Detection and Factors That Induce Stenocarpella spp. Survival in Maize Stubble and Soil Suppressiveness under Tropical Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the Inoculum Source for Stenocarpella spp. within Maize Stubble
2.2. Classification of Decomposition on Maize Stubble
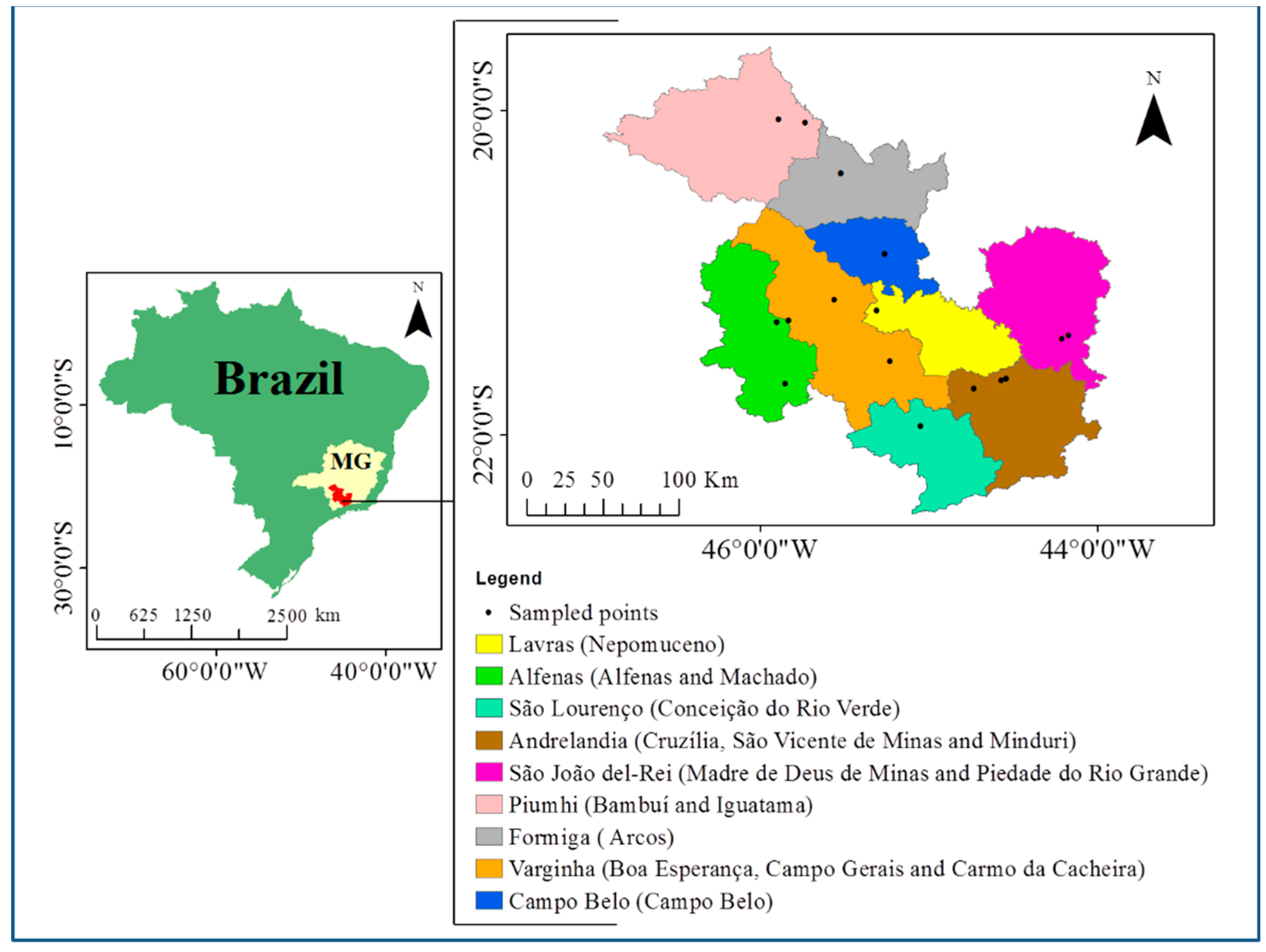
2.3. Sampling in Maize Fields
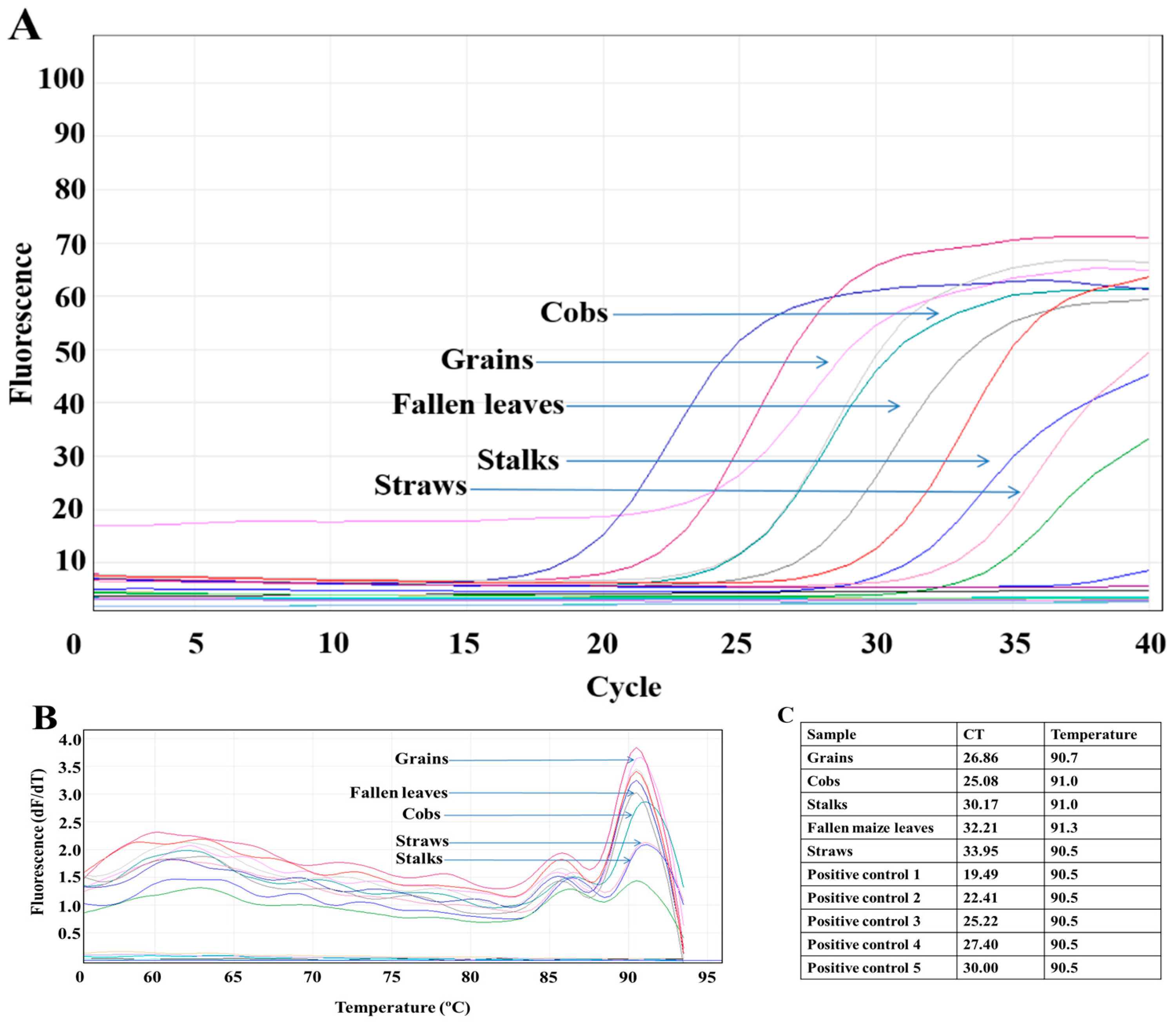
2.4. Sample Processing, DNA Extraction, and Quantitative Real-Time PCR (qPCR)
2.5. Suppressiveness against Soil-Borne Diseases
2.6. Data Processing and Statistical Analysis
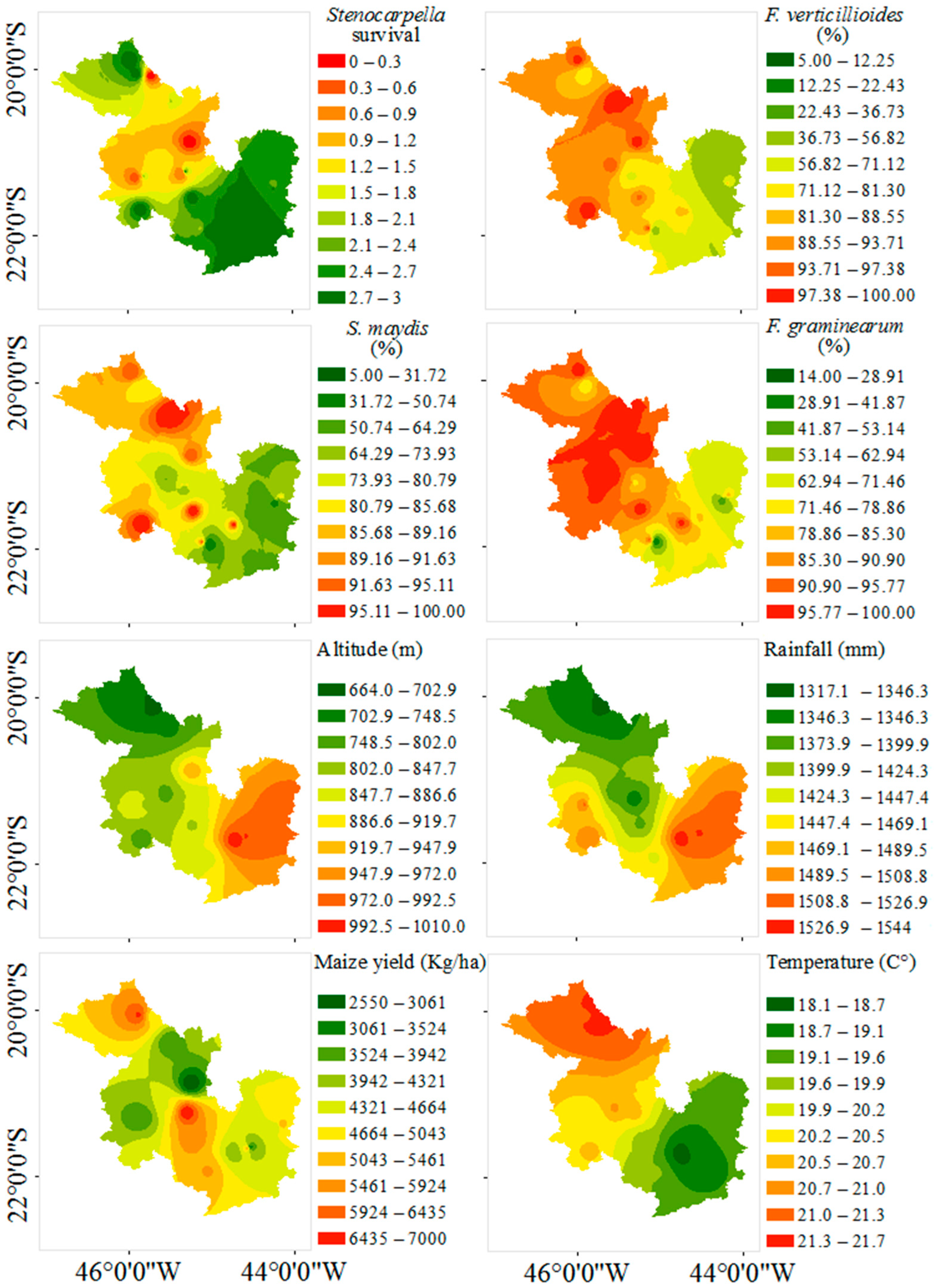
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matiello, R.R.; dos Santos, D.D.P.M.; Coelho, C.J.; Pria, M.D.; Gardingo, J.R. Damage in maize ears associated with methods of inoculation of Stenocarpella maydis. Afr. J. Agric. Res. 2015, 10, 2711–2716. [Google Scholar] [CrossRef]
- Flett, B.C.; McLaren, N.W.; Wehner, F.C. Incidence of Stenocarpella maydis ear rot of corn under crop rotation systems. Plant Dis. 2001, 85, 92–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, Z.; Achar, P.N. Random amplified polymorphic DNA and polymerase chain reaction markers for the differentiation and detection of Stenocarpella maydis in maize seeds. J. Phytopathol. 2001, 149, 35–44. [Google Scholar] [CrossRef]
- Barros, E.; Crampton, M.; Marais, G.; Lezar, S. A DNA-based method to quantify Stenocarpella maydis in maize. Maydica 2008, 53, 125. [Google Scholar]
