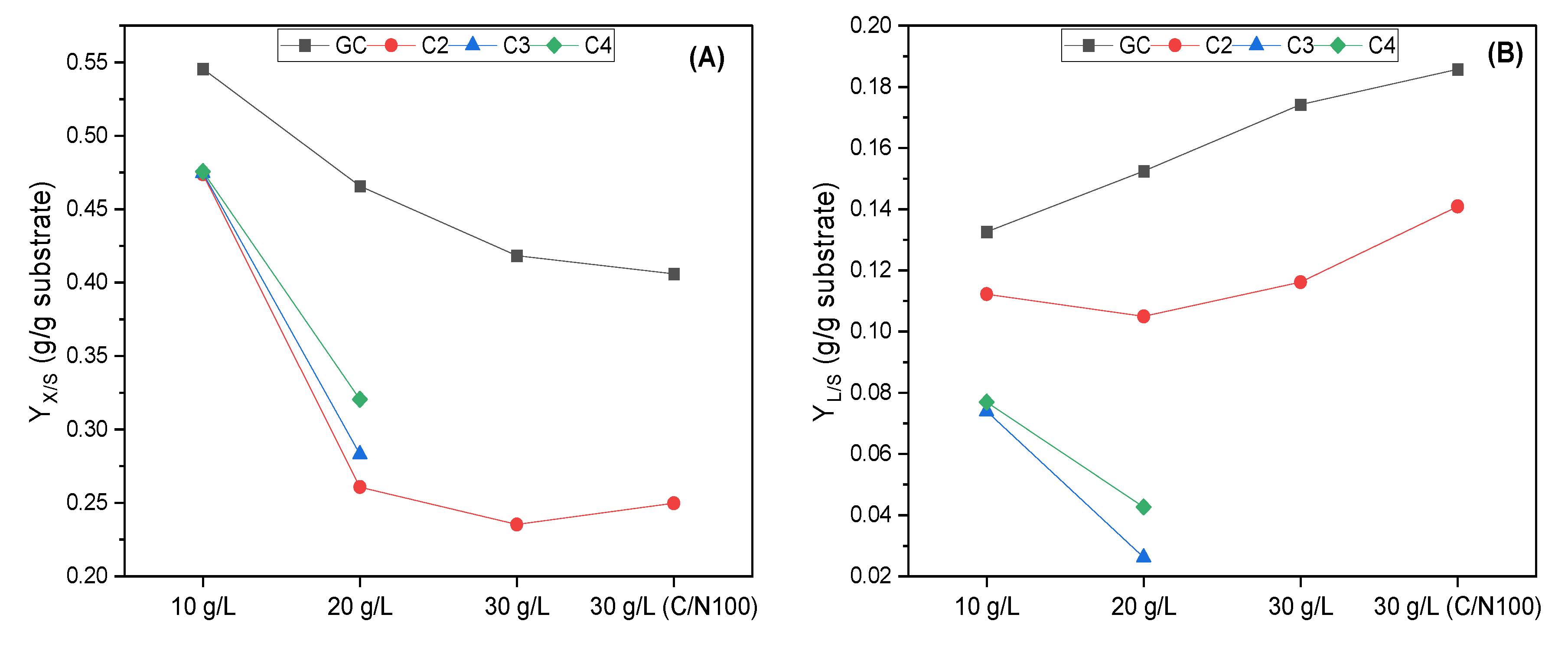
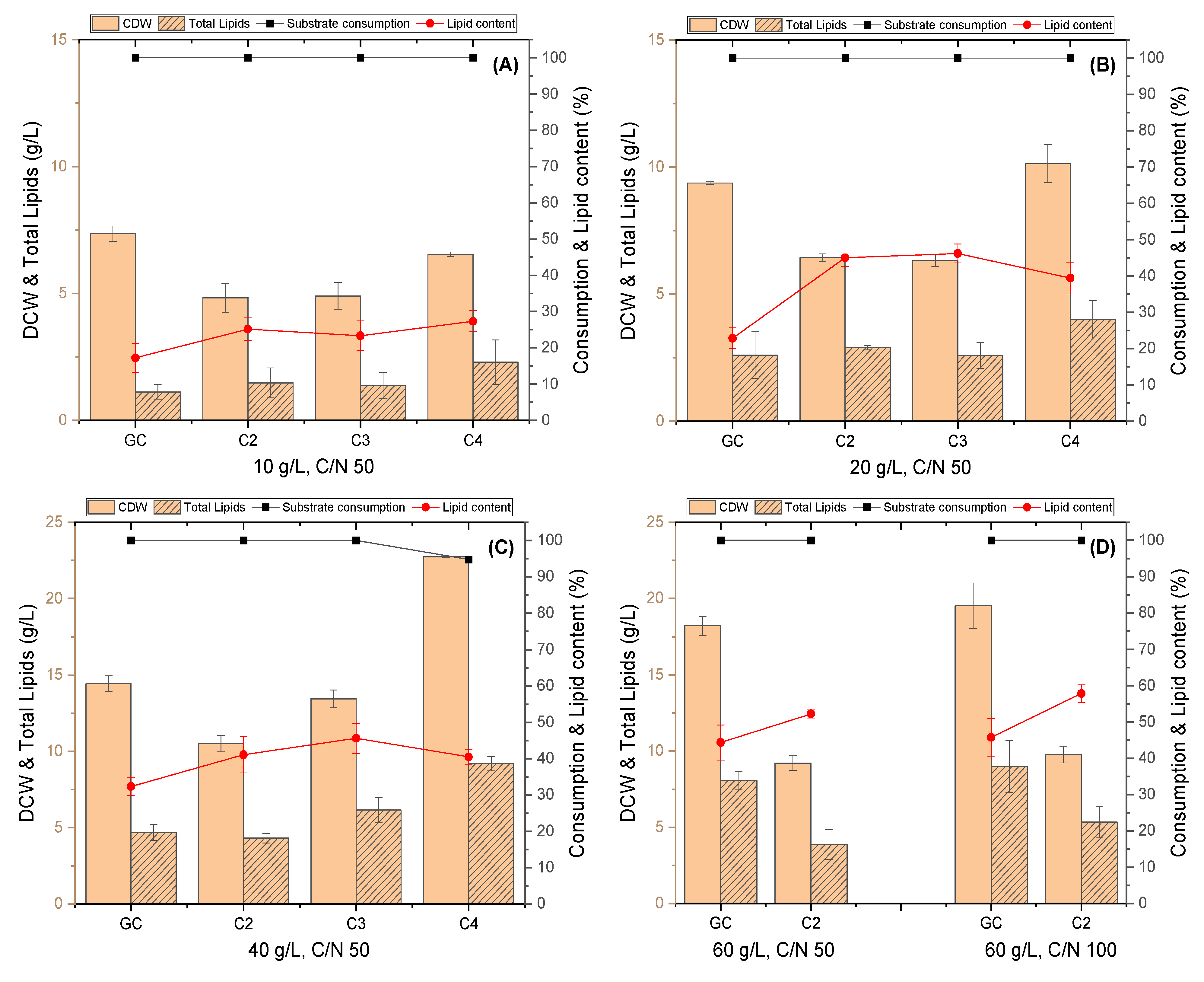
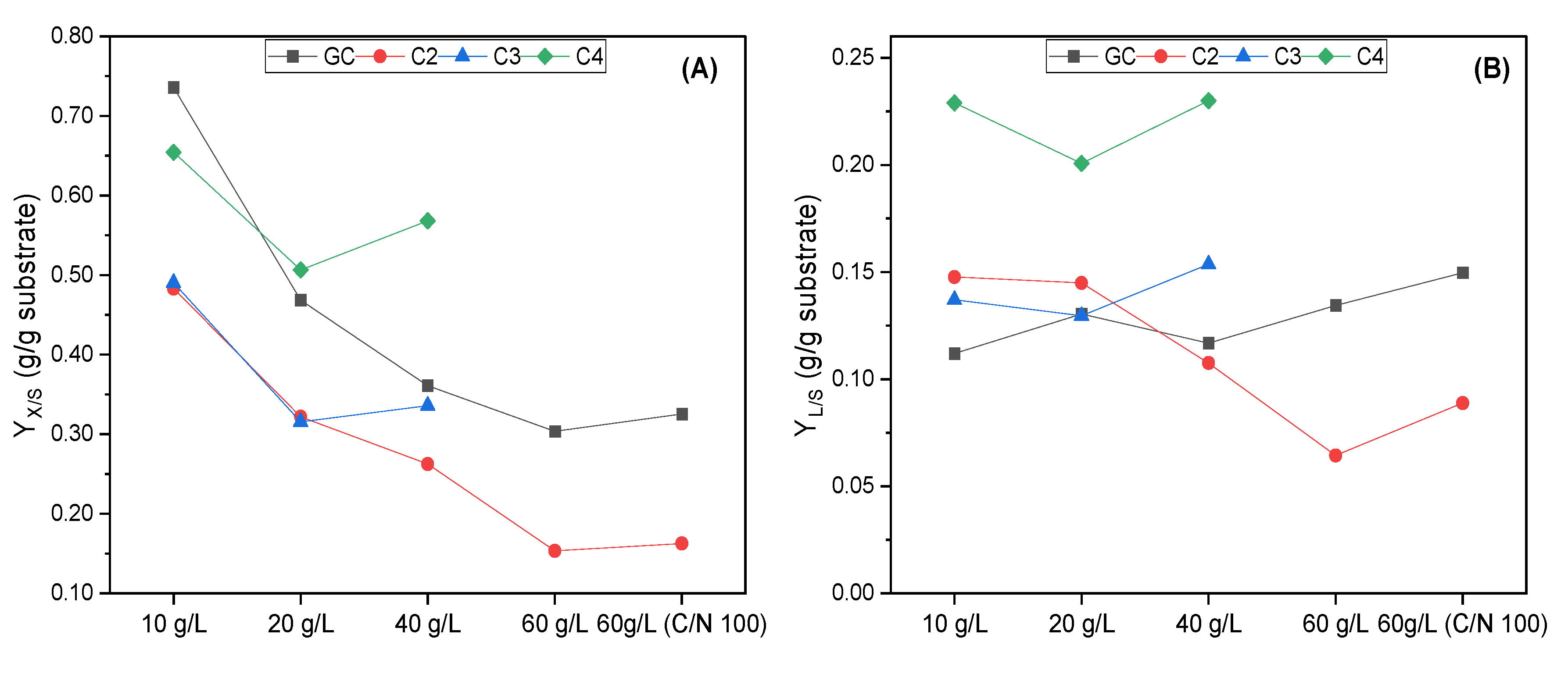
3.1. Evaluation of R. toruloides Growth on Different Concentrations of VFAs
3.2. Evaluation of C. curvatus Growth on Different Concentrations of VFAs
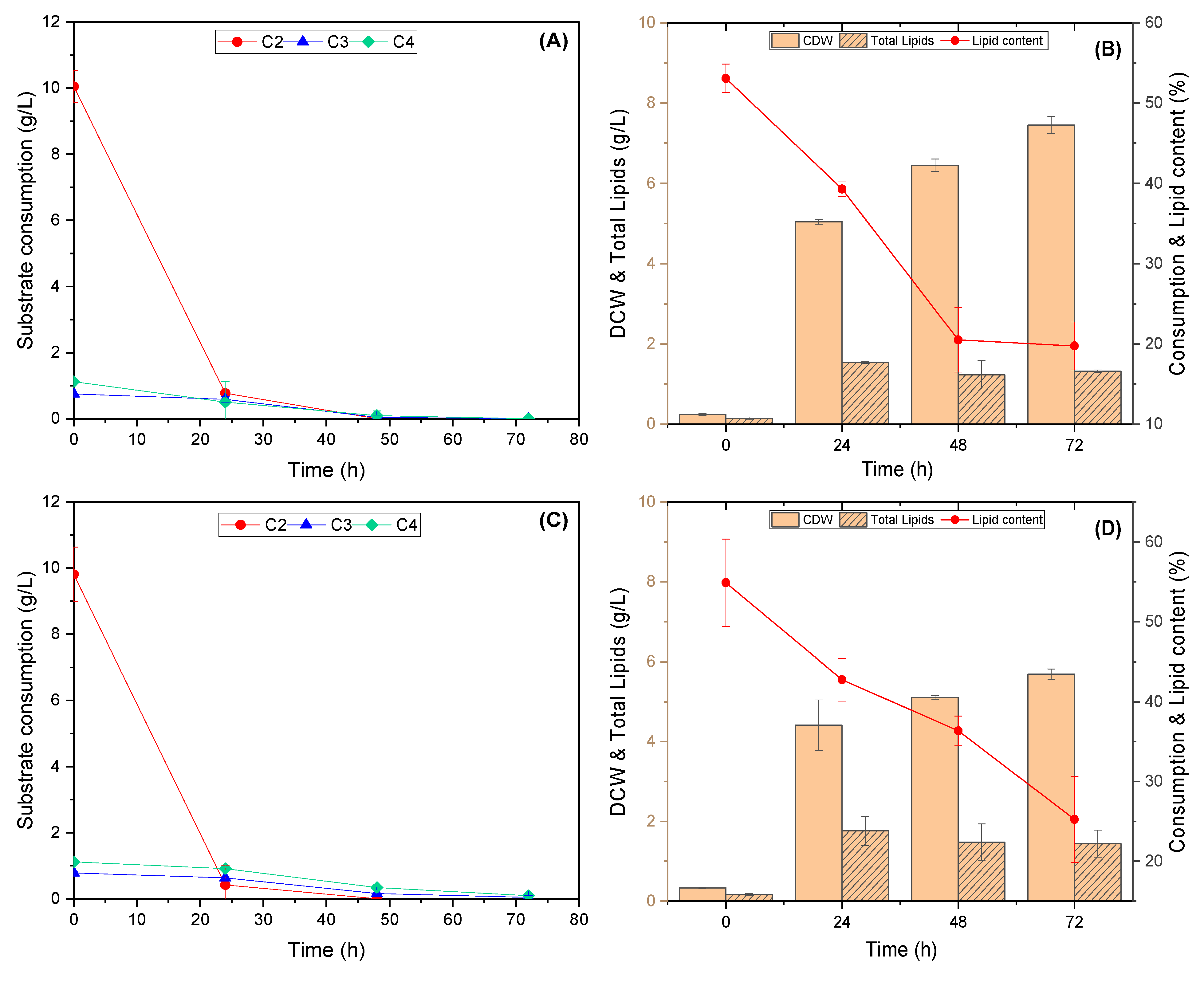
3.3. Evaluation of the Yeasts’ Growth on VFAs Derived from the Acidogenic Fermentation of BSG
3.4. Single-Cell Oil (SCO)
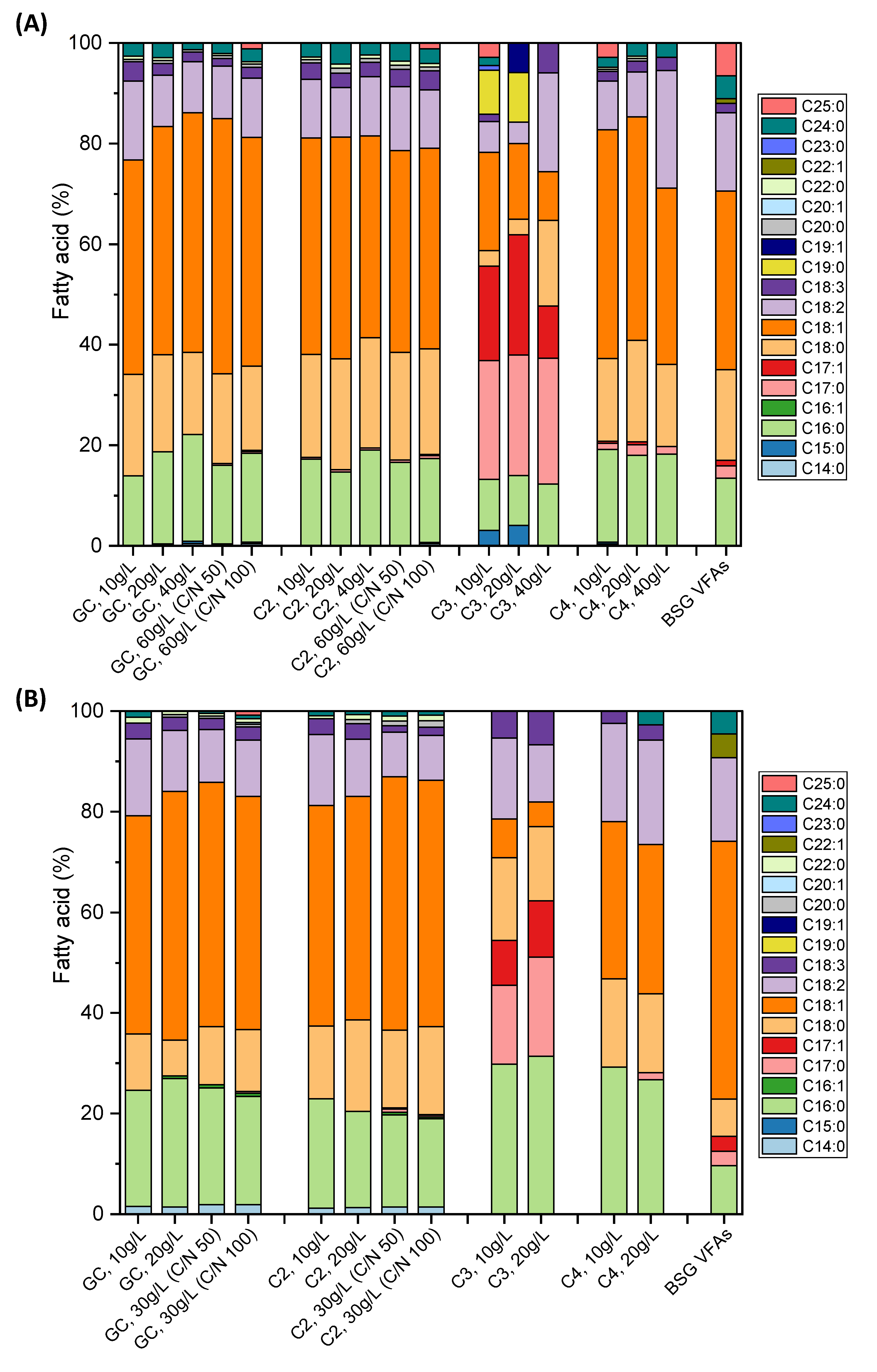
3.4.1. Fatty Acid Composition of the SCO
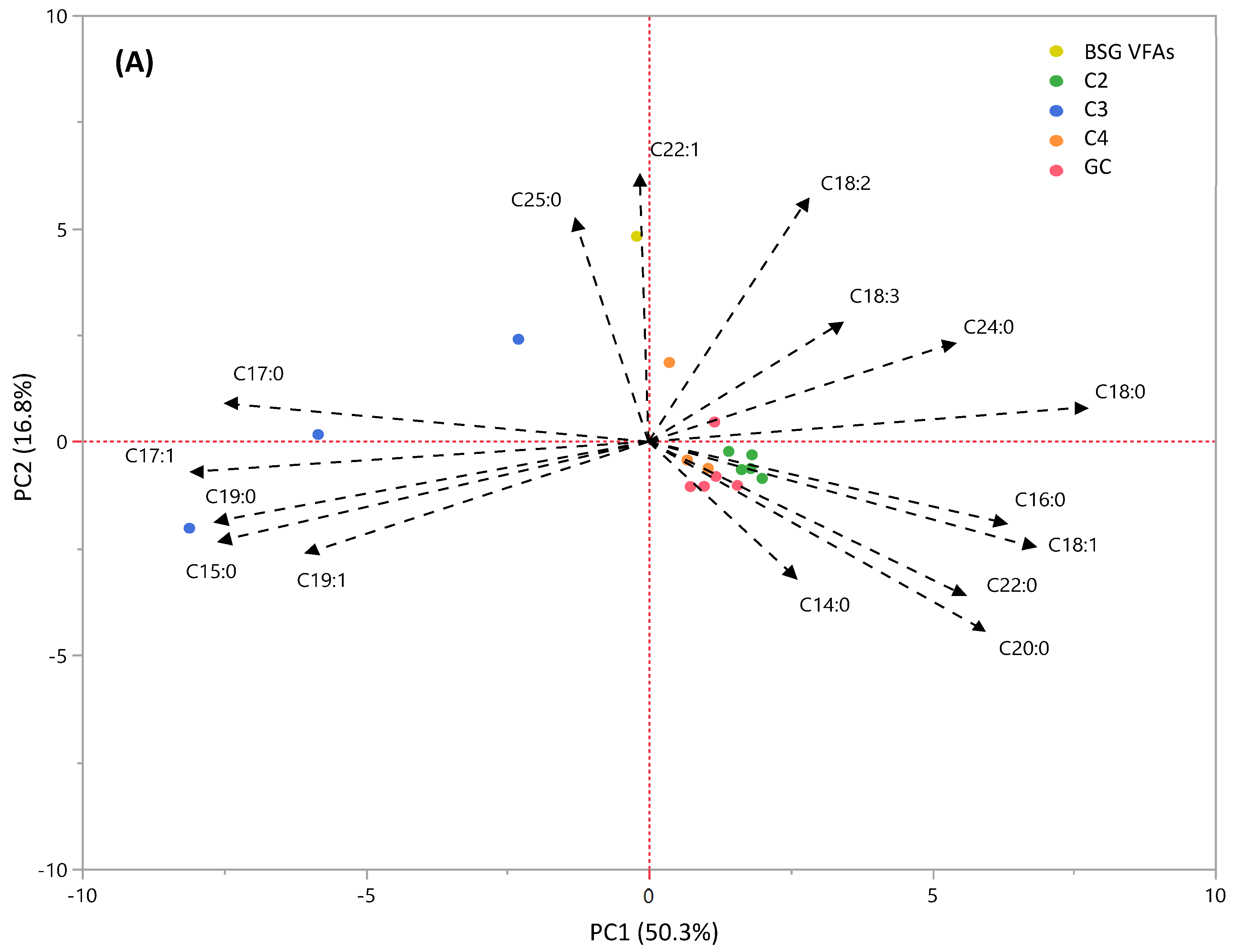
3.4.2. PCA Analysis
3.4.3. SCO from Oleaginous Yeasts towards the Production of Odd-Chain Fatty Acids (OCFAs)
3.4.4. Biodiesel Properties
3.4.5. Morphology of SCO within the Yeast Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papanikolaou, S. Oleaginous Yeasts: Biochemical Events Related with Lipid Synthesis and Potential Biotechnological Applications. Ferment. Technol. 2012, 1, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of Oleaginous Yeasts for Lipid Production Using Volatile Fatty Acids as Substrate. Biomass Bioenergy 2020, 138, 1–10. [Google Scholar] [CrossRef]
- Llamas, M.; Magdalena, J.A.; González-Fernández, C.; Tomás-Pejó, E. Volatile Fatty Acids as Novel Building Blocks for Oil-Based Chemistry via Oleaginous Yeast Fermentation. Biotechnol. Bioeng. 2020, 117, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-amaro, R.; Lazar, Z.; Rakicka, M.; Guo, Z.; Fouchard, F.; Coq, A.C.; Nicaud, J. Metabolic Engineering of Yarrowia Lipolytica to Produce Chemicals and Fuels from Xylose. Metab. Eng. 2016, 38, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; Silitonga, A.S.; Anjum, I.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A Comprehensive Review on Biodiesel as an Alternative Energy Resource and Its Characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Levering, J.; Broddrick, J.; Zengler, K. Engineering of Oleaginous Organisms for Lipid Production. Curr. Opin. Biotechnol. 2015, 36, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous Yeasts for Biodiesel: Current and Future Trends in Biology and Production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Antonopoulou, I.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Lipids Detection and Quantification in Oleaginous Microorganisms: An Overview of the Current State of the Art. BMC Chem. Eng. 2019, 1, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable Biodiesel Production from Oleaginous Yeasts Utilizing Hydrolysates of Various Non-Edible Lignocellulosic Biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. A Comparative Assessment of Biodiesel Cetane Number Predictive Correlations Based on Fatty Acid Composition. Energies 2019, 12, 422. