Abstract
Liquid dairy manure, which is produced in enormous quantities in flush dairy manure management systems, is commonly used as an alternative to chemical fertilizers. It provides nutrient benefits to crops and soils. While dairy waste is a well-accepted and widely used fertilizer, the presence of indicator organisms and human pathogens in manure may lead to pathogen contamination in crops and soils. This study is focused on the examination of ozone gas-based sterilization. In the past, ozone (O3) has been used for sanitizing various foods and solid surfaces, but the potential of O3 for eliminating human pathogens in liquid dairy waste is not studied yet. Pathogens such as Salmonella Typhimurium and Escherichia coli O157:H7 are reported to be present in liquid dairy manure, and this research evaluated the effects of various levels of ozone on the survival of these two pathogens. We designed a continuous type O3 treatment system that has four major components: (1) ozone generator using oxygen; (2) ozone concentration control by mixing with pure air; (3) continuous monitoring of ozone concentrations; and (4) ozone experiment chambers. Various levels of ozone (43.26, 87.40, and 132.46 mg·L−1) were produced in the ozone system, and subsequently, ozone was diffused through liquid manure. Liquid manure was exposed to ozone for multiple durations (30, 60, and 120 min). To determine the effectiveness of O3 in eliminating pathogens, time-series samples were collected and analyzed for determining the levels of S. typhimurium and E. coli O157:H7. Preliminary results showed that ozone concentrations of 132.46 mg/L, and exposure time of 120 min resulted in the reduced levels of E. coli and Salmonella. Low levels of ozone and limited exposure time were found to be less effective in pathogen removal potentially due to high solid contents. Additional studies carrying out experiments to evaluate the impacts of solids in combination with ozone concentrations will provide further insights into developing full-scale ozone-based treatment systems.
1. Introduction
In general, livestock manure provides valuable nutrients such as Nitrogen, Carbon, Phosphorous, Potassium, and micronutrients required for plant growth [1,2]. Previous studies showed that manure contains high levels of bioavailable carbon and nitrogen, and the application of manure in cropland can help in replenishing the soil nutrients [3,4,5,6]. While animal manure provides nutrients, animals such as dairy cattle are also known to shed pathogens, which is a concern and poses risks to animal and public health. Livestock waste-borne pathogens can be transmitted to the soil, and subsequently, these pathogens may be transported to surface and ground water through runoff and leachate causing contamination [7]. The results of these previous studies showed that the significant numbers of zoonotic pathogens (E. coli, Salmonella, Listeria monocytogenes, Campylobacter, Cryptosporidium parvum, Giardia intestinalis) are produced in livestock systems, which has the potential to contaminate water and soil [8,9]. Both fresh and stored animal waste (cattle, pig, poultry, and sheep) were found to contain various types of pathogens, which poses risks to food safety and fresh produce [7,10,11].
Over the past decades, the farm sizes have increased [12,13] and with the increasing size of farms, there is a large amount of animal waste in a confined area, which requires treatments and disposal. Because of public health concerns, and increasing emphasis on improving water quality, environment, and food safety, recently particular attention has been given to controlling pathogens from animal waste (i.e., dairy manure, swine manure, and poultry litter) [14,15]. To control public and animal health risks posed by animal waste, there is a requirement for effective disinfection tools and treatment methods, which can reduce the pathogen loads [16,17] in the environment. This will reduce the risk of zoonotic disease transmission [18,19]. Based on current guidelines, at least a 5-log10 pathogen reduction in animal waste is suggested to improve food, farm biosecurity, and control the spread of infectious diseases caused by zoonotic pathogens [20,21,22].
There are various treatment methods such as composting and anaerobic digestion, which are used for treating animal waste [23,24,25]. Temperature-based treatment methods, such as composting, which produces high temperature (>55 °C) and thermophilic anaerobic digestion, were found to be suitable for reducing pathogens in animal waste [26,27,28]. In many instances, however, pathogens such as E. coli O157:H7, Salmonella spp., and L. monocytogenes were present in composted organic waste [29,30,31,32,33] and animal-waste amended soil [34]. One possible reason for this could be that the composting process did not attain a high enough temperature for a sufficient duration [35,36]. In addition to this, cross contaminations are also known to cause pathogen contamination in cross products [37,38].
Because of pathogen survival issues in existing waste treatment methods, there is a growing demand to identify new technologies or improved methods capable of removing pathogens from waste such as dairy manure. Ozone treatment could be an option [39,40]; however, further studies are needed prior to its widespread application in treating dairy waste. Ozone has been used to sanitize a number of products including food products and ozone treatment has been studied in various food and waste treatment industries. Ozone is used for treating sewage and killing harmful bacteria [41,42,43]. Application of ozone treatment for wastewater treatment at a pilot scale showed significant removal of contaminants such as pharmaceuticals, nitrate, and micropollutants [44,45,46]. Ozone treatment is considered a cost-effective, and eco-friendly method for the removal of various contaminations including mycotoxins in grain products [47], pharmaceutical removal from water [48], and contaminants removal in food products [49].
The triatomic form of oxygen (O3) [ozone] is detrimental to pathogens and bacteria in food products because of the strong oxidation property of ozone. Ozone is considered to be highly effective against bacteria in many products, even with a low concentration of ozone (0.01 ppm). Further, ozone treatment is an approved food additive by the U.S. Food and Drug Administration (US FDA), which facilitates its use for treating food products for the removal of bacteria, viruses, and protozoa [7,39,50,51].
Ozone reduces microbial populations by 2–4 orders of magnitude and ozone treatment increases the shelf life of fruits and vegetables [22]. It has been found that E. coli would be more readily killed by ozone in juices used for human consumption. Pathogens reductions in wastewater have been widely reported when wastewater was exposed to ozone [42]. Further, the effects of ozone on reducing the populations of Salmonella, E. coli O157:H7, and L. monocytogenes on the surface of the lettuce, spinach, and green onion were studied and results showed that pathogens populations were reduced substantially [52].