- Siqueira, C.D.S.; Barrocas, E.N.; Machado, J.D.C.; Silva, U.A.D.; Dias, I.E. Effects of Stenocarpella maydis in seeds and in the initial development of corn. J. Seed Sci. 2014, 36, 79–86. [Google Scholar] [CrossRef]
- Li, L.; Qu, Q.; Cao, Z.; Guo, Z.; Jia, H.; Liu, N.; Wang, Y.; Dong, J. The Relationship Analysis on Corn Stalk Rot and Ear Rot According to Fusarium Species and Fumonisin Contamination in Kernels. Toxins 2019, 11, 320. [Google Scholar] [CrossRef]
- Flett, B.C.; Wehner, F.C. Incidence of Stenocarpella and Fusarium cob rots in monoculture maize under different tillage systems. J. Phytopathol. 1991, 133, 327–333. [Google Scholar] [CrossRef]
- Berghetti, J.; Casa, R.T.; Ferreira, E.Z.; Zanella, E.J.; Scheidt, B.T.; Sangoi, L. Incidence of stalk rots in corn hybrids influenced by sowing time and nitrogen rates. Bragantia 2019, 78, 371–378. [Google Scholar] [CrossRef]
- van Rensburg, B.J.; Mc Laren, N.W.; Schoeman, A.; Flett, B.C. The effects of cultivar and prophylactic fungicide spray for leaf diseases on colonisation of maize ears by fumonisin producing Fusarium spp. and fumonisin synthesis in South Africa. Crop Prot. 2016, 79, 56–63. [Google Scholar] [CrossRef]
- Barbosa, M.; Sousa-Ferraz, R.L.; Coutinho, E.L.M.; Neto, A.M.C.; da Silva, M.S.; Fernandes, C.; Rigobelo, E.C. Multivariate analysis and modeling of soil quality indicators in long-term management systems. Sci. Total Environ. 2019, 657, 457–465. [Google Scholar] [CrossRef]
- Derpsch, R.; Friedrich, T.; Kassam, A.; Li, H. Current status of adoption of no-till farming in the world and some of its main benefits. Int. J. Agric. Biol. Eng. 2010, 3, 1–25. [Google Scholar] [CrossRef]