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of Fuel Properties on the Basis of Fatty Acid Profiles of Oleaginous Yeast for Potential Biodiesel Production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Sierra-Cantor, J.F.; Guerrero-Fajardo, C.A. Methods for Improving the Cold Flow Properties of Biodiesel with High Saturated Fatty Acids Content: A Review. Renew. Sustain. Energy Rev. 2017, 72, 774–790. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A Statistical Investigation of Biodiesel Physical and Chemical Properties, and Their Correlation with the Degree of Unsaturation. Renew. Energy 2013, 50, 858–878. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” Biodiesel: Optimizing Fatty Ester Composition to Improve Fuel Properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel Production from Oleaginous Microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of Brewers’ Spent Grain for the Production of Lipids by Oleaginous Yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Krikigianni, E.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprocessing of Volatile Fatty Acids by Oleaginous Freshwater Microalgae and Their Potential for Biofuel and Protein Production. Chem. Eng. J. 2022, 438, 135529. [Google Scholar] [CrossRef]
- Patel, A.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Volatile Fatty Acids (VFAs) Generated by Anaerobic Digestion Serve as Feedstock for Freshwater and Marine Oleaginous Microorganisms to Produce Biodiesel and Added-Value Compounds. Front. Microbiol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential Applications of Brewery Spent Grain: Critical an Overview. J. Environ. Chem. Eng. 2022, 10. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of Initial Uncontrolled PH on Acidogenic Fermentation of Brewery Spent Grains to Biohydrogen and Volatile Fatty Acids Production: Optimization and Scale-Up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Valorization of Volatile Fatty Acids Derived from Low-Cost Organic Waste for Lipogenesis in Oleaginous Microorganisms—A Review. Bioresour. Technol. 2021, 321, 124457. [Google Scholar] [CrossRef] [PubMed]
- Dulermo, T.; Tréton, B.; Beopoulos, A.; Gnankon, A.P.K.; Haddouche, R.; Nicaud, J.M. Characterization of the Two Intracellular Lipases of Y. Lipolytica Encoded by TGL3 and TGL4 Genes: New Insights into the Role of Intracellular Lipases and Lipid Body Organisation. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2013, 1831, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Delgado Vellosillo, I.; Rova, U.; Matsakas, L.; Christakopoulos, P. A Novel Bioprocess Engineering Approach to Recycle Hydrophilic and Hydrophobic Waste under High Salinity Conditions for the Production of Nutraceutical Compounds. Chem. Eng. J. 2022, 431. [Google Scholar] [CrossRef]
