Concentrations of Beetroot (Beta vulgaris L.) Peel and Flesh Extracts by Reverse Osmosis Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Betalains, Phenolics, and Antioxidants from Peel and Flesh of Beetroot
2.2. Membrane Separation
2.3. Thermo-Instability of Betalains
2.4. Analytical Method
2.4.1. Total Betalains Compound (TBC), Betacyanin (BC), and Betaxanthin (BX)
2.4.2. Color Measurement (Values of L*, a*, and b*)
2.4.3. Browning Index (BI)
2.4.4. Total Phenolic Compound
2.4.5. Ferric Reduction Antioxidant Power (FRAP) Assay
2.4.6. Miscellaneous
3. Results
3.1. Betalains, TPC, and Antioxidant Compounds Recovery by RO Membrane (X20)
3.2. Color Retention and Tonality Measurement
3.3. Thermo-Instability of Betalains (Color Compounds)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malaeb, L.; George, M.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Gabelich, C.; Ishida, K.; Bold, R. Testing of water treatment copolymers for compatibility with polyamide reverse osmosis membranes. Environ. Prog. 2005, 24, 410–416. [Google Scholar] [CrossRef]
- Alsobh, A.; Zin, M.M.; Vatai, G.; Bánvölgyi, S. The application of membrane technology in the concentration and purification of plant extracts: A review. Period. Polytech. Chem. Eng. 2022, 66, 394–408. [Google Scholar] [CrossRef]
- Wenten, I.G.; Khoiruddin. Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Garud, R.M.; Kore, S.V.; Kore, V.S.; Kulkarni, G.S.A. Short Review on Process and Applications of Reverse Osmosis. Univers. J. Environ. Res. Technol. 2011, 1, 233–238. [Google Scholar]