During ozone treatment, the survival of pathogen depends on the O3 concentration and duration of exposure [53,54]. In addition to time and concentrations, the effect of O3 in pathogen reduction is influenced by the physicochemical characteristics of the material (i.e., water content, pH, microbial load, and organic matter content) [22,53,55,56,57]. Pathogens such as E. coli and Salmonella typhimurium in food products were reduced substantially after exposure to O3 [51,55]. The goal of this study was to understand the impacts of ozone on pathogen removal in dairy wastewater. Specific objectives of this study were: (1) to investigate the impacts of ozone concentrations and exposure time on E. coli O157:H7 reductions; (2) to determine the impacts of ozone S. Typhimurium reductions; and (3) to understand the impacts of ozone on the degradation of organic content of manure.
2. Materials and Methods
2.1. Bacterial Strains and Inoculum
In this study, we did use two pathogen strains: (1) S. typhimurium LT2 ATCC 700720, and (2) E. coli O157:H7 ATCC 35150. These pathogens were grown in Luria Bertani (LB) broth (Becton, Dickinson and Company, Sparks, MD, USA) with shaking (250 rpm) at 37 °C for 24 h prior to starting each experiment. Inoculums of these pathogens were prepared using the fresh pathogen cultures of E. coli O157:H7 (ATCC #35150), and S. typhimurium LT2 (ATCC #700720). The fresh cultures were prepared in the lab prior to starting the experiment. Difco LB Broth Miller (Luriae Bertani) growth media was used for growing E. coli O157:H7 and S. typhimurium LT2. The overnight growth of these pathogens in respective media was used to inoculate feedstock. The pathogen inoculated mixed feedstock was used for testing the pathogen levels in feedstock. Once the experiment started, ozonated samples were collected at regular intervals, and analyzed within 24 h using a petri dish with selective agar media. Samples for E. coli O157:H7, and S. Typhimurium were streaked (i.e., plated) on MacConkey II Agar (Becton, Dickinson and Company, Sparks, MD, USA), and Difco XLD Agar (Becton, Dickinson and Company, Sparks, MD, USA), respectively. All ozonation experiments were conducted at room temperature.
2.2. Dairy Manure Characteristics
Liquid dairy wastewater used in this study was collected from three dairy farms. These dairy farms were located in the Central Valley of California. The average number of cows on these farms is around 2000. Prior to starting the experiment, manure from three dairy farms was mixed to form homogenized manure streams representative of manure produced in the flush manure system in California. All these three dairy farms follow similar animal waste management practices (i.e., flush manure management system and mechanical liquid-solid separation). Each of these three farms has around 2000 dairy herd sizes and uses a flush system. Further, each of the three dairy farms have two lagoons and uses a liquid-solid separation system for separating the liquid and solid. The nitrate analysis of manure showed nitrate concentrations between 330 and 400 ppm, and the pH was 6.5–7.3. In manure, total solid (TS) was 1–2%, and volatile solid (VS) was 20–25% of TS. During the experiment, we formed two types of dairy wastewater for preparing feedstock for treatment: (1) raw liquid manure (no pretreatment); and (2) sterilized raw manure (pretreatment) followed by pathogen inoculation. Sterilization of manure feedstock was done by autoclaving raw manure for 15 min. Subsequently, pathogens were inoculated prior to the ozone treatments. Experiments with post-sterilization-inoculated pathogens (i.e., pretreated manure) were done to compare the impacts of ozone treatment on manure with pretreatment and without pretreatment.
2.3. Ozone Experiment Setup and Samples Analysis
A bench-scale experiment shown in Figure 1 was used to test pathogen inactivation under different treatment conditions. The experiment setup has various components including an ozone generator, ozone monitor, ozone distributor, ozone concentration controller, and treatment chamber. The O3 was generated using an O3 generator (Model NANO, AbsoluteOzone®, Edmonton, AB, Canada). The concentration of O3 was controlled by controlling the inflow of ultra-pure air. Mixing of ozone with air produced different levels of ozone, and concentrations of ozone were monitored continuously using an O3 monitor (Model 106-H Ozone Monitor™, 2B Technologies, Boulder, CO, USA). Three round bottom flasks (each flask volume of 250 mL named as a treatment chamber) with 20 mL of feedstock (i.e., liquid dairy manure) in each chamber were connected with the ozone tubes, which provided continuous and constant ozone flow to the chamber. The O3 concentration was set as 3%, 6%, and 9%, which were equal to 43.26, 87.40, and 132.46 mg·L−1, respectively. We selected this range because the ability of the ozone generator was to produce ozone concentrations within this range. The time of ozone exposure to manure was set to 0, 30, 60, and 120 min at each concentration of ozone. In the inoculated sterile manure, 1 mL per 10 mL of feedstock (1/10 dilution) of the pathogen solution (either E. coli or Salmonella) was added 12 h prior to O3 treatment and incubated for 12 h statically at 37 °C to obtain ~ 106–8 CFU/mL of each pathogen prior to treatment.
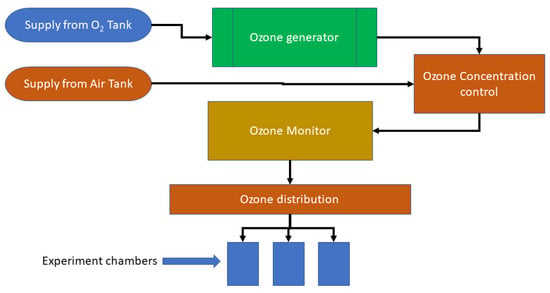
Figure 1.
Schematics of bench-scale ozone treatment system.
After the O3 treatment, 1 mL sample from the treatment chamber was collected, transferred to the centrifuge tube, and diluted 1/10 with 9 mL PBS. Subsequently, pathogens in the samples were examined by plating serially diluted samples onto specific agar plates. To enumerate S. typhimurium LT2, samples were plated on Difco XLD Agar (Becton, Dickinson, and Company) plates. To determine E. coli levels, samples were plated on MacConkey II Agar (Becton, Dickinson, and Company). The concentrations of the bacteria were reported as colony-forming units (CFU)/mL of dairy manure. The moisture of the samples was analyzed by weight loss on 102–105 °C heating. Total organic matter was determined by weight loss on ignition of dried ground samples at 450 °C.
2.4. Statistical Analysis
All experiments were done using three biologicals, in which each sample was duplicate-plated after extraction and dilution. Observations were analyzed with one-way ANOVA using the SPSS statistics 23 (IBM Institute, Armonk, NY, USA) and Duncan’s multiple range test. This analysis provided significant differences (p < 0.05) in mean values of pathogen reductions in different ozone exposure times. Bacterial counts were converted to log10 before statistical analysis.