- Lindmark, H. Fatty Acids in Bovine Milk Fat. Food Nutr. Res. 2008, 52, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Stephanie, V.W.; Richard, L.; Edward, A.D. Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors Affecting Odd- and Branched-Chain Fatty Acids in Milk: A Review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Imamura, F.; Fretts, A.; Marklund, M.; Korat, A.V.A.; Yang, W.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.; Wong, K.; et al. Fatty Acid Biomarkers of Dairy Fat Consumption and Incidence of Type 2 Diabetes: A Pooled Analysis of Prospective Cohort Studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty Acid Composition of Adipose Tissue and Blood in Humans and Its Use as a Biomarker of Dietary Intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzegorz, D.; Konopka, I. Update on Food Sources and Biological Activity of Odd-Chain, Branched and Cyclic Fatty Acids––A Review. Trends Food Sci. Technol. 2022, 119, 514–529. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R.; Nicaud, J.M. De Novo Biosynthesis of Odd-Chain Fatty Acids in Yarrowia Lipolytica Enabled by Modular Pathway Engineering. Front. Bioeng. Biotechnol. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Park, Y.K.; Bordes, F.; Letisse, F.; Nicaud, J.M. Engineering Precursor Pools for Increasing Production of Odd-Chain Fatty Acids in Yarrowia Lipolytica. Metab. Eng. Commun. 2021, 12, e00158. [Google Scholar] [CrossRef]
- Park, Y.K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.M. Optimization of Odd Chain Fatty Acid Production by Yarrowia Lipolytica. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee Chang, K.J.; Mansour, M.P.; Dunstan, G.A.; Blackburn, S.I.; Koutoulis, A.; Nichols, P.D. Odd-Chain Polyunsaturated Fatty Acids in Thraustochytrids. Phytochemistry 2011, 72, 1460–1465. [Google Scholar] [CrossRef]
- Wang, F.; Bi, Y.; Diao, J.; Lv, M.; Cui, J.; Chen, L.; Zhang, W. Metabolic Engineering to Enhance Biosynthesis of Both Docosahexaenoic Acid and Odd-Chain Fatty Acids in Schizochytrium sp. S31. Biotechnol. Biofuels 2019, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Oliver, L.; Fern, L.; Dietrich, T.; Villaran, M.C.; Barrio, J. Production of Docosahexaenoic Acid and Odd-Chain Fatty Acids by Microalgae Schizochytrium Limacinum Grown on Waste-Derived Volatile Fatty Acids. Appl. Sci. 2022, 12, 3976. [Google Scholar] [CrossRef]
- Wu, H.; San, K. Engineering Escherichia Coli for Odd Straight Medium Chain Free Fatty Acid Production. Appl. Microbiol. Biotechnol. 2014, 8145–8154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Liang, S.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Microbial Synthesis of Functional Odd-Chain Fatty Acids: A Review. World J. Microbiol. Biotechnol. 2020, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Wychen, S.; Ramirez, K.; Laurens, L.M.L.; Van Wychen, S.; Ramirez, K. