Fabricating a Novel Three-Dimensional Skin Model Using Silica Nonwoven Fabrics (SNF)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
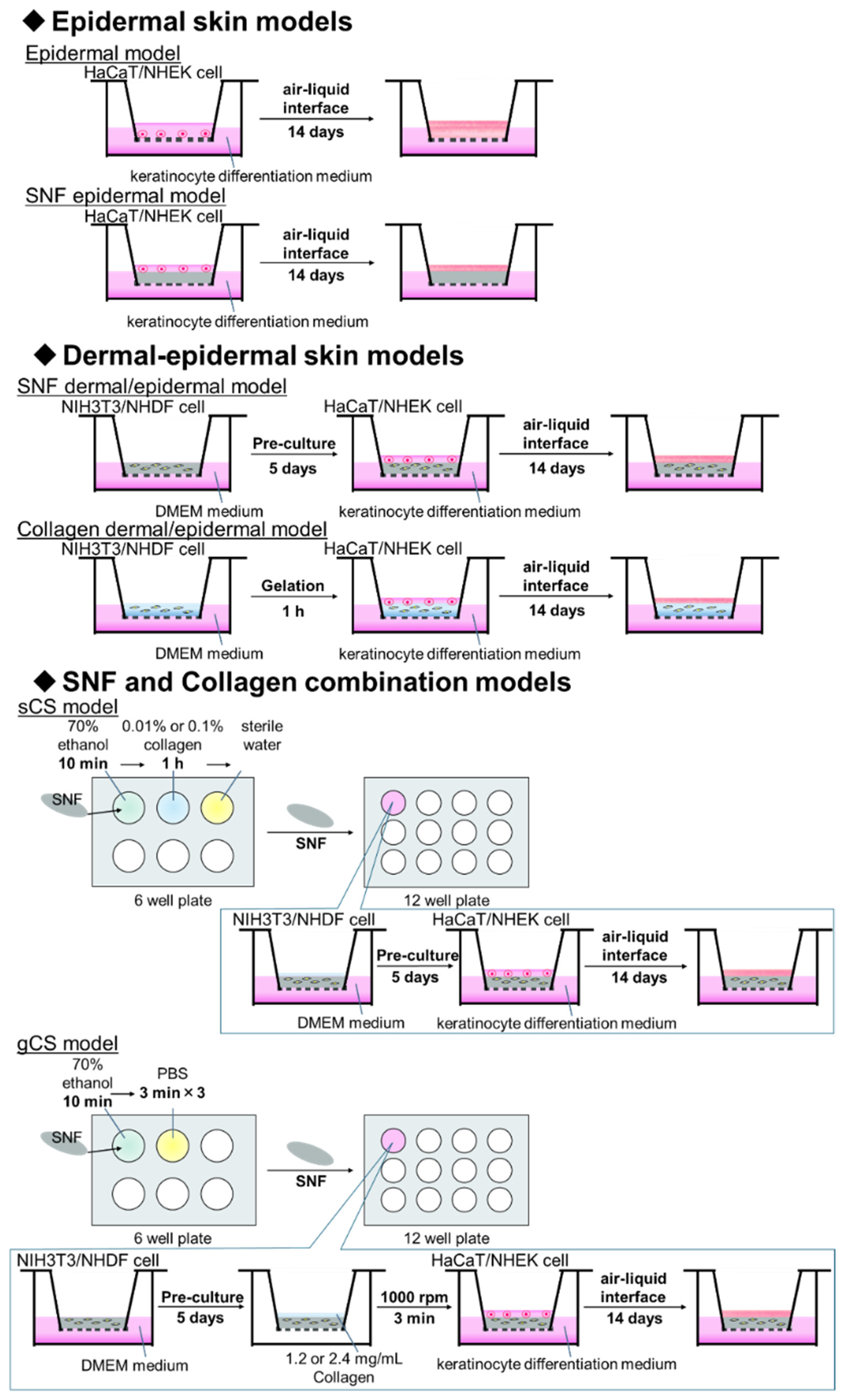
2.3. Preparation of Skin Models
2.3.1. Preparation of a Single Material Skin Models with Immortalized Cells or Primary Cells
2.3.2. Preparation of Skin Model with Primary Cells
2.3.3. Preparation of Skin Model Combining SNF and Collagen with Immortalized Cells
2.4. Histology
2.5. High Resolution Scanning Electron Microscopic (SEM) Observation
2.6. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.7. Skin Corrosion Test by MTT Assay
3. Results
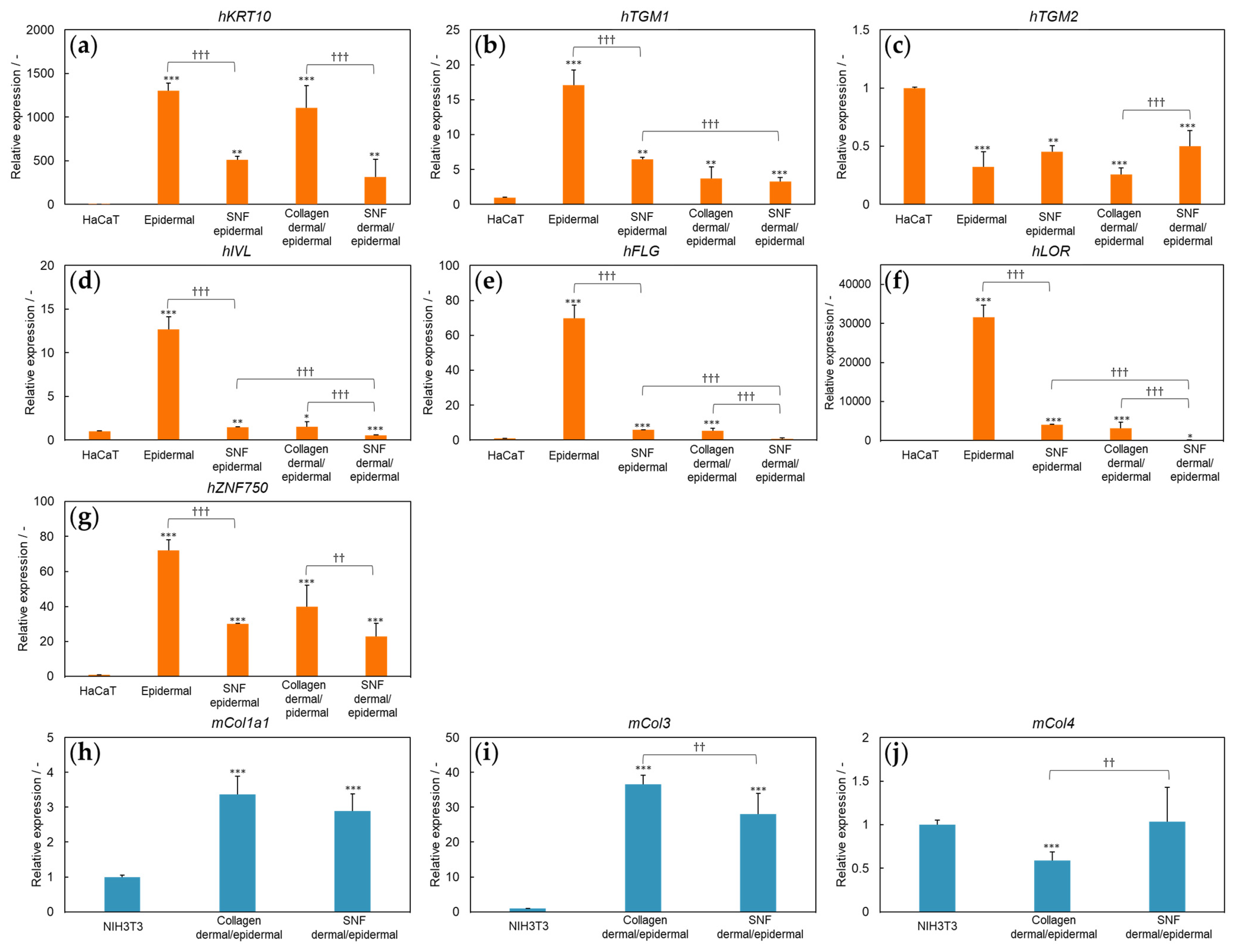
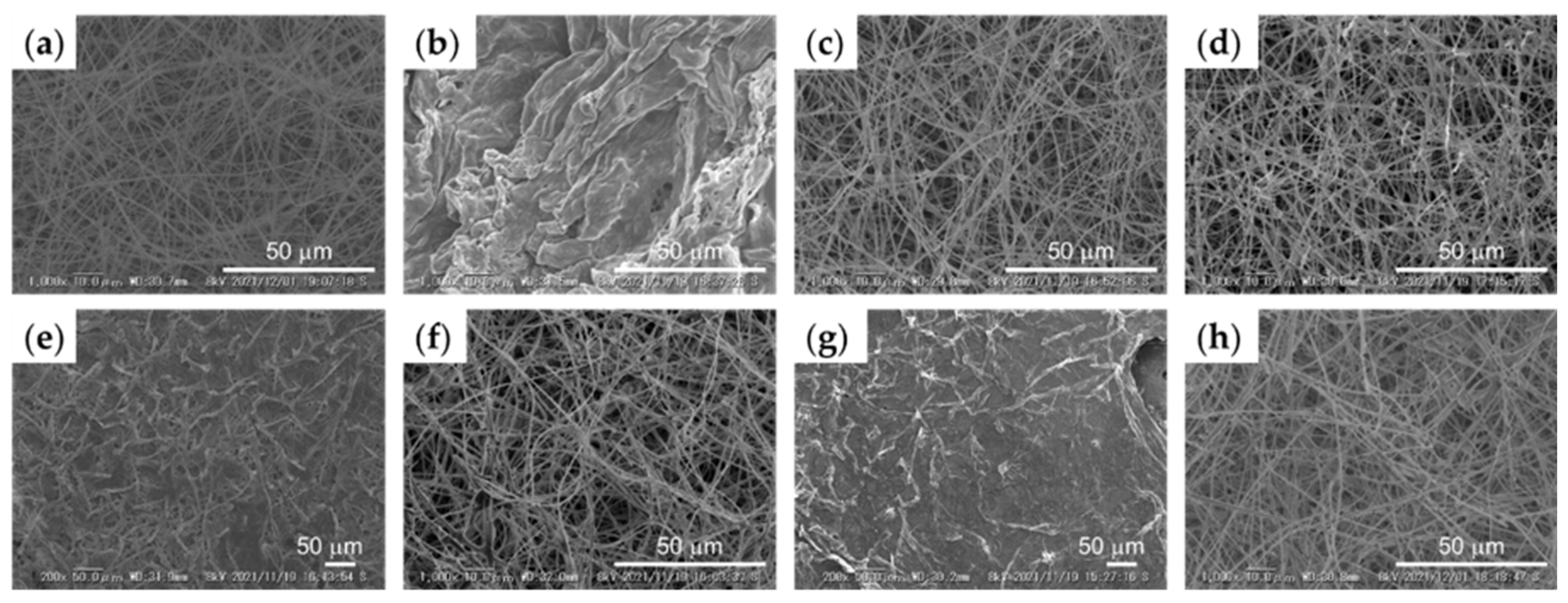
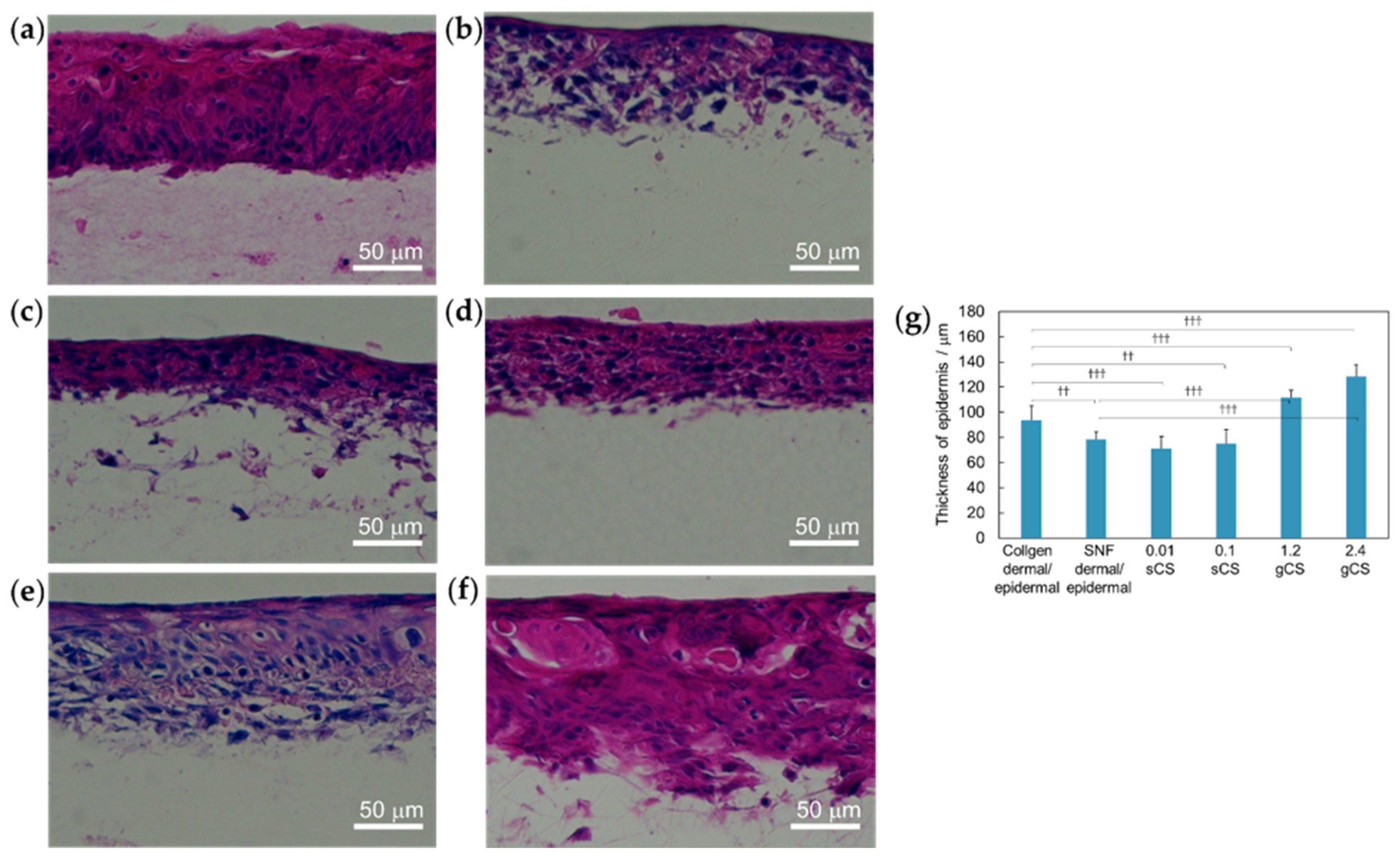
3.1. Three-Dimensional Model of SNF Skin Using Immortalized Cells
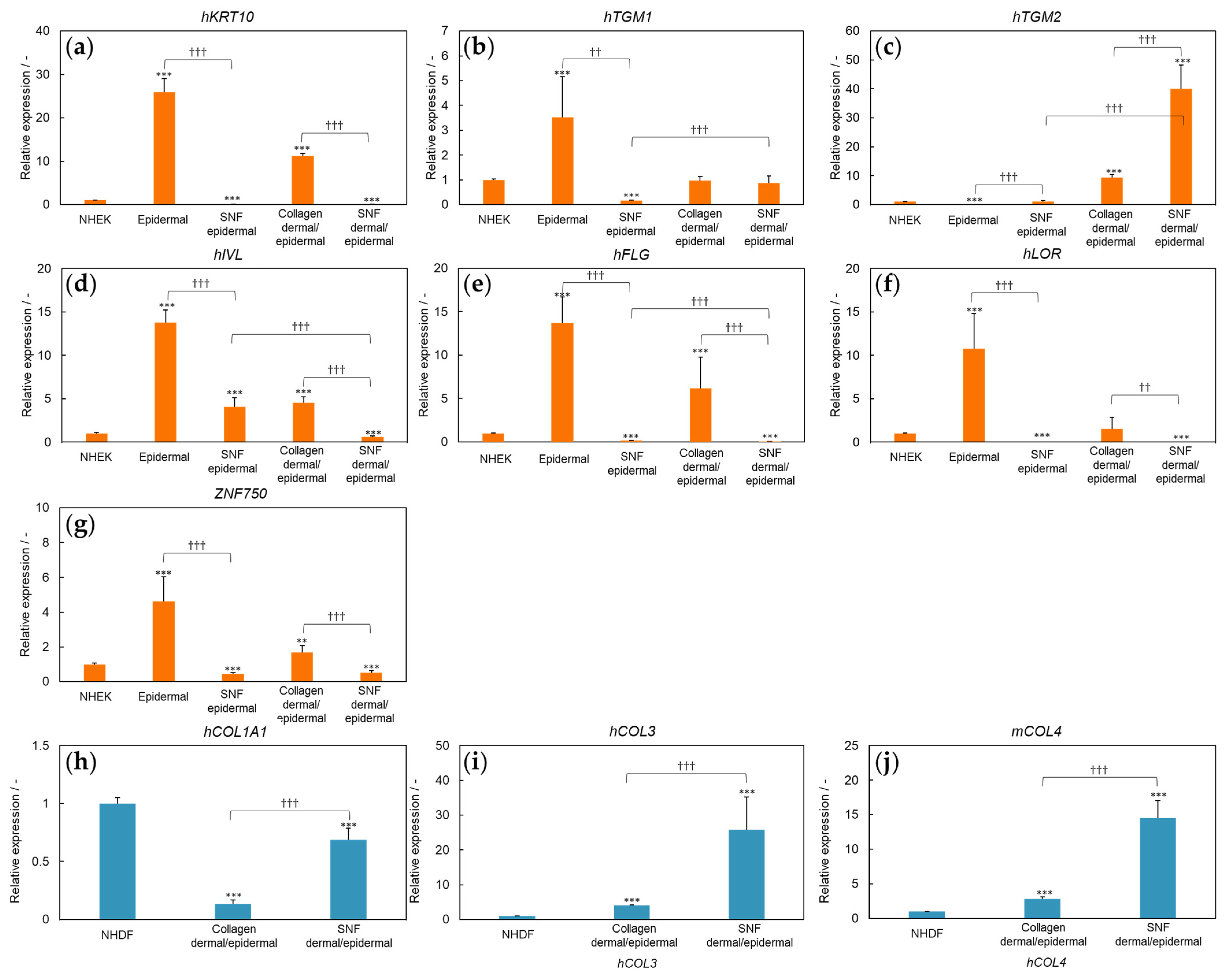
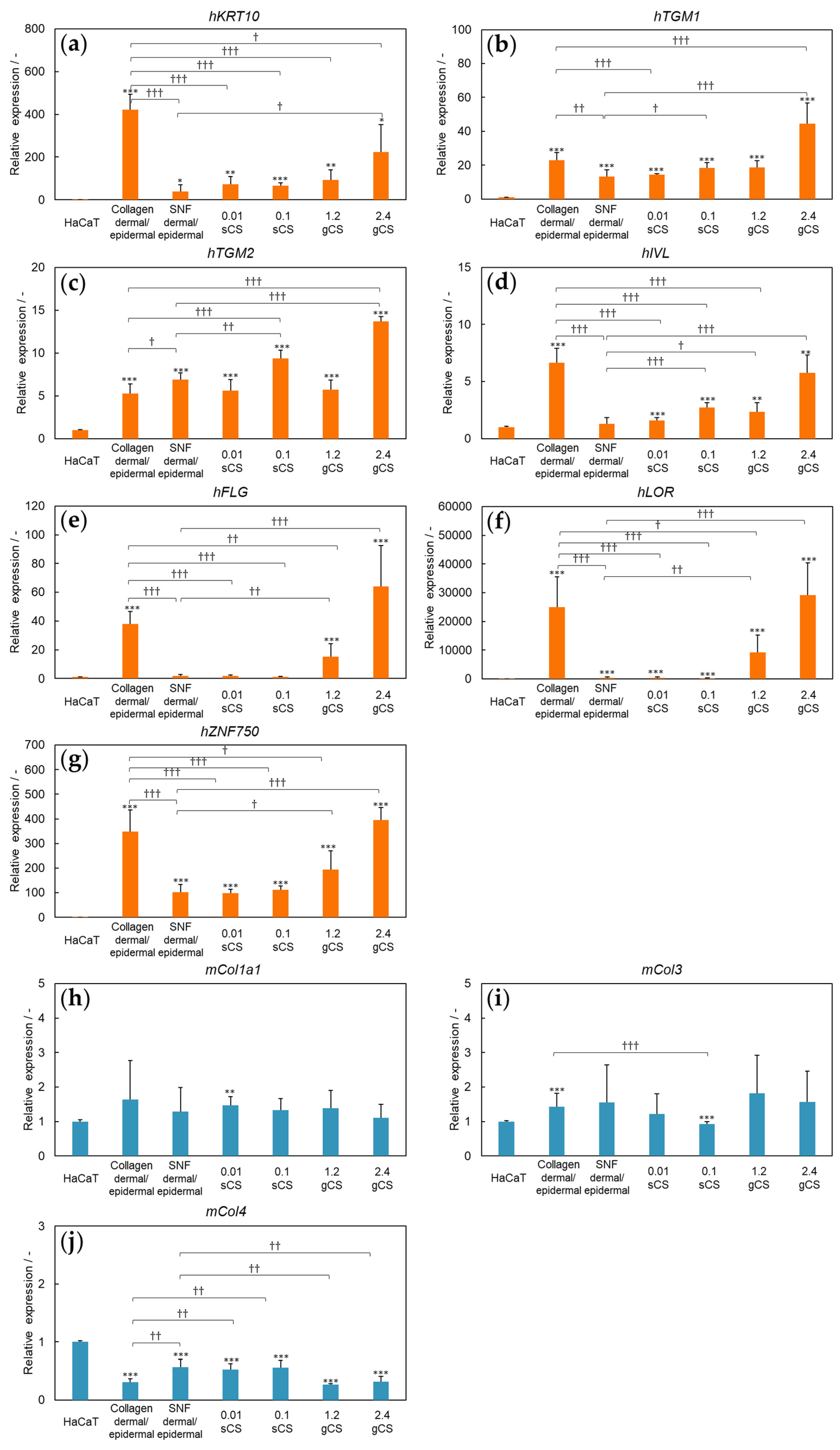
3.2. Three-Dimensional Model of SNF Skin Using Primary Cultured Cells
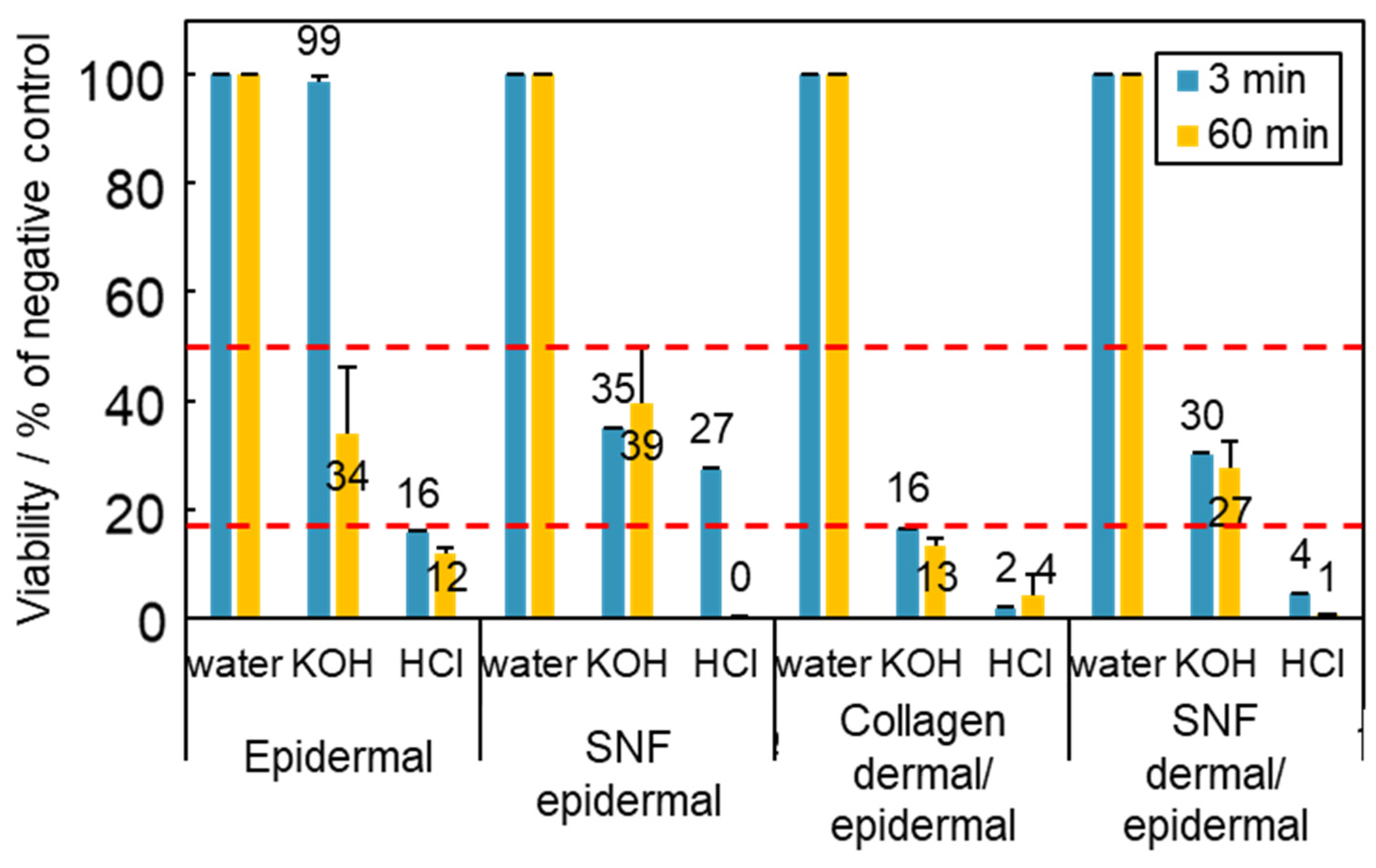
3.3. Application of Skin Models to Skin Corrosion Testing
3.4. Three-Dimensional Model of SNF and COLLAGEN Using Immortalized Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Smalley, K.S.M.; Lionic, M.; Herlyn, M. Life ins’t flat: Taking cancer biology to the next dimension. In Vitro Cell. Dev. Biol. Anim. 2006, 42, 242–247. [Google Scholar] [CrossRef]
- Mazzolen, G.; Lorenzo, D.D.; Steimberg, N. Modelling tissues in 3D: The next future of pharmaco-toxicology and food research? Genes Nutr. 2009, 4, 13–22. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications. J. Biomed. Mater. Res. A 2016, 104, 1002–1016. [Google Scholar] [CrossRef]
- Cheung, H.; Lau, K.; Lu, T.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. Part B 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef]