3. Results and Discussion
3.1. Effects of O3 Concentration and Duration on E. coli O157:H7 Inactivation
Previous studies have shown that the ozone treatment is effective in pathogen reduction in wastewater or sludge [58,59,60]. The current study sought to determine the effects of O3 on the survival populations of E. coli O157:H7 in liquid dairy manure by using different O3 concentrations and exposure times (Figure 2).
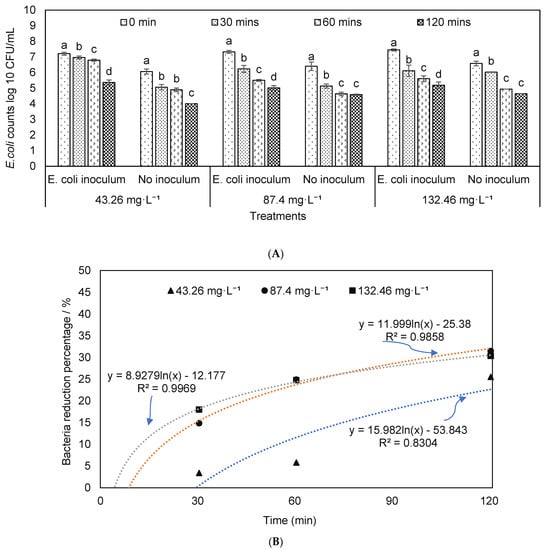
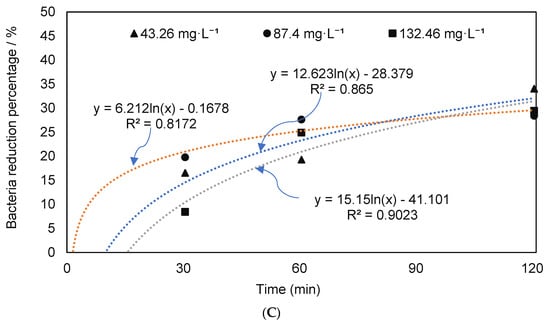
Figure 2.
Change in E. coli levels: (A) E. coli O157:H7 counts; (B,C) reduction percentages in the dairy manure with E. coli inoculation (B) or without E. coli inoculation (C) under different ozone concentrations and durations (note: the letters above columns showed the differences among different lasting times, which was the same as the next figures; means denoted by a different letter indicate significant differences between treatments (p < 0.05)).
The ozone treatment reduced the amount of E. coli O157: H7, and the reductions increased with the exposure time, particularly at 87.40 or 132.46 mg·L−1 (Figure 2). As shown in Figure 2B,C, the pathogen reduction percentages were similar regardless of the initial concentrations of pathogens. To remove the potential interference between the native microbial population and inoculated pathogens, first dairy manure was autoclaved, and then inoculated with E. coli O157: H7 prior to treatment by O3 (Figure 3). In this condition, the reduction percentages at O3 concentrations of 87.40 and 132.46 mg·L−1 were significantly higher than that of 43.26 mg·L−1. O3 is reported to eliminate bacteria by attacking the glycoproteins and glycolipids in the cell membrane, and the sulfhydryl groups of certain enzymes, therefore, the effects of ozone in complex feedstock such as manure where multiple microorganisms are present depends on the pretreatment of manure [22].
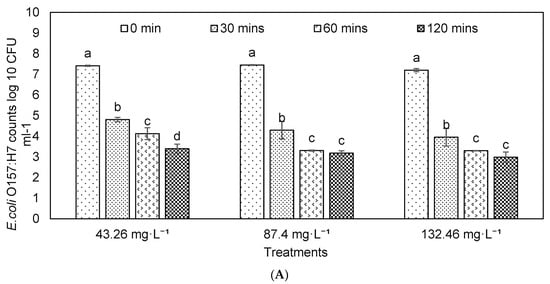
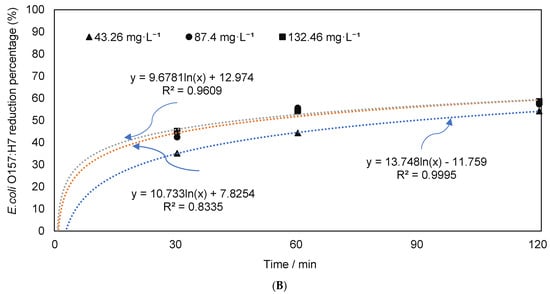
Figure 3.
Change in E. coli levels in autoclaved manure. (A) E. coli O157:H7 counts; (B) reduction percentages in the autoclaved dairy manure inoculated with E. coli O157:H7 under different ozone concentrations and durations (means denoted by a different letter indicate significant differences between treatments (p < 0.05)).
The results of this study suggest that when potential interference between the native microbial population of manure and inoculated pathogens was reduced by autoclaving the manure, the effects of O3 were more apparent. Further, pretreatment such as autoclave may have an influence on the structure of the organic contents of manure, which may have allowed increased pathogen reduction [59,60,61]. While pretreatment of manure (i.e., inoculation followed by sterilization) was more effective in pathogen removal, the application of pretreatment requires additional cost and facilities. Therefore, for manure management, the application of sterilization could be cost-prohibitive. In this study, we implemented a sterilization process to understand the impacts of ozone on mixed species versus known species, and complex waste material such as manure. To understand the full potential of pretreatment, and its cost-effectiveness in manure treatment, additional studies carrying out pilot-scale implementation are needed.
Under standard temperature and pressure conditions, the O3 concentration of 132.46 mg·L−1 could be converted to ≈61,855 ppm, which was significantly higher than the concentrations of O3 used in previous experiments to remove the pathogens in foods [22,62,63,64,65,66], and in liquid organic wastes [21]. Pathogen reductions in foods were considerably higher than those in our study. This indicates that the impacts of ozone may vary depending on the feedstocks, and a higher level of ozone concentrations may be needed for waste material. The previous study, while investigating O3 treatments for pathogen reduction, also suggest that the needed ozone concentrations depend on material types [60].