- Deshwal, G.K.; Akshit; Kadyan, S.; Sharma, H.; Singh, A.K.; Panjagari, N.R.; Meena, G.S. Applications of reverse osmosis in dairy processing: An Indian perspective. J. Food Sci. Technol. 2021, 58, 3676–3688. [Google Scholar] [CrossRef]
- El-Gazzar, F.E.; Marth, E.H. Ultrafiltration and Reverse Osmosis in Dairy Technology: A Review. J. Food Prot. 1991, 54, 801–809. [Google Scholar] [CrossRef]
- Matta, V.M.; Moretti, R.H.; Cabral, L.M.C. Microfiltration and reverse osmosis for clarification and concentration of acerola juice. J. Food Eng. 2004, 61, 477–482. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Falguera, V.; Torras, C.; Berdún, C.; Pagán, J.; Ibarz, A. Ultrafiltration and reverse osmosis for clarification and concentration of fruit juices at pilot plant scale. LWT-Food Sci. Technol. 2012, 46, 189–195. [Google Scholar] [CrossRef]
- Massota, A.; Mietton-Peuchota, M.; Peuchotb, C.; Milisica, V. Nanofiltration and reverse osmosis in winemaking. Desalination 2008, 231, 283–289. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zin, M.M.; Anucha, C.B.; Bánvölgyi, S. Recovery of phytochemicals via electromagnetic irradiation (Microwave-Assisted-Extraction): Betalain and phenolic compounds in perspective. Foods 2020, 9, 918. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Chauhan, D.S.; Thakur, D.; Chhikara, S.; Prabhakar, P.K. Status of beetroot processing and processed products: Thermal and emerging technologies intervention. Trends Food Sci. Technol. 2021, 114, 443–458. [Google Scholar] [CrossRef]
- Shuaibu, B.S.; Aremu, M.O.; Kalifa, U.J. Chemical composition and antioxidant activities of beetroot peel. Afr. J. Eng. Environ. Res. 2021, 2, 62–73. [Google Scholar]