- Coulomb, B.; Lebreton, C.; Dubertret, L. Influence of Human Dermal Fibroblasts on Epidermalization. J. Investig. Dermatol. 1989, 92, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Muta, K.; Matsunaga, Y.; Hirao, T.; Amano, S. In Vitro Reconstruction of 3-D Elastic Fiber in a Novel Dermal Equivalent. J. Soc. Cosmet. Chem. Jpn. 2010, 44, 278–284. [Google Scholar] [CrossRef]
- Ponec, M. Skin constructs for replacement of skin tissues for in vitro testing. Adv. Drug Deliv. Rev. 2002, 54, 19–30. [Google Scholar] [CrossRef]
- Heisenberg, C.P.; Bellaiche, Y. Forces in tissue morphogenesis and patterning. Cell 2013, 153, 948–962. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lak ins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Anlas, A.A.; Nelson, C.M. Tissue mechanics regulates form, function, and dysfunction. Curr. Opin. Cell Biol. 2018, 54, 98–105. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Gandhi, M.R.; Ko, F.K.; Weiss, A.S.; Lelkes, P.I. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 2005, 26, 5999–6008. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. Engineered human skin fabricated using electrospun collagen-PCL blends: Morphogenesis and mechanical properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.S.; Choi, W.; Jeon, J.; Wona, J.; Sung, G.Y.; Kim, B.; Sung, J.H. In vitro 3D skin model using gelatin methacrylate hydrogel. J. Ind. Eng. Chem. Res. 2018, 66, 254–261. [Google Scholar] [CrossRef]
- Kimura, S.; Tsuchiya, A.; Ogawa, M.; Ono, M.; Suda, N.; Sekimoto, K.; Takeo, M.; Tsuji, T. Tissue-scale tensional homeostasis in skin regulates structure and physiological function. Commun. Biol. 2020, 3, 637. [Google Scholar] [CrossRef]