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by In Situ Transesterification (NREL/TP-5100-60958); Laboratory Analytical Procedures (LAP): Gold, CO, USA, 2013. [Google Scholar]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer©: A User-Friendly Software for Predicting the Properties of Prospective Biodiesel. Biofuel Res. J. 2014, 1, 55–57. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient Conversion of Acetate into Lipids by the Oleaginous Yeast Cryptococcus Curvatus. Biotechnol. Biofuels 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Huang, X.F.; Liu, J.N.; Lu, L.J.; Peng, K.M.; Yang, G.X.; Liu, J.N. Culture Strategies for Lipid Production Using Acetic Acid as Sole Carbon Source by Rhodosporidium Toruloides. Bioresour. Technol. 2016, 206, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Afonso, V.; Tangerino, L.; Oliveira, D.; Raposo, S. Culture Strategies for Lipid Production Using Low-Cost Carbon Sources by Rhodosporidium Toruloides. In INCREaSE; Mortal, A., Aníbal, J., Monteiro, J., Sequeira, C., Semião, J., Silva, M.M., Oliveira, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 103–116. [Google Scholar]
- Liu, J.; Huang, X.; Chen, R.; Yuan, M.; Liu, J. Efficient Bioconversion of High-Content Volatile Fatty Acids into Microbial Lipids by Cryptococcus Curvatus ATCC 20509. Bioresour. Technol. 2017, 239, 394–401. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, M.; Liu, J.N.; Huang, X.F. Bioconversion of Mixed Volatile Fatty Acids into Microbial Lipids by Cryptococcus Curvatus ATCC 20509. Bioresour. Technol. 2017, 241, 645–651. [Google Scholar] [CrossRef]
- Zheng, Y.; Chi, Z.; Ahring, B.K.; Chen, S. Oleaginous Yeast Cryptococcus Curvatus for Biofuel Production: Ammonia’s Effect. Biomass Bioenergy 2012, 37, 114–121. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous Yeast Yarrowia Lipolytica Culture with Synthetic and Food Waste-Derived Volatile Fatty Acids for Lipid Production. Biotechnol. Biofuels 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Fernandez-Castane, A.; Oleskowicz-Popiel, P. Production of Microbial Lipids Utilizing Volatile Fatty Acids Derived from Wastepaper: A Biorefinery Approach for Biodiesel Production. Fuel 2020, 276, 118087. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.N.; Yuan, M.; Shen, Z.H.; Peng, K.M.; Lu, L.J.; Huang, X.F. Bioconversion of Volatile Fatty Acids Derived from Waste Activated Sludge into Lipids by Cryptococcus Curvatus. Bioresour. Technol. 2016, 211, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Béligon, V.; Noblecourt, A.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Proof of Concept for Biorefinery Approach Aiming at Two Bioenergy Production Compartments, Hydrogen and Biodiesel, Coupled by an External Membrane. Biofuels 2018, 9, 163–174. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, M.; Liu, J.N.; Lu, L.J.; Peng, K.M.; Huang, X.F. Microbial Conversion of Mixed Volatile Fatty Acids into Microbial Lipids by Sequencing Batch Culture Strategy. Bioresour. Technol. 