3.2. Effects of O3 on S. typhimurium Inactivation
Effects of ozone on S. typhimurium reduction were estimated (Figure 4), and results showed that more than 1–2 log reduction was achieved depending on the ozone concentrations and exposure time. The S. typhimurium reduction was compared in the dairy manure with and without autoclave (i.e., pretreatment) to understand the impacts of microbial interference in treatment. As shown in Figure 4, the bacteria counts were significantly decreased along with the exposure time. The reduction percentages in treatment with ozone concentrations of 132.46 mg·L−1 were significantly higher than that at 43.26 or 87.40 mg·L−1 (p = 0.05). Figure 5 showed Salmonella reductions in the dairy manure, which was autoclaved before O3 treatment. The reduction percentages in treatments of 87.40 and 132.46 mg·L−1 were significantly higher than that in 43.26 mg·L−1. The reduction percentages in autoclaved dairy manure were significantly higher than the reductions in the material without an autoclave, and similar findings are presented elsewhere [67]. These results showed that the effects of ozone on the removal of the studied two pathogens were similar for both species (E. coli and Salmonella), and ozone may eliminate a range of pathogenic species [68].
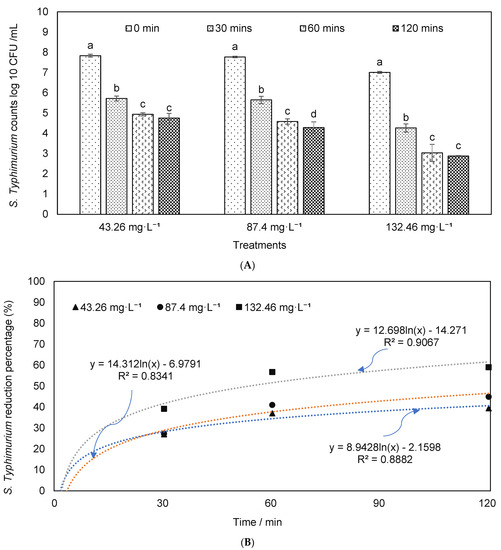
Figure 4.
S. typhimurium (A) counts and (B) reduction percentages in the dairy manure inoculated with S. typhimurium under different ozone concentrations and durations (means denoted by a different letter indicate significant differences between treatments (p < 0.05)).
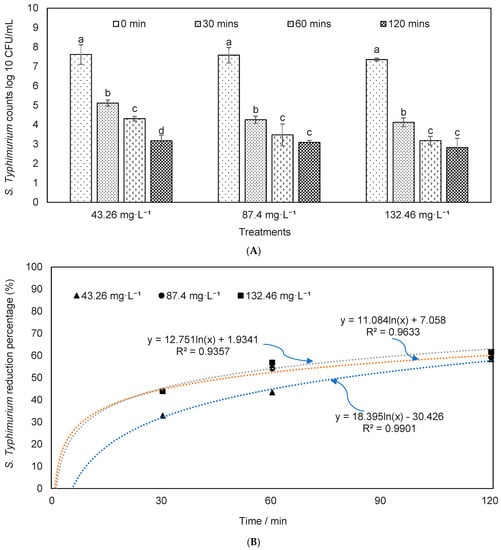
Figure 5.
S. typhimurium (A) counts and (B) reduction percentages in the autoclaved dairy manure inoculated with S. typhimurium under different ozone concentrations and durations (means denoted by a different letter indicate significant differences between treatments (p < 0.05)).
In previous studies, pathogen removal through ozone treatments has been demonstrated on various surface types and bacteria. As an example, ozone treatments were used on personal protective equipment (PPE) to study coronavirus 2 (SARS-CoV-2) and ozone impacts on it. To generate O3 concentrations a medical ozone generator that produced higher ozone concentrations at 500 and 40,000 ppm (1–80 g/m3) was used and a special ozone chamber was designed that produced low concentrations of ozone 8–12 ppm (0.016–0.024 g/m3). While at high concentrations, there was a virucidal effect after a few minutes of exposure, the effects of ozone were low at low concentrations. Further, the virucidal effects of ozone treatment on SARS-CoV-2 were also dependent on humidity levels [69]. Other studies displayed reductions of Escherichia coli O157:H7 and Salmonella in apple cider and orange juice at thermophilic conditions. E. coli and Salmonella populations were reduced below the detection limit dependent on temperature conditions. In apple cider, impacts of ozone were greater in E. coli and Salmonella reductions at 50 °C than at 4 °C (p < 0.05). In orange juice, however, the effectiveness of E. coli and Salmonella reductions between 4 °C and ambient temperature did not differ substantially. These results were statistically insignificant (p > 0.05). Also, the type of juice (apple cider or orange juice) did not alter the results [70].
In surfaces such as wheat, ozone levels (0–20 µg m = 0–120 ppb v/v 7 h per day)−3 degraded the leaf pigments prematurely [71]. Specifically, in medium, hard wheat, Triticum aestivum L., ozone gas levels at 5 mg/L at 3.3 L/min in time intervals of 0, 0.5, 1.0, 1.5, and 2.0 h were influential in the degradation of the wheat endosperm starch granule but not against the protein or fat contents in the wheat (p < 0.05) [72].
In foods, ozone treatments of 0.35 ppm for 3 days at 2 °C were partially effective in preventing fungal growth in strawberries [73] and reduced E. coli and Listeria spp. in spinach when it came in contact with ozone exposure of 1 ppm for 10 min and 10 ppm for 20 min [64].
Other studies have shown that ozone treatments were efficacious against multiple pathogenic bacterial strains such as Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. The concentrations of the O3 treatments were split into separate groups: an ozone-oxygen mixture group containing 20 μg of O3/mL for 5 min, 100% oxygen group for 5 min, and a baseline group where no gas was used. As a result, the ozone-oxygen mixture stopped all the bacterial strains present [74]. Ozone treatments have also been proved to reduce antibiotic resistance genes and pathogenic bacteria in wastewater [73,74,75,76,77].