- Kawakami, K.; Yoshida, S. Thermal Stabilization of Lipase by Sol-Gel Entrapment in Organically Modified Silicate Formed on Kiselguhr. J. Ferment. Bioeng. 1996, 82, 239–245. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakai, S.; Kawakami, K. Application of silicate electrospun nanofibers for cell culture. J. Sol-Gel Sci. Technol. 2008, 48, 350–355. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakai, S.; Watanabe, R.; Tarao, T.; Kawakami, K. Heat Treatment of Electrospun Silicate Fiber Substrates Enhances Cellular Adhesion and Proliferation. J. Biosci. Bioengin. 2010, 109, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Ahola, M.S.; Säilynoja, E.S.; Raitavuo, M.H.; Vaahtio, M.M.; Salonen, J.I.; Yli-Urpo, A.U. In vitro release of heparin from silica xerogels. Biomaterials 2001, 22, 2163–2170. [Google Scholar] [CrossRef]
- Xue, M.; Yang, S.; Chen, Y.; Yang, L.; Zhao, F.; Ding, B.; Yu, J. Silica nanofibrous membranes with robust flexibility and thermal stability for high-efficiency fine particulate filtration. RSC Adv. 2012, 2, 12216–12223. [Google Scholar] [CrossRef]
- Otsuka, H.; Sasaki, K.; Okimura, S.; Nagamura, M.; Watanabe, R.; Kawabe, M. Contribution of fibroblasts cultured on 3D silica nonwoven fabrics to cocultured hepatocytes function. Chem. Lett. 2014, 43, 343–345. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Rubenstein, D.; Han, D.; Goldgraben, S.; Elgendi, H.; Gouma, P.I.; Frame, M.D. Bioassay Chamber for Angiogenesis with Perfused Explanted Arteries and Electrospun Scaffolding. Microcirculation 2007, 14, 723–737. [Google Scholar] [CrossRef]
- Ikari, R.; Mukaisho, K.; Kageyama, S.; Nagasawa, M.; Kubota, S.; Nakayama, T.; Murakami, S.; Taniura, N.; Tanaka, H.; Kushima, R.P.; et al. Differences in the Central Energy Metabolism of Cancer Cells between Conventional 2D and Novel 3D Culture Systems. Int. J. Mol. Sci. 2021, 22, 1805. [Google Scholar] [CrossRef]