- Zin, M.M.; Márki, E.; Bánvölgyi, S. Evaluation of reverse osmosis membranes in concentration of beetroot peel extract. Period. Polytech. Chem. Eng. 2020, 64, 340–348. [Google Scholar] [CrossRef]
- Zin, M.M.; Bánvölgyi, S. Portfolio of Beetroot (Beta vulgaris L.) Peel Extracts concentrated by nanofiltration membrane. Int. J. Food Stud. 2021, 10, 334–345. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoey, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Lele, S.S. Dehydration of red beet root (Beta vulgaris) by hot air drying: Process optimization and mathematical modeling. Food Sci. Biotechnol. 2011, 20, 955–964. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Bergamasco, R.D.C.; Madrona, G.S. Effect of pH on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Trends Food Sci. Technol. 2018, 38, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Ling, Y.S. Browning assessment methods and polyphenol oxidase in UV-C irradiated Berangan banana fruit. Int. Food Res. J. 2014, 21, 1667–1674. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley & Sons Ltd.: New York, NY, USA, 2017; pp. 77–106. [Google Scholar] [CrossRef]
- Hájos, T.M.; Rubóczki, T. Evaluation of mineral element content of beetroot during the different stages of the growing season. Acta Agrar. Debr. 2018, 150, 459–469. [Google Scholar] [CrossRef]
- Hussain, E.A.; Sadiq, Z.; Zia-Ul-Haq, M. Sources of Betalains. In Betalains: Biomolecular Aspects, 1st ed.; Hussain, E.A., Sadiq, Z., Zia-Ul-Haq, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 15–32. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. In Free Radical Medicine and Biology, 1st ed.; InTech: Rijeca, Croatia, 2019. [Google Scholar] [CrossRef] [Green Version]