Using 1 g of ozone/g, the ozonation reduced the micro-pollutants like antibiotic drugs or biocides and effectively removed approximately 2 log units of the micro-pollutants [40]. Previous studies have tested the effectiveness of a domestic ozone generator in reducing bacterial and fungal species of E coli, Proteus sp., Pseudomonas aeruginosa, Serratia sp., three strains of Staphylococcus aureus, Candida albicans, and Aspergillus fumigatus using ozone concentrations of 0.3 to 0.9 ppm for 4 h. In these conditions, there was a bactericidal effect in all of the species except Candida albicans which showed more resistance. In other experiments that dealt with 0.9 ppm ozone exposure for 0–4 h, bacteria was most rapidly reduced in S. aureus within the first few hours until the bactericidal effect rate was slowed significantly. At 1 ppm, ozone effectively reduced the bacterial numbers of most of the tested species by 95% [78]. Pathogen reduction by ozone treatment is likely to be affected by the type of material to be treated, its physical properties, and the mode of application. In a previous study [53], we designed an ozone chamber to expose solid poultry litter to ozone gas. Because liquid dairy manure has a higher amount of moisture, the current research needed substantial modification in previous experiments for diffusing ozone uniformly inside the liquid manure. In contrast to the liquid dairy manure treatment experiment presented here, diffusion of ozone inside of poultry litter was not needed because poultry litter moisture content was considerably low, and poultry litter was in the form of solid pellets. Published results showed that pathogen reduction was reduced when moisture level was increased [53], which indicates that the ozone impacts on pathogen removal may vary from one material to another depending on moisture level and type of material (liquid vs. solid surface). In addition to bacteria removal, ozone treatments are also proven suitable for virus removal. For example, several human pathogenic viruses in sewage were reduced to undetectable levels, when sewage was exposed to ozone treatment suggesting that ozone can be a promising technique for reducing the transmission of human viruses [21].
3.3. Effects of O3 Treatments on Organic Content Degradation
The effects of O3 in pathogen removal are influenced by the material characteristics, like pH, water temperature, free chlorine, moisture, and the organic load of the materials [56,57]. To assess the effects of O3 treatments on the organic matter of dairy manure, we evaluated moisture content and organic matter during O3 treatment. Results showed that the increase in O3 concentrations resulted in decreased organic matter contents in dairy manure (Figure 6). The results suggest that increased ozone exposure reduces the organic matter in manure. During treatment, O3 may firstly be consumed by the dissolved organic carbon of organic material, which results in high chemical oxygen demand (COD). This increased COD demand can lower pathogen reduction during ozone treatment [52]. Another study reported that aqueous O3 can directly react with the dissolved organic compounds and generate radical species such as a hydroxyl radical (•OH) that have higher oxidative potential (2.83 volts) than O3, and ozone treatment can influence available organic carbon in the feedstock [79]. The increased oxidative potential may be due to the fact that conversion of O3 to aqueous O3 resulted in a low concentration of O3 in the materials [61].
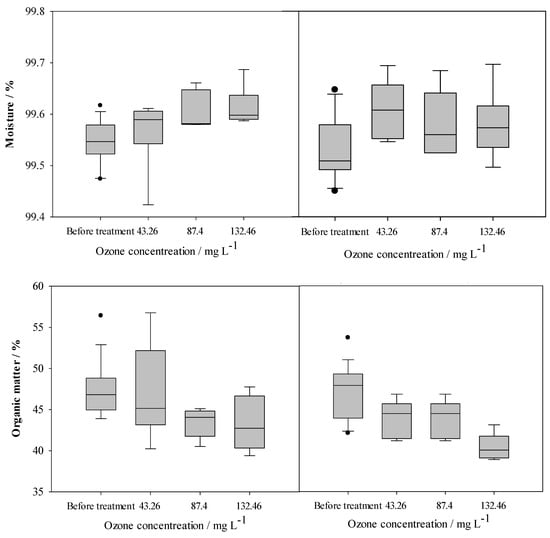
Figure 6.
The change in moisture content and organic matter of dairy manure during O3 treatment (left side figures showed moisture content and organic matter in raw manure; right side figures showed moisture and organic matter in autoclaved manure, and black dots indicate outliers).
4. Conclusions
To determine the impacts of ozone treatment on pathogen reduction in dairy manure, we executed a series of experiments at a range of exposure times and ozone concentrations. The effects of O3 concentrations and exposure times on E. coli O157:H7 and S. Typhimurium reduction in liquid dairy manure were determined. Results showed that the O3 treatment could be used as a potential option for liquid dairy manure treatment. The effects of O3 are likely to change with moisture and concentrations of ozone, therefore, the treatment time and ozone concentrations will vary with the characteristics of feedstocks. Under the tested conditions, the increase in O3 concentration and prolonged exposure time resulted in more reduction of E. coli O157:H7 and S. typhimurium.
Author Contributions
Conceptualization, P.P. (Pramod Pandey), Y.L., R.Z. and B.C.W.; Funding acquisition, P.P. (Pramod Pandey); Investigation, R.C., P.J. and P.P. (Prachi Pandey); Methodology, P.P. (Pramod Pandey); Project administration, P.P. (Pramod Pandey); Resources, B.C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the USDA National Institute of Food and Agriculture, Hatch Project. Accession Number 1019323.
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
This study did not require informed consent.
Data Availability Statement
This study did not require data availability statement.