- Casa, R.T.; Reis, E.M.; Zambolim, L. Doenças do milho causadas por fungos do gênero Stenocarpella. Fitopatol. Bras. 2006, 31, 427–439. [Google Scholar] [CrossRef]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ghini, R.; Kimati, H. Método de Iscas Para Obtenção de Isolados de Trichoderma Antagônicos a Botrytis cinerea; EMBRAPA-CNPDA: Jaguariúna, Brazil, 1989. [Google Scholar]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Webster, R.; Oliver, M.A. Statistical Methods in Soil and Land Resource Survey; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Shurtleff, M.C. Compendium of Maize Diseases; American Phytopathological Society: St. Paul, MI, USA, 1992. [Google Scholar]
- Reis, E.M.; Mario, J.L. Quantificação do inóculo de Diplodia macrospora e de D. maydis em restos culturais, no ar, e sua relação com a infecção em grãos de milho. Fitopatol. Bras. 2003, 28, 143–147. [Google Scholar] [CrossRef][Green Version]
- Casa, R.T.; Reis, E.M.; Zambolim, L. Decomposição dos restos culturais do milho e sobrevivência saprofítica de Stenocarpella macrospora e S. maydis. Fitopatol. Bras. 2003, 28, 355–361. [Google Scholar] [CrossRef]
- Handayanto, E.; Giller, K.E.; Cadisch, G. Regulating N release from legume tree prunings by mixing residues of different quality. Soil Biol. Biochem. 1997, 29, 1417–1426. [Google Scholar] [CrossRef]
- Santiago, R.; Barros-Rios, J.; Malvar, R.A. Impact of cell wall composition on maize resistance to pests and diseases. Int. J. Mol. Sci. 2013, 14, 6960–6980. [Google Scholar] [CrossRef]