- Couto, D.S.; Corrêa Cabral, L.M.; da Matta, V.M.; Deliza, R.; de Grandi Castro Freitas, D. Concentration of pineapple juice by reverse osmosis: Physicochemical characteristics and consumer acceptance. Food Sci. Technol. 2011, 31, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Elimelech, M. Colloidal Fouling of Reverse Osmosis Membranes: Measurements and Fouling Mechanisms. Environ. Sci. Technol. 1997, 31, 3654–3662. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Hafidi, A.; Pioch, D.; Ajana, H. Adsorptive fouling of inorganic membranes during microfiltration of vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 138–148. [Google Scholar] [CrossRef]
- Mulder, M.H.V. Membrane preparation: Phase Inversion Membranes. In Encyclopedia of Separation Science, 1st ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 3331–3346. [Google Scholar] [CrossRef]
- Mereddy, R.; Chan, A.; Fanning, K.; Nirmal, N.; Sultanbawa, Y. Betalain rich functional extract with reduced salts and nitrate content from red beetroot (Beta vulgaris L.) using membrane separation technology. Food Chem. 2017, 215, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Rodriguesa, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; Costa, S.C.; Madrona, G.S. Camu-camu bioactive compounds extraction by ecofriendly sequential processes (ultrasound assisted extraction and reverse osmosis). Ultrason. Sonochem. 2020, 64, 105017. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leavesthrough simultaneous extraction: Modelling andprocess optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Otálora, C.M.; Bonifazi, E.L.; Fissore, E.N.; Basanta, M.F.; Gerschenson, L.N. Thermal Stability of Betalains in By-Products of the Blanching and Cutting of Beta vulgaris L. Var conditiva. Pol. J. Food Nutr. Sci. 2020, 70, 15–24. [Google Scholar] [CrossRef]