- Ishikawa, S.; Iijima, K.; Sasaki, K.; Kawabe, M.; Osawa, S.; Otsuka, H. Silica-Based Nonwoven Fiber Fabricated by Electrospinning to Promote Fibroblast Functions. Bull. Chem. Soc. Jpn. 2020, 93, 477–481. [Google Scholar] [CrossRef]
- Ishikawa, S.; Iijima, K.; Sasaki, K.; Kawabe, M.; Otsuka, H. Improvement of Hepatic Functions by Spheroids Coculture with Fibroblasts in 3D Silica Nonwoven Fabrics. J. Nanosci. Nanotechnol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Ishikawa, S.; Sasaki, K.; Hashizume, M.; Kawabe, M.; Otsuka, H. Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Electrospun Silica Nonwoven Fabrics. ACS Omega 2018, 3, 10180–10187. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Iijima, K.; Sasaki, K.; Hashizume, M.; Kawabe, M.; Otsuka, H. Cartilage Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Three-Dimensional Silica Nonwoven Fabrics. Appl. Sci. 2018, 8, 1398. [Google Scholar] [CrossRef]
- Schoop, V.M.; Mirancea, N.; Fusenig, N.E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; Arcangelis, A.D.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- OECD. Test No. 431: In Vitro Skin Corrosion (Human Skin Model Test); OECD: Paris, France, 2014. [Google Scholar]
- Strudwick, X.L.; Lang, D.L.; Smith, L.E.; Cowin, A.J. Combination of Low Calcium with Y-27632 Rock Inhibitor Increases the Proliferative Capacity, Expansion Potential and Lifespan of Primary Human Keratinocytes while Retaining Their Capacity to Differentiate into Stratified Epidermis in a 3D Skin Model. PLoS ONE 2014, 10, e0123651. [Google Scholar] [CrossRef]
- Song, S.; Raja, I.; Lee, Y.; Kang, M.; Seo, H.; Lee, H.; Han, D. Comparison of cytotoxicity of black phosphorus nanosheets in different types of fibroblasts. Biomater. Res. 2019, 23, 1–7. [Google Scholar] [CrossRef]
- Chermnykh, E.S.; Alpeeva, E.V.; Vorotelyak, E.A. Transglutaminase 3: The Involvement in Epithelial Differentiation and Cancer. Cells 2020, 9, 1996. [Google Scholar] [CrossRef]