2016, 222, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Christophe, G.; Deo, J.L.; Kumar, V.; Nouaille, R.; Fontanille, P.; Larroche, C. Production of Oils from Acetic Acid by the Oleaginous Yeast Cryptococcus Curvatus. Appl. Biochem. Biotechnol. 2012, 167, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, W.; Shen, H.; Yang, Z.; Wang, G.; Zuo, Z.; Hou, Y.; Zhao, Z.K. Co-Fermentation of Acetate and Sugars Facilitating Microbial Lipid Production on Acetate-Rich Biomass Hydrolysates. Bioresour. Technol. 2016, 207, 102–108. [Google Scholar] [CrossRef]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Improvement and Modeling of Culture Parameters to Enhance Biomass and Lipid Production by the Oleaginous Yeast Cryptococcus Curvatus Grown on Acetate. Bioresour. Technol. 2015, 192, 582–591. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Cho, H.U.; Park, H.R.; Park, J.M. Bioconversion of Volatile Fatty Acids from Macroalgae Fermentation into Microbial Lipids by Oleaginous Yeast. Chem. Eng. J. 2015, 264, 735–743. [Google Scholar] [CrossRef]
- Lian, J.; Garcia-Perez, M.; Coates, R.; Wu, H.; Chen, S. Yeast Fermentation of Carboxylic Acids Obtained from Pyrolytic Aqueous Phases for Lipid Production. Bioresour. Technol. 2012, 118, 177–186. [Google Scholar] [CrossRef]
- Kolouchová, I.; Schreiberová, O.; Sigler, K.; Masák, J.; Řezanka, T. Biotransformation of Volatile Fatty Acids by Oleaginous and Non-Oleaginous Yeast Species. FEMS Yeast Res. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hoekman, S.K.; Robbins, C. Review of the Effects of Biodiesel on NOx Emissions. Fuel Processing Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of Biodiesel Fuel Properties on the Structure of Fatty Acid Alkyl Esters. Fuel Processing Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Mohamed Shameer, P. A Comparative Assessment of Biodiesel Cetane Number Predictive Correlations Based on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. Dependence of Oil Stability Index of Fatty Compounds on Their Structure and Concentration and Presence of Metals. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 1021–1026. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Smith, J.C.; Wilson, J.R.; Salley, S.O.; Ng, K.Y.S. Investigation of the Parameters Affecting the Cetane Number of Biodiesel. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 1073–1081. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic Viscosity of Biodiesel Fuel Components and Related Compounds. Influence of Compound Structure and Comparison to Petrodiesel Fuel Components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A Review of the Effect of the Composition of Biodiesel on NOx Emission, Oxidative Stability and Cold Flow Properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- McCormick, R.L.; Ratcliff, M.; Moens, L.; Lawrence, R. Several Factors Affecting the Stability of Biodiesel in Standard Accelerated Tests. Fuel Processing Technol. 2007, 88, 651–657. [Google Scholar] [CrossRef]