- Jung, H.G.; Casler, M.D. Maize stem tissues: Impact of development on cell wall degradability. Crop Sci. 2006, 46, 1801–1809. [Google Scholar] [CrossRef]
- Köhl, J.; Lombaers, C.; Moretti, A.; Bandyopadhyay, R.; Somma, S.; Kastelein, P. Analysis of microbial taxonomical groups present in maize stalks suppressive to colonization by toxigenic Fusarium spp.: A strategy for the identification of potential antagonists. Biol. Control 2015, 83, 20–28. [Google Scholar] [CrossRef]
- Kerdraon, L.; Laval, V.; Suffert, F. Microbiomes and pathogen survival in crop residues, an ecotone between plant and soil. Phytobiomes J. 2019, 3, 246–255. [Google Scholar] [CrossRef]
- Lapsansky, E.R.; Milroy, A.M.; Andales, M.J.; Vivanco, J.M. Soil memory as a potential mechanism for encouraging sustainable plant health and productivity. Curr. Opin. Biotechnol. 2016, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.R.D.; Guimarães, R.A.; Pinto, F.A.M.F.; Siqueira, C.D.S.; Silva, C.A.; Medeiros, F.H.V.; Bettiol, W. Contribution of organic amendments to soil properties and survival of Stenocarpella on maize stalk. Sci. Agric. 2020, 77, e20180289. [Google Scholar] [CrossRef]
- Malusha, J.M.; Karama, M.; Makokha, A.O. Household maize storage practices and aflatoxin contamination in Makueni County, Kenya. Afr. J. Health Sci. 2016, 29, 89–105. [Google Scholar]
- Romero-Luna, M.P.; Wise, K.A. Development of molecular assays for detection of Stenocarpella maydis and Stenocarpella macrospora in corn. Plant Dis. 2015, 99, 761–769. [Google Scholar] [CrossRef]