- Choi, E.; Kang, Y.G.; Hwang, S.H.; Kim, J.K.; Hong, Y.D.; Park, W.S.; Kim, D.; Kim, E.; Cho, J.Y. In Vitro Effects of Dehydrotrametenolic Acid on Skin Barrier Function. Molecules 2019, 24, 4583. [Google Scholar] [CrossRef]
- Scholz, G.M.; Sulaiman, N.S.; Baiiaty, S.A.; Kwa, M.Q.; Reynolds, E.C. A novel regulatory relationship between RIPK4 and ELF3 in keratinocytes. Cell. Signal. 2016, 28, 1916–1922. [Google Scholar] [CrossRef]
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)—A wound response enzyme. Front. Biosci. 2006, 11, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, D.; Paulsson, M. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. J. Biol. Chem. 1991, 266, 15308–15317. [Google Scholar] [CrossRef]
- Klicksa, J.; Molitora, E.; Ertongur-Fauthb, T.; Rudolf, R. In vitro skin three-dimensional models and their applications. J. Cell. Biotechnol. 2017, 3, 21–39. [Google Scholar] [CrossRef]
- Eckert, R.L.; Sturniolo, M.T.; Broome, A.; Ruse, M.; Rorke, E.A. Transglutaminase function in epidermis. J. Investig. Dermatol. 2005, 124, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Birnbaum, R.Y.; Leibson, K.; Taube, R.; Sivan, S.; Birk, O.S. ZNF750 Is Expressed in Differentiated Keratinocytes and Regulates Epidermal Late Differentiation Genes. PLoS ONE 2012, 7, e42628. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Ohto-Fujita, E.; Shimizu, M.; Sano, S.; Kurimoto, M.; Yamazawa, K.; Atomi, T.; Sakurai, T.; Murakami, Y.; Takami, T.; Murakami, T.; et al. Solubilized eggshell membrane supplies a type III collagen-rich elastic dermal papilla. Cell Tissue Res. 2019, 376, 123–135. [Google Scholar] [CrossRef]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Petrova, M.M.; Gavrilyuk, O.A.; Karpova, E.I.; Trefilova, V.V.; Demina, O.M.; Popova, T.E.; Shnayder, N.A. Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning. Cosmetics 2021, 8, 92. [Google Scholar] [CrossRef]