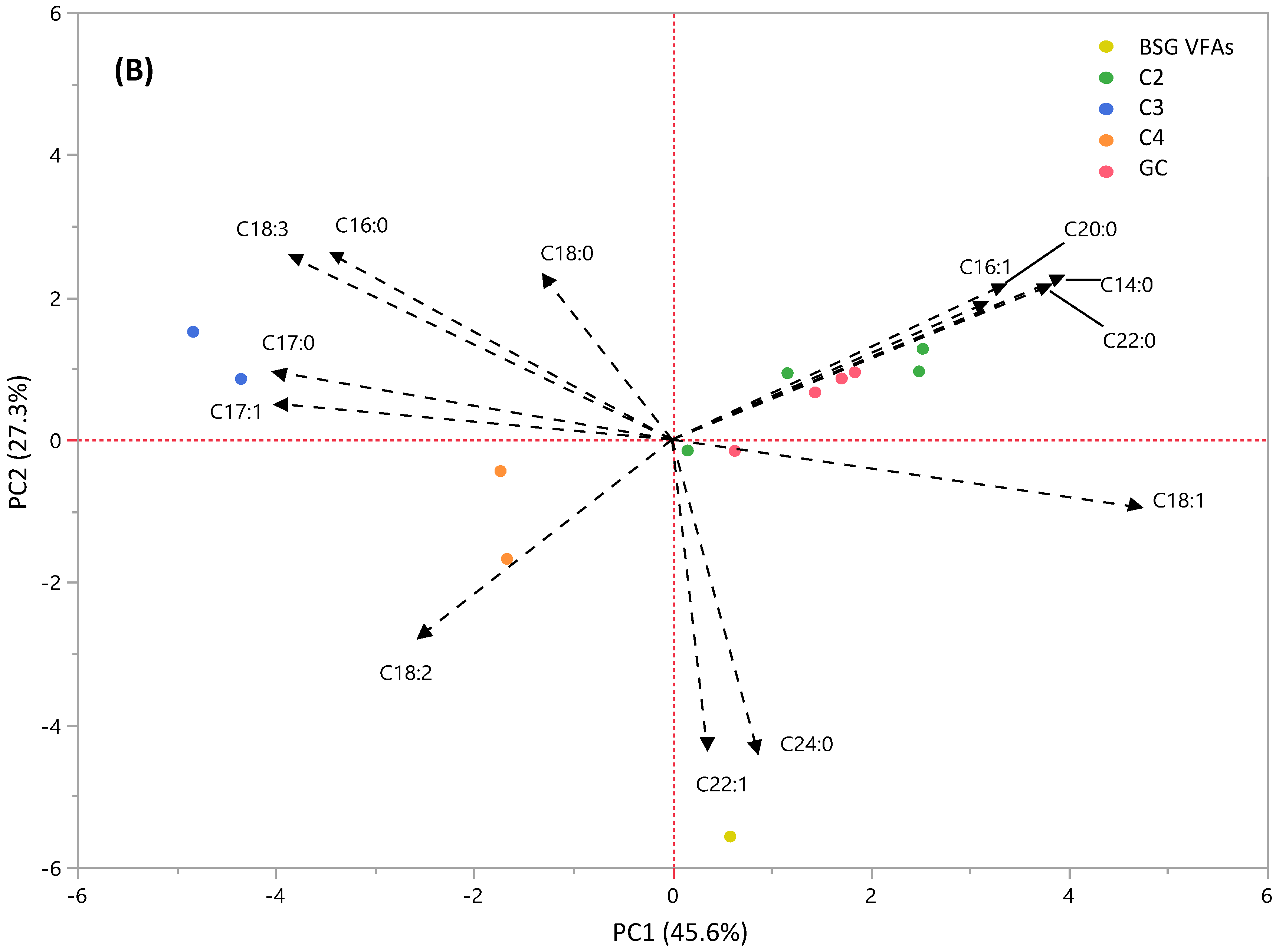
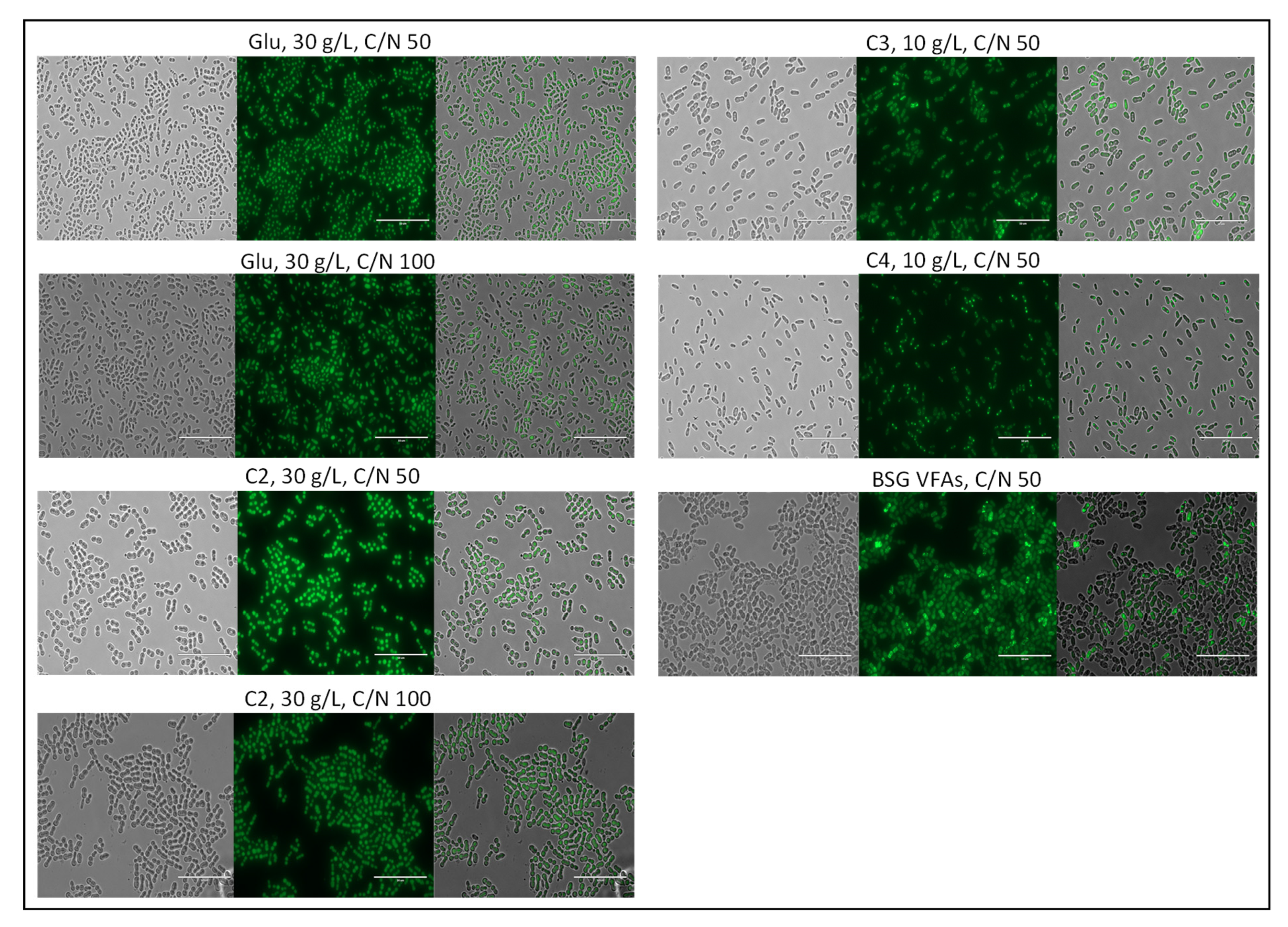
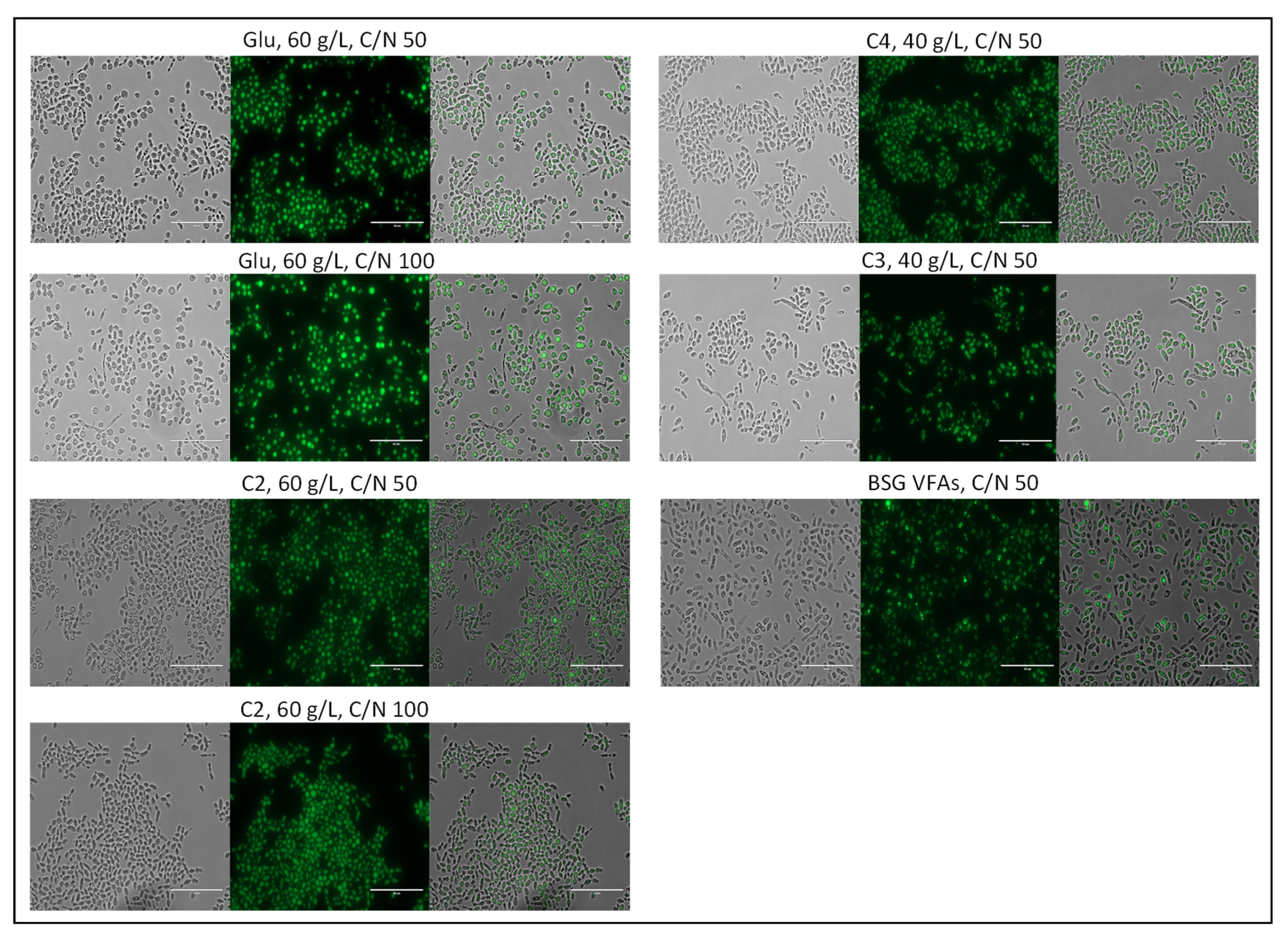
| Yeast Strain | Culture Strategy | Carbon Source | Concentration (g/L) | Lipid Content (%) | Reference |
|---|---|---|---|---|---|
| C. curvatus NRRL-Y-1511 | Batch | VFAs from anaerobic digestion effluent | 5, 10, 15 | 29.2; 32.6; 36.9 | [3] |
| C. curvatus DSM 70022 | Batch | VFAs mix (C2:C3:C4) | 2 (5:1:4) | 36.3 | [52] |
| 5 (5:1:4) | 28.1 | ||||
| 10 (5:1:4) | 17.0 | ||||
| 5 (5:1:4) | 23.6 | ||||
| 5 (5:2:3) | 26.2 | ||||
| 5 (6:2:2) | 30.1 | ||||
| 5 (6:1:3) | 38.6 | ||||
| VFAs from waste office paper | 17.28 (5.3 C2: 3.6 C3: 1.1 C4) | 41.2 | |||
| VFAs from waste newspaper | 10.23 (4.9 C2: 3.6 C3: 1.7 C4) | 27.7 | |||
| C. curvatus MUCL 29819 | Batch | VFAs from waste activated sludge (WAS) | 1.7 C2; 0.7 C3 | 13.86 | [53] |
| sequencing batch culture SBC (5 cycles) | VFAs from waste activated sludge (WAS) | 3.35 | 39.6 | ||
| C. curvatus MUCL 29819 | sequencing batch culture SBC (4 cycles) | VFAs mix (C2:C3:C4) | 4 (6:3:1) | 42.7 | [55] |
| C. curvatus MUCL 29819 | Two-stage batch | Glu + C2 | 5 + 5 | 47.3 | [56] |
| pH-stat two-stage fed-batch bioreactor | Glu + C2 | 15 + 5 | 50.9 | ||
| C. curvatus ATCC 20509 | Batch | C2 (5–40 g/L) | 30; 40 | 58.0; 62.5 | [48] |
| Batch | VFAs mix (C2:C3:C4) | 30; 40 (6:3:1) | 53.3; 63.2 | ||
| C. curvatus ATCC 20509 | Batch | C2 (5–40 g/L) | 30 | 57.9 | [49] |
| C3 (5–40 g/L) | |||||
| C4 (5–40 g/L) | |||||
| VFAs mix (C2:C3:C4) | 30 (15:5:10) | 56.9 | |||
| C. curvatus ATCC 20509 | Fed-batch | VFAs from dark fermentation | 12 | 42 | [54] |
| C. curvatus ATCC 20509 | Batch | Glu + C2 | 30 + 5 | 31.7 | [57] |
| Glu + C2 | 30 + 10 | 37.9 | |||
| Glu + C2 | 30 + 20 | 47.6 | |||
| Glu + C2 | 30 + 30 | 56.0 | |||
| Xylose + C2 | 30 + 5 | 28.1 | |||
| C. curvatus ATCC 20509 | Fed-batch | C2 | 5 | 53 | [58] |
| C. curvatus ATCC 20509 | Batch | C2 | 30 | 73.4 | [45] |
| Continuous culture | C2 | 30 | 66.4 | ||
| C. curvatus ATCC 20509 | Repeated fed-batch | VFAs mix (C2:C3:C4) | 9.8 | 61 | [59] |
| C. curvatus ATCC 20509 | Batch | C2 (5–60 g/L) | 20 | 41.3 | [60] |
| C. curvatus DSM 101032 | Batch | C2 (10–80 g/L) | 10, 20, 40, 60 | 25.2; 45; 41.1; 52.2 | This study |
| C2 (C/N 100) | 60 | 57.9 | |||
| C3 (10–60 g/L) | 10, 20, 40 | 23.3; 46.2; 45.6 | |||
| C4 (10–60 g/L) | 10, 20, 40 | 27.3; 39.5; 40.5 | |||
| VFAs from BSG | (10.1 C2: 0.8 C3: 1.2 C4) | 39.3 (24 h) |
| Yeast Strain | Culture Strategy | Carbon Source | Concentration | Lipid Content (%) | Reference |
|---|---|---|---|---|---|