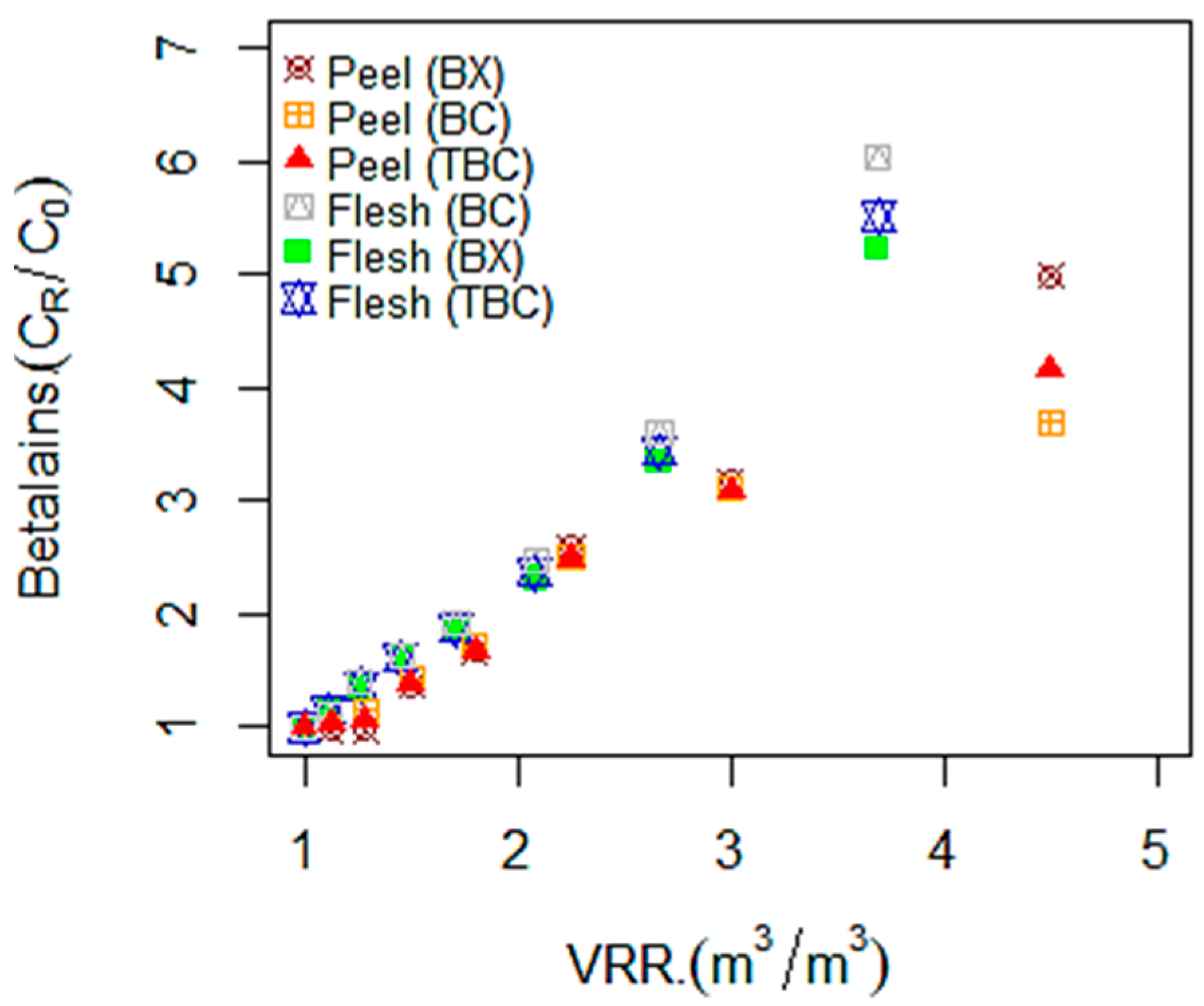
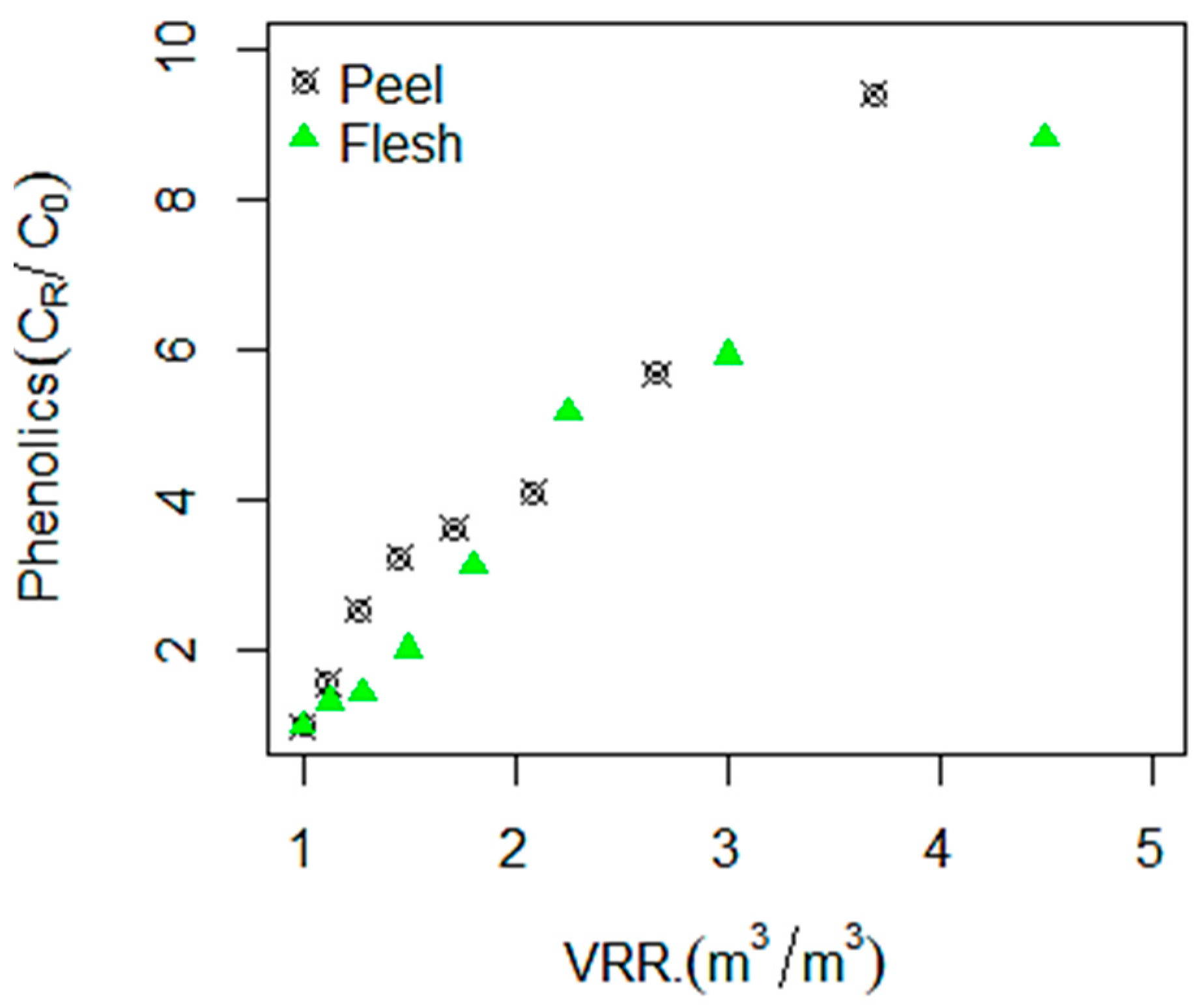
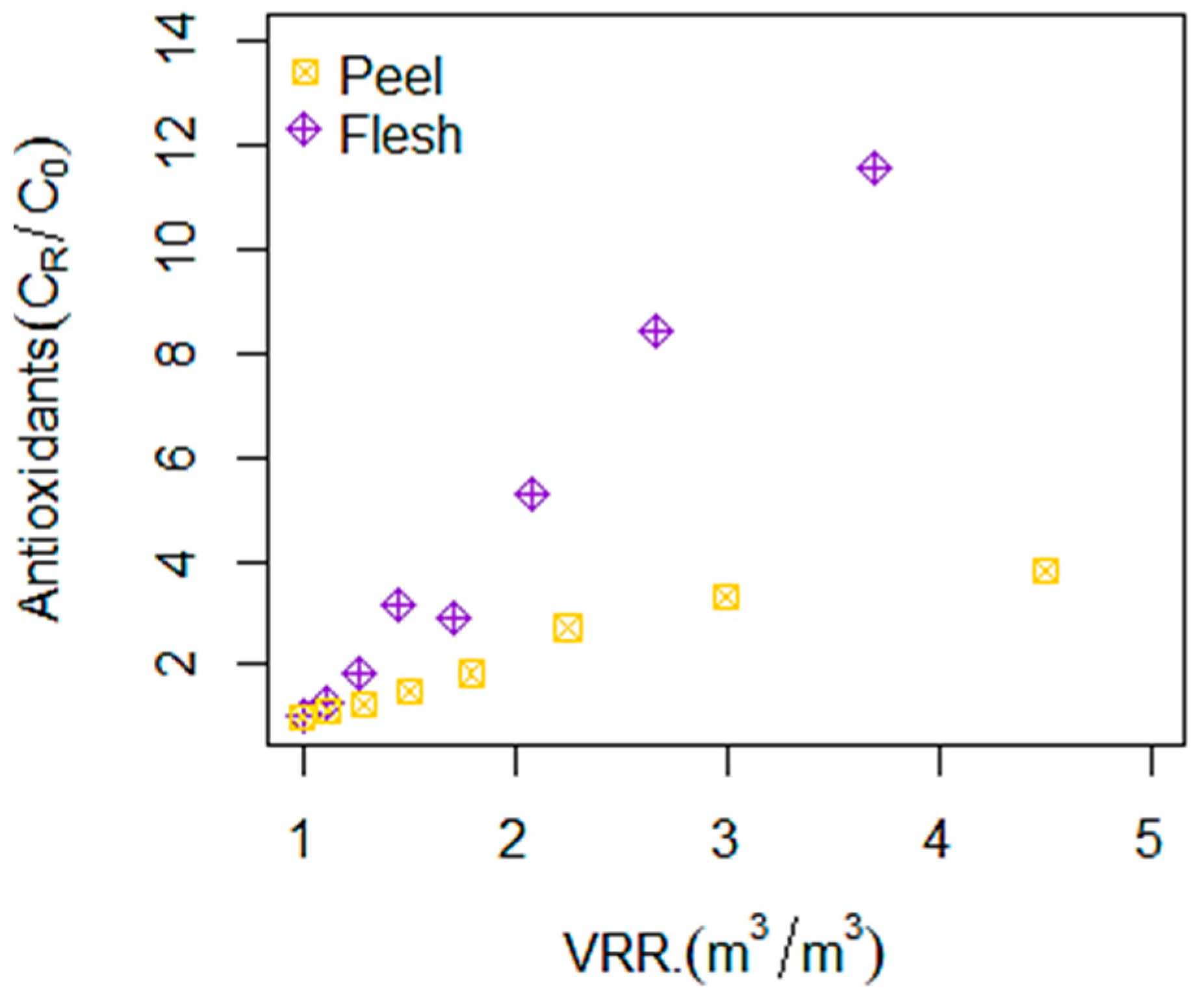

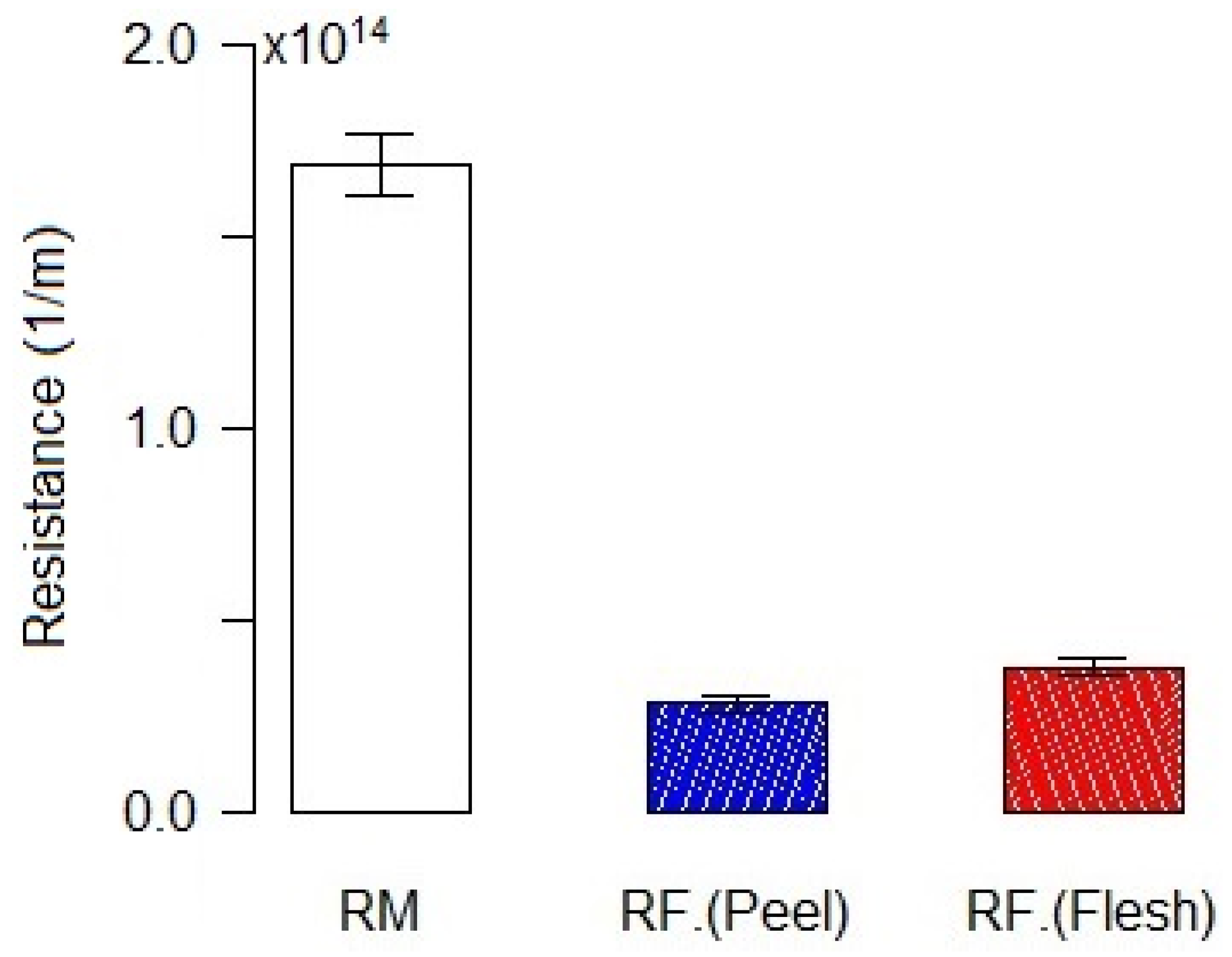


| Materials | Concentrates | BC (mg·g−1 dm) | BX (mg·g−1 dm) | TBC (mg·g−1 dm) | TPC (mg GAE·g−1 dm) | AA (mg ASE·g−1 dm) |
|---|---|---|---|---|---|---|
| Peel | Initial | 4.06 ± 0.11 | 3.02 ± 0.15 | 7.22 ± 0.06 | 3.91 ± 0.74 | 6.5 ± 0.09 |
| 500 mL | 4.45 ± 0.06 | 2.94 ± 0.04 | 7.37 ± 0.1 | 5.2 ± 0.06 | 7.2 ± 0.05 | |
| 1000 mL | 4.66 ± 0.13 | 2.97 ± 0.1 | 7.61 ± 0.22 | 5.63 ± 0.46 | 8.11 ± 0.19 | |
| 1500 mL | 5.78 ± 0.34 | 4.09 ± 0.21 | 9.86 ± 0.55 | 7.86 ± 0.41 | 9.68 ± 0.24 | |
| 2000 mL | 6.94 ± 0.1 | 5.04 ± 0.18 | 11.96 ± 0.27 | 12.26 ± 0.53 | 11.97 ± 0.28 | |
| 2500 mL | 10.15 ± 0.14 | 7.84 ± 0.11 | 17.97 ± 0.24 | 20.17 ± 1.0 | 17.7 ± 0.05 | |
| 3000 mL | 12.61 ± 0.24 | 9.61 ± 0.28 | 22.2 ± 0.42 | 23.19 ± 1.65 | 21.61 ± 0.33 | |
| Final | 15 ± 0.17 | 15.06 ± 0.13 | 30.02 ± 0.28 | 34.47 ± 0.19 | 24.65 ± 1.42 | |
| Flesh | Initial | 1.72 ± 0.03 | 0.89 ± 0.03 | 2.6 ± 0.05 | 1.35 ± 0.0 | 1.00 ± 0.04 |
| 500 mL | 2.00 ± 0.04 | 0.99 ± 0.05 | 2.98 ± 0.08 | 2.13 ± 0.0 | 1.28 ± 0.1 | |
| 1000 mL | 2.32 ± 0.01 | 1.24 ± 0.04 | 3.54 ± 0.05 | 3.45 ± 0.45 | 1.82 ± 0.11 | |
| 1500 mL | 2.74 ± 0.03 | 1.46 ± 0.02 | 4.18 ± 0.01 | 4.38 ± 0.34 | 3.16 ± 0.16 | |
| 2000 mL | 3.17 ± 0.04 | 1.71 ± 0.07 | 4.87 ± 0.11 | 4.92 ± 0.67 | 2.91 ± 0.21 | |