Acknowledgments
The authors thank the Center for Food Animal Health (CFAH), University of California, Davis, and the Division of Agriculture and Natural Resources (ANR), University of California, Davis, for supporting this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Varma, V.S.; Parajuli, R.; Scott, E.; Canter, T.; Lim, T.T.; Popp, J.; Thoma, G. Dairy and swine manure management–Challenges and perspectives for sustainable treatment technology. Sci. Total Environ. 2021, 778, 146319. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, X.; Liao, Y.; Lu, Y.; Nie, J.; Cao, W. Co-incorporation of Rice Straw and Green Manure Benefits Rice Yield and Nutrient Uptake. Crop Sci. 2019, 59, 749–759. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokhrel, B.; Laursen, K.H.; Petersen, K.K. Yield, Quality, and Nutrient Concentrations of Strawberry (Fragaria ×ananassa Duch. cv. ‘Sonata’) Grown with Different Organic Fertilizer Strategies. J. Agric Food Chem. 2015, 63, 5578–5586. [Google Scholar] [CrossRef]
- Overbeek, L.S.; Wichers, J.H.; Amerongen, A.; Roermund, H.J.W.; Zouwen, P.; Willemsen, P.T.J. Circulation of Shiga Toxin-Producing Escherichia Coli Phylogenetic Group B1 Strains Between Calve Stable Manure and Pasture Land With Graz-ing Heifers. Front. Microbiol. 2020, 11, 1355. [Google Scholar] [CrossRef]
- Kumar, G.D.; Williams, R.C.; Sumner, S.S.; Eifert, J.D. Effect of ozone and ultraviolet light on Listeria monocytogenes populations in fresh and spent chill brines. Food Control 2016, 59, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Buelow, E.; Ploy, M.-C.; Dagot, C. Role of pollution on the selection of antibiotic resistance and bacterial pathogens in the environment. Curr. Opin. Microbiol. 2021, 64, 117–124. [Google Scholar] [CrossRef]
- Amato, H.K.; Wong, N.M.; Pelc, C.; Taylor, K.; Price, L.B.; Altabet, M.; Jordan, T.E.; Graham, J.P. Effects of concen-trated poultry operations and cropland manure application on antibiotic resistant Escherichia Coli and nutrient pollution in Chesapeake Bay watersheds. Sci. Total Environ. 2020, 735, 139401. [Google Scholar] [CrossRef]
- Haack, S.K.; Duris, J.W.; Kolpin, D.W.; Fogarty, L.R.; Johnson, H.E.; Gibson, K.E.; Focazio, M.; Schwab, K.J.; Hubbard, L.E.; Foreman, W.T. Genes Indicative of Zoonotic and Swine Pathogens Are Persistent in Stream Water and Sediment following a Swine Manure Spill. Appl. Environ. Microbiol. 2015, 81, 3430–3441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsbottom, G.; Läpple, D.; Pierce, K.M. Financial benchmarking on dairy farms: Exploring the relationship be-tween frequency of use and farm performance. J. Dairy Sci. 2021, 104, 3169–3180. [Google Scholar] [CrossRef]
- Nehring, R.F.; Gillespie, J.; Greene, C.; Law, J. The Economics and Productivity of Organic versus Conventional U.S. Dairy Farms. J. Agric. Appl. Econ. 2021, 53, 134–152. [Google Scholar] [CrossRef]
- Holden, N.; Wright, F.; MacKenzie, K.; Marshall, J.; Mitchell, S.; Mahajan, A.; Wheatley, R.; Daniell, T. Prevalence and diversity of Escherichia coli isolated from a barley trial supplemented with bulky organic soil amendments: Green compost and bovine slurry. Lett. Appl. Microbiol. 2014, 58, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jiang, X. The growth potential of Escherichia coli O157:H7, Salmonella spp.; Listeria monocytogenes in dairy manure-based compost in a greenhouse setting under different seasons. J. Appl. Microbiol. 2010, 109, 2095–2104. [Google Scholar] [CrossRef]
- Lin, M.; Wang, A.; Ren, L.; Qiao, W.; Wandera, S.M.; Dong, R. Challenges of pathogen inactivation in animal manure through anaerobic digestion: A short review. Bioengineered 2022, 13, 1149–1161. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The chal-lenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Rossi, G.; Smith, R.L.; Pongolini, S.; Bolzoni, L. Modelling farm-to-farm disease transmission through personnel movements: From visits to contacts, and back. Sci. Rep. 2017, 7, 2375. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.K.; Biswas, S.; Kass, P. Microbial pathogen quality criteria of rendered products. Appl. Microbiol. Biotechnol. 2016, 100, 5247–5255. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Presence and survival of Escherichia coli O157:H7 on lettuce leaves and in soil treated with contaminated compost and irrigation water. Int. J. Food Microbiol. 2012, 156, 133–140. [Google Scholar] [CrossRef]
- Wang, H.; Sikora, P.; Rutgersson, C.; Lindh, M.; Brodin, T.; Bjorlenius, B.; Larsson, D.G.J.; Norder, H. Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int. J. Hyg. Environ. Health 2018, 221, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.; Aldridge, B.; Lowe, J. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS ONE 2018, 13, e0196555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silwadi, M.; Mousa, H.; AL-Hajji, B.Y.; AL-Wahaibi, S.S.; AL-Harrasi, Z.Z. Enhancing biogas production by an-aerobic digestion of animal manure. Int. J. Green Energy 2022, 1–8. [Google Scholar] [CrossRef]
- Burch, T.R.; Firnstahl, A.D.; Spencer, S.K.; Larson, R.A.; Borchardt, M.A. Fate and seasonality of antimicrobial resistance genes during full-scale anaerobic digestion of cattle manure across seven livestock production facilities. J. Environ. Qual. 2022, 51, 352–363. [Google Scholar] [CrossRef]
- Lepesteur, M. Human and livestock pathogens and their control during composting. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1639–1683. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.W.; Nomanbhay, S.; Ho, Y.C.; Show, P.L. Transformation of biomass waste into sustainable organic fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef] [Green Version]
- Sowah, R.A.; Bradshaw, K.; Snyder, B.; Spidle, D.; Molina, M. Evaluation of the soil and water assessment tool (SWAT) for simulating E. coli concentrations at the watershed-scale. Sci. Total Environ. 2020, 746, 140669. [Google Scholar] [CrossRef]
- Zhu, L.D.; Li, Z.H.; Guo, D.B.; Huang, F.; Nugroho, Y.; Xia, K. Cultivation of Chlorella sp. with livestock waste com-post for lipid production. Bioresour. Technol. 2017, 223, 296–300. [Google Scholar] [CrossRef]