| R. toruloides NRRL-Y-27012 | Batch | VFAs from anaerobic digestion effluent | 5, 10, 15 | 25.7; 19.9; no growth | [3] |
| R. toruloides CECT 1499 | Batch | C2 | 5–18 g/L | [47] | |
| Fed-batch | C2 | 15 | 20 | ||
| R. toruloides AS 2.1389 | Batch | C2 | 20 | 48.2 | [46] |
| Batch | C2 | 4 | 15.2 | ||
| Two-stage batch | Glu + C2 | 40 + 20 | 50.1 | ||
| Two-stage batch | Glu + C2 | 40 + 5 | 13.7 | ||
| Sequencing batch | C2 | 4 | 38.6 | ||
| R. toruloides AS 2.1389 | Batch | VFAs from waste activated sludge (WAS) | 1.7 C2; 0.7 C3 | 10.43 | [53] |
| R. toruloides ATCC 10788 | Batch | C2 | 30 | 33 | [45] |
| R. toruloides Y4 | C2 | 30 | 54.9 | ||
| R. toruloides NCYC 1576 | Batch | C2 (10–40 g/L) | 10, 20, 30 | 27.9; 40.3; 49.3 | This study |
| C2 (C/N 100) | 30 | 56.4 | |||
| C3 (10–40 g/L) | 10, 20 | 9.3; 9.2 | |||
| C4 (10–40 g/L) | 10, 20 | 8.8; 13.3 | |||
| VFAs from BSG | (10.1 C2: 0.8 C3: 1.2 C4) | 42.8 (24 h) |
| C. curvatus | ||||||
|---|---|---|---|---|---|---|
| Concentration (g/L) | Substrate | SFA (%) | MUFA (%) | PUFA (%) | OCFAs/Total Lipids (%) | OCFAs (g/L) |
| 10 | GC | 37.8 | 42.7 | 19.6 | 0.0 | 0.00 |
| C2 | 42.1 | 43.0 | 14.9 | 0.4 | 0.01 | |
| C3 | 54.1 | 38.4 | 7.5 | 58.1 | 0.80 | |
| C4 | 42.5 | 45.9 | 11.6 | 4.8 | 0.11 | |
| 20 | GC | 42.1 | 45.3 | 12.6 | 0.0 | 0.00 |
| C2 | 43.2 | 44.1 | 12.7 | 0.5 | 0.01 | |
| C3 | 50.9 | 44.8 | 4.4 | 67.7 | 1.75 | |
| C4 | 43.8 | 45 | 11.1 | 2.7 | 0.11 | |
| 40 | GC | 40.2 | 47.7 | 12.1 | 0.4 | 0.02 |
| C2 | 45.6 | 39.9 | 14.5 | 0.4 | 0.02 | |
| C3 | 54.4 | 20.0 | 25.6 | 35.4 | 2.17 | |
| C4 | 39.0 | 35.0 | 26.0 | 1.5 | 0.14 | |
| 60 (C/N 50) | GC | 37.3 | 50.7 | 12.0 | 0.4 | 0.03 |
| C2 | 43.9 | 40.0 | 16.1 | 0.4 | 0.02 | |
| 60 (C/N 100) | GC | 40.4 | 45.7 | 13.9 | 2.0 | 0.18 |
| C2 | 45.0 | 40.6 | 15.6 | 2.1 | 0.11 | |
| (10.1 C2; 0.8 C3; 1.2 C4) | BSG VFAs | 45.1 | 37.5 | 17.4 | 10.0 | 0.15 |
| R. toruloides | ||||||
| Concentration (g/L) | Substrate | SFA (%) | MUFA (%) | PUFA (%) | OCFAs/Total Lipids (%) | OCFAs (g/L) |
| 10 | GC | 38.2 | 43.3 | 18.5 | 0.0 | 0.00 |
| C2 | 38.9 | 43.8 | 17.3 | 0.0 | 0.00 | |
| C3 | 61.9 | 16.6 | 21.4 | 24.6 | 0.18 | |
| C4 | 47.9 | 30.5 | 21.6 | 0.0 | 0.00 | |
| 20 | GC | 35.3 | 49.9 | 14.8 | 0.0 | 0.00 |
| C2 | 41.2 | 44.3 | 14.5 | 0.0 | 0.00 | |
| C3 | 65.9 | 16.0 | 18.1 | 30.8 | 0.16 | |
| C4 | 46.6 | 29.7 | 23.7 | 1.4 | 0.01 | |
| 30 (C/N 50) | GC | 38.1 | 49.1 | 12.7 | 0.0 | 0.00 |
| C2 | 38.8 | 51.0 | 10.2 | 0.8 | 0.03 | |
| 30 (C/N 100) | GC | 38.9 | 47.2 | 13.9 | 1.2 | 0.07 |
| C2 | 39.9 | 49.5 | 10.6 | 0.5 | 0.02 | |
| (10.1 C2; 0.8 C3; 1.2 C4) | BSG VFAs | 23.6 | 57.2 | 16.0 | 5.7 | 0.10 |
| C. curvatus | Standard Fuel Parameters | ||||||
|---|---|---|---|---|---|---|---|
| 60 g/L GC (C/N100) | 60 g/L C2 (C/N100) | 40 g/L C3 | 40 g/L C4 | BSG VFAs | ASTM D6751-02 | EN 14214 | |