| 2500 mL | 3.98 ± 0.13 | 2.21 ± 0.09 | 6.17 ± 0.21 | 5.57 ± 0.17 | 5.31 ± 0.08 | |
| 3000 mL | 5.75 ± 0.14 | 3.22 ± 0.04 | 8.94 ± 0.18 | 7.7 ± 0.67 | 8.44 ± 0.58 | |
| Final | 8.98 ± 0.11 | 5.39 ± 0.04 | 14.33 ± 0.15 | 12.74 ± 0.42 | 11.6 ± 0.1 |
| Material | Sample | L* | a* | b* | Hue° | BI | ||
|---|---|---|---|---|---|---|---|---|
| Flesh | Initial β | 11.1 ± 0.67 | 35.23 ± 1.13 | 18.44 ± 1.23 | 27.61 ± 0.84 | 39.77 ± 1.57 | 60.89 ± 0.36 | 165.32 ± 2.69 |
| Final β | 10 ± 0.25 | 18.91 ± 1.18 | 14.5 ± 0.67 | 37.51 ± 0.48 | 23.83 ± 1.34 | 57.7 ± 0.24 | 119.34 ± 7.96 | |
| Peel | Initial θ | 3.53 ± 0.33 | 11.6 ± 0.78 | 4.14 ± 0.33 | 19.4 ± 0.13 | 12.04 ± 0.49 | 66.72 ± 0.38 | 163.26 ± 6.41 |
| Final θ | 4.58 ± 0.21 | 9.93 ± 0.16 | 4.22 ± 0.03 | 23.04 ± 0.23 | 10.79 ± 0.16 | 65.66 ± 0.18 | 126.77 ± 0.6 |
| Material | Sample | Moisture (%) | Dry Matter (%) | Dry Matter (g) | Yield (%) | Density (g·cm−2) | TSS (Brix%) |
|---|---|---|---|---|---|---|---|
| Flesh | Initial | 99.6 | 0.4 | 0.004 | 3.44 | 0.9998 | 0.02 |
| Final | 97.75 | 2.25 | 0.023 | 19.57 | 1.0073 | 4.1 | |
| Peel | Initial | 99.91 | 0.09 | 0.001 | 0.55 | 0.9992 | 0.1 |
| Final | 98.5 | 1.5 | 0.015 | 9.11 | 1.0039 | 3 |
| Material | Heating Time (min) | BC (mg·g−1 dm) | BX (mg·g−1 dm) | TBC (mg·g−1 dm) | |||
|---|---|---|---|---|---|---|---|
| Peel | Feed | Retentate | Feed | Retentate | Feed | Retentate | |
| 0 | 4.06 ± 0.11 | 15 ± 0.17 | 3.02 ± 0.15 | 15.06 ± 0.13 | 7.07 ± 0.26 | 30.02 ± 0.28 | |
| 15 | 3.73 ± 0.13 | 12.87 ± 5.17 | 2.75 ± 0.09 | 14.88 ± 2.47 | 6.47 ± 0.21 | 29.31 ± 2.34 | |
| 30 | 1.41 ± 0.18 | 6.77 ± 0.19 | 1.22 ± 0.07 | 6.27 ± 0.2 | 2.72 ± 0.13 | 13.02 ± 0.39 | |