- Varkey, M.; Ding, J.; Tredget, E.E. Superficial dermal fibroblasts enhance basement membrane and epidermal barrier formation in tissue-engineered skin: Implications for treatment of skin basement membrane disorders. Tissue Eng. Part A 2014, 20, 540–552. [Google Scholar] [CrossRef]
- el-Ghalbzouri, A.; Gibbs, S.; Lamme, E.; Van Blitterswijk, C.A.; Ponec, M. Effect of fibroblasts on epidermal regeneration. Br. J. Dermatol. 2002, 147, 230–243. [Google Scholar] [CrossRef]
- Seo, M.; Kang, T.; Lee, C.; Lee, A.; Noh, M. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol. Ther. 2012, 20, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Straley, K.S.; Heilshorn, S.C. Design and adsorption of modular engineered proteins to prepare customized, neuron-compatible coatings. Front. Neuroeng. 2009, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spargo, B.J.; Testoff, M.A.; Nielsen, T.B.; Stenger, D.A.; Hickman, J.J.; Rudolph, A.S. Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc. Natd. Acad. Sci. USA 1994, 91, 11070–11074. [Google Scholar] [CrossRef]
- Fujisaki, H.; Futaki, S.; Yamada, M.; Sekiguchi, K.; Hayashi, T.; Ikejima, T.; Hattori, S. Respective optimal calcium concentrations for proliferation on type I collagen fibrils in two keratinocyte line cells, HaCaT and FEPE1L-8. Regen. Ther. 2018, 8, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Küttner, V.; Mack, C.; Gretzmeier, C.; Bruckner-Tuderman, L.; Dengjel, J. Loss of collagen VII is associated with reduced transglutaminase 2 abundance and activity. J. Investig. Dermatol. 2014, 134, 2381–2389. [Google Scholar] [CrossRef]