| DU | 73.371 | 71.531 | 60.848 | 87.074 | 70.298 | - | - |
| SV | 198.115 | 199.814 | 182.656 | 200.917 | 182.257 | - | - |
| IV | 68.180 | 68.094 | 60.551 | 81.037 | 65.153 | - | 120 (Max) |
| CN | 58.509 | 58.294 | 62.557 | 55.232 | 61.587 | 47 (Min) | 51 (Min) |
| LCSF | 16.626 | 20.088 | 9.752 | 15.717 | 19.548 | - | - |
| CFPP | 35.758 | 46.635 | 14.161 | 32.901 | 44.936 | country specific | |
| CP | 4.295 | 3.894 | 1.490 | 4.615 | 2.114 | country specific | |
| PP | −2.158 | −2.594 | −5.204 | −1.811 | −4.526 | - | - |
| APE | 73.371 | 71.531 | 60.848 | 87.074 | 70.298 | - | - |
| BAPE | 17.442 | 20.905 | 31.535 | 28.623 | 19.235 | - | - |
| OS | 11.048 | 10.166 | 7.200 | 7.121 | 9.375 | ≥3 | ≥6 |
| HHV | 39.079 | 39.526 | 35.423 | 39.565 | 36.278 | - | - |
| υ | 4.063 | 4.160 | 3.460 | 4.034 | 3.726 | 1.9–6 | 3.5–5.0 |
| ρ | 0.861 | 0.871 | 0.783 | 0.874 | 0.799 | 0.88 | 0.86–0.9 |
| R. toruloides | Standard Fuel Parameters | ||||||
| GC 30 g/L (CN100) | C2 30 g/L, (CN100) | C3 10 g/L | C4 10 g/L | BSG VFAs | ASTM D6751-02 | EN 14214 | |
| DU | 74.995 | 70.438 | 50.568 | 73.648 | 81.704 | - | - |
| SV | 201.314 | 201.159 | 188.023 | 203.134 | 177.267 | - | - |
| IV | 70.096 | 65.011 | 50.754 | 68.703 | 73.678 | - | 120 (Max) |
| CN | 57.640 | 58.805 | 63.909 | 57.711 | 60.512 | 47 (Min) | 51 (Min) |
| LCSF | 11.408 | 15.082 | 11.203 | 15.708 | 13.279 | - | - |
| CFPP | 19.364 | 30.905 | 18.720 | 32.873 | 25.241 | country specific | |
| CP | 6.354 | 4.243 | 10.685 | 10.058 | −0.097 | country specific | |
| PP | 0.077 | −2.215 | 4.778 | 4.097 | −6.926 | - | - |
| APE | 74.051 | 70.077 | 50.568 | 73.648 | 81.704 | - | - |
| BAPE | 17.520 | 14.717 | 26.855 | 23.962 | 16.014 | - | - |
| OS | 11.066 | 13.745 | 8.089 | 8.053 | 9.955 | ≥3 | ≥6 |
| HHV | 39.191 | 39.506 | 35.910 | 39.523 | 35.400 | - | - |
| υ | 3.964 | 4.116 | 3.494 | 4.022 | 3.563 | 1.9–6 | 3.5–5.0 |
| ρ | 0.866 | 0.871 | 0.794 | 0.873 | 0.781 | 0.88 | 0.86–0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krikigianni, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Patel, A. Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids. Appl. Sci. 2022, 12, 6541. https://doi.org/10.3390/app12136541
Krikigianni E, Matsakas L, Rova U, Christakopoulos P, Patel A. Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids. Applied Sciences. 2022; 12(13):6541. https://doi.org/10.3390/app12136541
Chicago/Turabian StyleKrikigianni, Eleni, Leonidas Matsakas, Ulrika Rova, Paul Christakopoulos, and Alok Patel. 2022. "Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids" Applied Sciences 12, no. 13: 6541. https://doi.org/10.3390/app12136541
APA StyleKrikigianni, E., Matsakas, L., Rova, U., Christakopoulos, P., & Patel, A. (2022). Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids. Applied Sciences, 12(13), 6541. https://doi.org/10.3390/app12136541