| 45 | 1.32 ± 0.05 | 6.31 ± 0.61 | 1.22 ± 0.03 | 6.44 ± 0.0 | 2.12 ± 0.66 | 12.28 ± 0.0 | |
| 60 | 0.94 ± 0.11 | 5.34 ± 0.43 | 1.02 ± 0.16 | 6.5 ± 0.48 | 1.95 ± 0.27 | 11.81 ± 0.91 | |
| 75 | 0.77 ± 0.09 | 4.29 ± 0.34 | 0.99 ± 0.08 | 5.78 ± 0.39 | 1.81 ± 0.11 | 10.05 ± 0.72 | |
| 90 | 0.71 ± 0.05 | 3.23 ± 0.00 | 0.95 ± 0.0 | 4.89 ± 0.00 | 1.64 ± 0.03 | 8.1 ± 0.00 | |
| Flesh | 0 | 1.72 ± 0.03 | 8.98 ± 0.11 | 0.89 ± 0.03 | 5.39 ± 0.04 | 2.6 ± 0.05 | 14.33 ± 0.15 |
| 15 | 1.96 ± 0.14 | 9.27 ± 1.23 | 1.1 ± 0.11 | 5.67 ± 0.79 | 3.05 ± 0.24 | 14.42 ± 2.04 | |
| 30 | 1.56 ± 0.18 | 8.27 ± 0.71 | 1.09 ± 0.18 | 6.24 ± 0.56 | 2.73 ± 0.32 | 14.48 ± 1.28 | |
| 45 | 0.67 ± 0.04 | 3.17 ± 0.34 | 0.51 ± 0.31 | 2.82 ± 0.34 | 1 ± 0.38 | 5.97 ± 0.68 | |
| 60 | 0.54 ± 0.08 | 2.51 ± 0.24 | 0.34 ± 0.1 | 2.7 ± 0.28 | 0.87 ± 0.18 | 5.19 ± 0.51 | |
| 75 | 0.43 ± 0.04 | 1.88 ± 0.65 | 0.27 ± 0.02 | 2.64 ± 0.74 | 0.67 ± 0.05 | 4.5 ± 1.39 | |
| 90 | 0.43 ± 0.02 | 1.49 ± 0.00 | 0.46 ± 0.07 | 2.46 ± 0.00 | 0.88 ± 0.1 | 3.94 ± 0.00 | |
| Material | Sample | L* | a* | b* | Hue° | BI | ||
|---|---|---|---|---|---|---|---|---|
| Flesh | before heat treatment β | 27.14 ± 0.31 | 30.91 ± 0.92 | 32.57 ± 1.87 | 46.47 ± 0.81 | 44.91 ± 1.99 | 43.03 ± 0.21 | 82.24 ± 1.84 |
| after heat treatment β | 17.04 ± 0.87 | 35.11 ± 1.31 | 28.56 ± 1.44 | 39.11 ± 0.45 | 43.8 ± 4.44 | 54.04 ± 0.34 | 128.97 ± 1.74 | |
| Peel | before heat treatment θ | 4.12 ± 0.06 | 14.11 ± 0.05 | 6.74 ± 0.04 | 25.53 ± 0.14 | 15.64 ± 0.06 | 65.28 ± 0.05 | 172.65 ± 1.38 |
| after heat treatment θ | 4.61 ± 0.44 | 9.81 ± 0.19 | 6.4 ± 0.29 | 33.09 ± 0.72 | 11.71 ± 0.32 | 64.69 ± 0.33 | 134.46 ± 5.28 |