- Regl, G.; Kasper, M.; Schnidar, H.; Eichberger, T.; Neill, G.; Ikram, M.; Quinn, A.; Philpott, M.; Frischauf, A.; Aberger, F. The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene 2004, 23, 1263–1274. [Google Scholar] [CrossRef]
- Bause, A.; Matsui, M.; Haigis, M. The Protein Deacetylase SIRT3 Prevents Oxidative Stress-induced Keratinocyte Differentiation. J. Biol. Chem. 2013, 288, 36484–36491. [Google Scholar] [CrossRef]
- Fontana, R.; Raccosta, L.; Rovati, L.; Steffensen, K.R.; Paniccia, A.; Jakobsson, T.; Melloni, G.; Bandiera, A.; Mangili, G.; Bergamini, A.; et al. Nuclear receptor ligands induce TREM-1 expression on dendritic cells: Analysis of their role in tumors. Oncoimmunology 2019, 8, 1554967. [Google Scholar] [CrossRef]
- Cai, P.; Otten, A.B.C.; Cheng, B.; Ishii, M.A.; Zhang, W.; Huang, B.; Qu, K.; Sun, B.K. A genome-wide long noncoding RNA CRISPRi screen identifies PRANCR as a novel regulator of epidermal homeostasis. Genome Res. 2020, 30, 22–34. [Google Scholar] [CrossRef]
- Wang, W.; Yu, X.; Wu, C.; Jin, H. IL-36γ inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int. J. Med. Sci. 2017, 14, 1002–1007. [Google Scholar] [CrossRef]
- Park, C.; Min, S.; Yu, H.; Kim, K.; Kim, S.; Lee, H.; Kim, J.; Park, Y. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef] [PubMed]
- Nambara, S.; Masuda, T.; Tobo, T.; Kidogami, S.; Komatsu, H.; Sugimachi, K.; Saeki, H.; Oki, E.; Maehara, Y. Clinical significance of ZNF750 gene expression, a novel tumor suppressor gene, in esophageal squamous cell carcinoma. Oncology 2017, 14, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jiang, X.; Zhou, Y.; Dai, Y. Effects of A2BR on the biological behavior of mouse renal fibroblasts during hypoxia. Mol. Med. Rep. 2015, 11, 4397–4402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pickard, A.; Adamson, A.; Lu, Y.; Chang, J.; Garva, R.; Hodson, N.; Kadler, K.E. Collagen assembly and turnover imaged with a CRISPR-Cas9 engineered Dendra2 tag. bioRxiv 2018, 331496. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.; Cai, Y.; Wang, W.; Jiang, J.X.; Varga, Z.V.; Feng, D.; Pacher, P.; Kunos, G.; Torok, N.J.; et al. Neutrophil–Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 399–413. [Google Scholar] [CrossRef]
- Rachmawati, H.; Novel, M.; Nisa, R.M.; Berlian, G.; Tandrasasmita, O.M.; Rahma, A.; Riani, C.; Tjandrawinata, R.R. Co-delivery of curcumin-loaded nanoemulsion and Phaleria macrocarpa extract to NIH 3T3 cell for antifibrosis. J. Drug. Deliv. Sci. Technol. 2017, 39, 123–130. [Google Scholar] [CrossRef]
- François, A.; Chatelus, E.; Wachsmann, D.; Sibilia, J.; Bahram, S.; Alsaleh, G.; Gottenberg, J. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res. Ther. 2013, 15, R168. [Google Scholar] [CrossRef]
- Wanga, T.; Sunb, J.; Huanga, Y.; Wua, H.; Chend, L.; Lin, F. Skin basement membrane and extracellular matrix proteins characterization and quantification by real time RT-PCR. Biomaterials 2006, 27, 5059–5068. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iijima, M.; Iijima, K. Fabricating a Novel Three-Dimensional Skin Model Using Silica Nonwoven Fabrics (SNF). Appl. Sci. 2022, 12, 6537. https://doi.org/10.3390/app12136537
Iijima M, Iijima K. Fabricating a Novel Three-Dimensional Skin Model Using Silica Nonwoven Fabrics (SNF). Applied Sciences. 2022; 12(13):6537. https://doi.org/10.3390/app12136537
Chicago/Turabian StyleIijima, Mizuki, and Kazutoshi Iijima. 2022. "Fabricating a Novel Three-Dimensional Skin Model Using Silica Nonwoven Fabrics (SNF)" Applied Sciences 12, no. 13: 6537. https://doi.org/10.3390/app12136537
APA StyleIijima, M., & Iijima, K. (2022). Fabricating a Novel Three-Dimensional Skin Model Using Silica Nonwoven Fabrics (SNF). Applied Sciences, 12(13), 6537. https://doi.org/10.3390/app12136537