- Hutchison, M.; Walters, L.; Avery, S.; Moore, A. Decline of zoonotic agents in livestock waste and bedding heaps. J. Appl. Microbiol. 2005, 99, 354–362. [Google Scholar] [CrossRef]
- Liu, N.; Xu, L.; Han, L.; Huang, G.; Ciric, L. Microbiological safety and antibiotic resistance risks at a sustainable farm under large-scale open-air composting and composting toilet systems. J. Hazard. Mater. 2021, 401, 123391. [Google Scholar] [CrossRef]
- Gabriel, M.L.; Nicolas, B.; Will, V.; Nicholas, G.; Zoran, N.; Smukler, S.M. Greater Impacts of Incubation Temperature and Moisture on Carbon and Nitrogen Cycling in Poultry Relative to Horse Manure-based Soil Amendments. J. Environ. Qual. 2018, 47, 914–921. [Google Scholar]
- Xia, L.; Lam, S.K.; Yan, X.; Chen, D. How Does Recycling of Livestock Manure in Agroecosystems Affect Crop Productivity, Reactive Nitrogen Losses, and Soil Carbon Balance? Environ. Sci. Technol. 2017, 51, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Shepherd Jr, M.W.; Liang, P.; Jiang, X.; Doyle, M.P.; Erickson, M.C. Microbiological analysis of composts produced on South Carolina poultry farms. J. Appl. Microbiol. 2010, 108, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.; Thorn, C.; Ashekuzzaman, S.; Kavanagh, I.; Nag, R.; Bolton, D.; Cummins, E.; O’Flaherty, V.; Abram, F.; Richards, K. Landspreading with co-digested cattle slurry, with or without pasteurisation, as a mitigation strategy against pathogen, nutrient and metal contamination associated with untreated slurry. Sci. Total Environ. 2020, 744, 140841. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Derksen, T.; Biswas, S.; Nazmi, A.; Rejmanek, D.; Crossley, B.; Pandey, P.; Gallardo, R.A. Persistence of low and highly pathogenic avian influenza virus in reused poultry litter, effects of litter amendment use, and composting temperatures. J. Appl. Poult. Res. 2021, 30, 100096. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Guo, H.; Li, E.; Yan, Y. Characteristics and optimization of dairy manure composting for reuse as a dairy mattress in areas with large temperature differences. J. Clean. Prod. 2019, 232, 1053–1061. [Google Scholar] [CrossRef]
- Agga, G.E.; Couch, M.; Parekh, R.R.; Mahmoudi, F.; Appala, K.; Kasumba, J.; Loughrin, J.H.; Conte, E.D. Lagoon, Anaerobic Digestion, and Composting of Animal Manure Treatments Impact on Tetracycline Resistance Genes. Antibiotics 2022, 11, 391. [Google Scholar] [CrossRef]
- Dong, R.; Qiao, W.; Guo, J.; Sun, H. Manure treatment and recycling technologies. In Circular Economy and Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–180. [Google Scholar]
- Itzel, F.; Jewell, K.S.; Leonhardt, J.; Gehrmann, L.; Nielsen, U.; Ternes, T.A.; Schmidt, T.C.; Tuerk, J. Comprehensive analysis of antagonistic endocrine activity during ozone treatment of hospital wastewater. Sci. Total Environ. 2018, 624, 1443–1454. [Google Scholar] [CrossRef]
- Hembach, N.; Alexander, J.; Hiller, C.; Wieland, A.; Schwartz, T. Dissemination prevention of antibiotic resistant and facultative pathogenic bacteria by ultrafiltration and ozone treatment at an urban wastewater treatment plant. Sci. Rep. 2019, 9, 12843. [Google Scholar] [CrossRef] [Green Version]
- Paucar, N.E.; Kim, I.; Tanaka, H.; Sato, C. Ozone treatment process for the removal of pharmaceuticals and personal care products in wastewater. Ozone Sci. Eng. 2019, 41, 3–16. [Google Scholar] [CrossRef]
- Gomes, J.; Matos, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review. Catalysts 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Chen, Z.; Liu, H.; Lu, Y.; Li, K.; Shi, Y.; Mao, Y.; Hu, H.Y. Efficient synergistic disinfection by ozone, ultra-violet irradiation and chlorine in secondary effluents. Sci. Total Environ. 2021, 758, 143641. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Anumol, T.; Vagliasindi, F.G.; Snyder, S.A.; Roccaro, P. Comparison of the new Cl2/O3/UV process with different ozone-and UV-based AOPs for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter. Sci. Total Environ. 2021, 765, 142720. [Google Scholar] [CrossRef]
- Krakkó, D.; Illés, Á.; Licul-Kucera, V.; Dávid, B.; Dobosy, P.; Pogonyi, A.; Demeter, A.; Mihucz, V.G.; Dóbé, S.; Záray, G. Application of (V)UV/O3 technology for post-treatment of biologically treated wastewater: A pilot-scale study. Chemosphere 2021, 275, 130080. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Angelotti, B.; Brooks, M.; Dowbiggin, B.; Evans, P.J.; Devins, B.; Wang, Z.-W. A pilot-scale investigation of disinfection by-product precursors and trace organic removal mechanisms in ozone-biologically activated carbon treatment for potable reuse. Chemosphere 2018, 210, 539–549. [Google Scholar] [CrossRef]
- Zhu, F. Effect of ozone treatment on the quality of grain products. Food Chem. 2018, 264, 358–366. [Google Scholar] [CrossRef]
- de Wilt, A.; van Gijn, K.; Verhoek, T.; Vergnes, A.; Hoek, M.; Rijnaarts, H.; Langenhoff, A. Enhanced pharmaceu-tical removal from water in a three step bio-ozone-bio process. Water Res. 2018, 138, 97–105. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K.B. Ozone in the food industry: Principles of ozone treatment, mechanisms of action, and applications: An overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef]
- Zhou, Z.; Zuber, S.; Cantergiani, F.; Sampers, I.; Devlieghere, F.; Uyttendaele, M. Inactivation of foodborne pathogens and their surrogates on fresh and frozen strawberries using gaseous ozone. Front. Sustain. Food Syst. 2018, 2, 51. [Google Scholar] [CrossRef] [Green Version]
- Selma, M.V.; Ibáñez, A.M.; Allende, A.; Cantwell, M.; Suslow, T. Effect of gaseous ozone and hot water on microbial and sensory quality of cantaloupe and potential transference of Escherichia coli O157:H7 during cutting. Food Microbiol. 2008, 25, 162–168. [Google Scholar] [CrossRef]