| Material | Compound | Initial Feed | Final Concentrate | ||||
|---|---|---|---|---|---|---|---|
| Half-Life (t1/2, min) | Degradation Constant (k, min−1) | Retention (%) | Half-Life (t1/2, min) | Degradation Constant (k, min−1) | Retention (%) | ||
| Flesh | BC | 35.19 | 0.02 | 25.00 | 29.88 | 0.02 | 16.62 |
| BX | 49.51 | 0.01 | 52.06 | 61.89 | 0.01 | 45.68 | |
| TBC | 39.16 | 0.02 | 33.90 | 40.30 | 0.02 | 27.48 | |
| Peel | BC | 33.16 | 0.02 | 17.60 | 41.26 | 0.02 | 21.51 |
| BX | 51.34 | 0.01 | 31.47 | 57.76 | 0.01 | 32.47 | |
| TBC | 40.07 | 0.02 | 23.14 | 47.15 | 0.01 | 26.97 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zin, M.M.; Alsobh, A.; Nath, A.; Csighy, A.; Bánvölgyi, S. Concentrations of Beetroot (Beta vulgaris L.) Peel and Flesh Extracts by Reverse Osmosis Membrane. Appl. Sci. 2022, 12, 6360. https://doi.org/10.3390/app12136360
Zin MM, Alsobh A, Nath A, Csighy A, Bánvölgyi S. Concentrations of Beetroot (Beta vulgaris L.) Peel and Flesh Extracts by Reverse Osmosis Membrane. Applied Sciences. 2022; 12(13):6360. https://doi.org/10.3390/app12136360
Chicago/Turabian StyleZin, Moh Moh, Areej Alsobh, Arijit Nath, Attila Csighy, and Szilvia Bánvölgyi. 2022. "Concentrations of Beetroot (Beta vulgaris L.) Peel and Flesh Extracts by Reverse Osmosis Membrane" Applied Sciences 12, no. 13: 6360. https://doi.org/10.3390/app12136360
APA StyleZin, M. M., Alsobh, A., Nath, A., Csighy, A., & Bánvölgyi, S. (2022). Concentrations of Beetroot (Beta vulgaris L.) Peel and Flesh Extracts by Reverse Osmosis Membrane. Applied Sciences, 12(13), 6360. https://doi.org/10.3390/app12136360