- Liu, T.; Wang, D.; Liu, H.; Zhao, W.; Wang, W.; Shao, L. Rotating packed bed as a novel disinfection contactor for the inactivation of E. coli by ozone. Chemosphere 2018, 214, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Pandey, P.; Li, Y.; Venkitasamy, C.; Chen, Z.; Gallardo, R.; Weimer, B.; Jay-Russell, M.; Weimer, B. Assess-ment of gaseous ozone treatment on Salmonella Typhimurium and Escherichia Coli O157:H7 reductions in poultry litter. Waste Manag. 2020, 117, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Castillo, A. Reduction of Salmonella and Shiga toxin-producing Escherichia Coli on alfalfa seeds and sprouts using an ozone generating system. Int. J. Food Microbiol. 2019, 289, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yousef, A. Inactivation kinetics of foodborne spoilage and pathogenic bacteria by ozone. J. Food Sci. 2000, 65, 521–528. [Google Scholar] [CrossRef]
- Gibson, K.E.; Almeida, G.; Jones, S.; Wright, K.; Lee, J.A. Inactivation of bacteria on fresh produce by batch wash ozone sanitation. Food Control 2019, 106, 106747. [Google Scholar] [CrossRef]
- Song, W.J.; Sung, H.J.; Kang, D.H. Inactivation of Escherichia coli O157:H7 and Salmonella typhimurium in apple juices with different soluble solids content by combining ozone treatment with mild heat. J. Appl. Microbiol. 2015, 118, 112–122. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Jäger, T.; Alexander, J.; Kirchen, S.; Dötsch, A.; Wieland, A.; Hiller, C.; Schwartz, T. Live-dead discrimination analysis, qPCR assessment for opportunistic pathogens, and population analysis at ozone wastewater treatment plants. Environ. Pollut. 2018, 232, 571–579. [Google Scholar] [CrossRef]
- Wolf, C.; von Gunten, U.; Kohn, T. Kinetics of Inactivation of Waterborne Enteric Viruses by Ozone. Environ. Sci. Technol. 2018, 52, 2170–2177. [Google Scholar] [CrossRef]
- Ai, P.; Zhang, X.; Dinamarca, C.; Elsayed, M.; Yu, L.; Xi, J.; Mei, Z. Different effects of ozone and aqueous ammonia in a combined pretreatment method on rice straw and dairy manure fiber for enhancing biomethane production. Bioresour. Technol. 2019, 282, 275–284. [Google Scholar] [CrossRef]
- Akata, I.; Torlak, E.; Erci, F. Efficacy of ozone for reducing microflora and foodborne pathogens on button mush-room. Postharvest Biol. Technol. 2015, 109, 40–44. [Google Scholar] [CrossRef]
- Song, W.J.; Shin, J.Y.; Ryu, S.; Kang, D.H. Inactivation of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes in apple juice at different pH levels by gaseous ozone treatment. J. Appl. Microbiol. 2015, 119, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Maker, J.K.; Thompson, J.R.; Barnes, J.; Singleton, I. Effect of Ozone Treatment on Inactivation of Escherichia coli and Listeria sp. on Spinach. Agriculture 2015, 5, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Yesil, M.; Kasler, D.R.; Huang, E.; Yousef, A.E. Efficacy of Gaseous Ozone Application during Vacuum Cooling against Escherichia coli O157:H7 on Spinach Leaves as Influenced by Bacterium Population Size. J. Food Prot. 2017, 80, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Kalchayanand, N.; Worlie, D.; Wheeler, T. A Novel Aqueous Ozone Treatment as a Spray Chill Intervention against Escherichia Coli O157:H7 on Surfaces of Fresh Beef. J. Food Prot. 2019, 82, 1874–1878. [Google Scholar] [CrossRef]
- Lezcano, I.; Pérez Rey, R.; Baluja, C.; Sánchez, E. Ozone Inactivation of Pseudomonas aeruginosa, Escherichia Coli, Shigella sonnei and Salmonella typhimurium in Water. Ozone: Sci. Eng. 1999, 21, 293–300. [Google Scholar] [CrossRef]
- Choi, M.R.; Liu, Q.; Lee, S.Y.; Jin, J.H.; Ryu, S.; Kang, D.H. Inactivation of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes in apple juice with gaseous ozone. Food Microbiol. 2012, 32, 191–195. [Google Scholar] [CrossRef]
- Clavo, B.; Córdoba-Lanús, E.; Rodríguez-Esparragón, F.; Cazorla-Rivero, S.E.; García-Pérez, O.; Piñero, J.E.; Villar, J.; Blanco, A.; Torres-Ascensión, C.; Martín-Barrasa, J.L. Effects of Ozone Treatment on Personal Protective Equipment Contaminated with SARS-CoV-2. Antioxidants 2020, 9, 1222. [Google Scholar] [CrossRef]
- Williams, R.C.; Sumner, S.S.; Golden, D.A. Survival of Escherichia coli O157:H7 and Salmonella in apple cider and orange juice as affected by ozone and treatment temperature. J. Food Prot. 2004, 67, 2381–2386. [Google Scholar] [CrossRef]
- Tiedemann, A. Single and combined effects of nitrogen fertilization and ozone on fungal leaf diseases on wheat. J. Plant Dis. Prot. 1996, 103, 409–419. [Google Scholar]
- Mei, J.; Liu, G.; Huang, X.; Ding, W. Effects of ozone treatment on medium hard wheat (Triticum aestivum) flour quali-ty and performance in steamed bread making. J. Food 2016, 14, 449–456. [Google Scholar]
- Pérez, A.G.; Sanz, C.; Ríos, J.J.; Olías, R.; Olías, J.M. Effects of Ozone Treatment on Postharvest Strawberry Quality. J. Agric. Food Chem. 1999, 47, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Fontes, B.; Heimbecker, A.M.C.; Brito, G.D.S.; Costa, S.F.; van der Heijden, I.M.; Levin, A.S.; Rasslan, S. Effect of low-dose gaseous ozone on pathogenic bacteria. BMC Infect. Dis. 2012, 12, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, L.; Xie, Y.; Kong, X.; Li, C.; Liu, D. Investigation of reduction in risk from antibiotic resistance genes in labor-atory wastewater by using O3, ultrasound, and autoclaving. Water Environ. Res. 2021, 93, 479–486. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Wen, Q.; Fu, Q.; Bao, H. Mechanism concerning the occurrence and removal of antibiotic resistance genes in composting product with ozone post-treatment. Bioresour. Technol. 2021, 321, 124433. [Google Scholar] [CrossRef]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Dyas, A.; Boughton, B.J.; Das, B.C. Ozone killing action against bacterial and fungal species; microbiological testing of a domestic ozone generator. J. Clin. Pathol. 1981, 36, 1102–1104. [Google Scholar] [CrossRef] [Green Version]
- Glaze, W.H.; Kang, J.W. Advanced oxidation processes. Description of a kinetic model for the oxidation of hazardous materials in aqueous media with ozone and hydrogen peroxide in a semibatch reactor. Ind. Eng. Chem. Res. 1989, 28, 1573–1580. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).