- Soltani, N.; Nurse, R.E.; Sikkema, P.H. Response of glyphosate-resistant soybean to dicamba spray tank contamination during vegetative and reproductive growth stages. Can. J. Plant Sci. 2016, 96, 160–164. [Google Scholar] [CrossRef]

| Field 1 | Crop in Sequence 2 | Tillage 3 | Maize Stubble | Baits Colonization in Soils (%) | ||
|---|---|---|---|---|---|---|
| RQ of Stenocarpella spp. | Stenocarpella maydis | Fusarium graminearum | Fusarium verticillioides | |||
| 1 | M-S | No | 1 | 95 a * | 85 b | 33 c |
| 2 | M-S | No | 8.49 × 10−11 | 100 a | 100 a | 100 a |
| 3 | M-B-S | No | 6.06 × 10−11 | 10 c | 14 c | 10 c |
| 4 | M-S | No | 7.10 × 10−10 | 5 c | 24 c | 5 c |
| 5 | M-B | No | 1.61 × 10−10 | 71 b | 62 b | 38 c |
| 6 | M-S | No | 1.31 × 10−10 | 95 a | 95 a | 100 a |
| 7 | S-M-S | No | 3.93 × 10−9 | 71 b | 100 a | 90 b |
| 8 | S-M-S | No | 8.59 × 10−9 | 33 c | 67 b | 29 c |
| 9 | M-W-S | No | 4.87 × 10−11 | 71 b | 62 b | 71 b |
| 10 | M-M | No | 6.34 × 10−11 | 76 b | 95 a | 86 b |
| 11 | M-M | No | 1.58 × 10−10 | 100 a | 100 a | 86 b |
| 12 | M-W-M | No | 3.90 × 10−10 | 29 c | 29 c | 43 c |
| 13 | S-W-M | No | 9.80 × 10−7 | 95 a | 95 a | 100 a |
| 14 | M-M | No | 7.89 × 10−11 | 86 b | 71 b | 76 b |
| 15 | M-B | Yes | 1 | 38 c | 76 b | 33 c |
| 16 | M-S | No | 1 | 100 a | 100 a | 100 a |
| 17 | M-M | No | 1.70 × 10−11 | 33 c | 100 a | 100 a |
| 18 | M-S | No | 1 | 95 a | 100 a | 95 a |
| 19 | M-M | No | 2.15 × 10−12 | 100 a | 100 a | 100 a |
| 20 | M-S | No | 1 | 76 b | 100 a | 81 b |
| 21 | M-M | No | 1 | 86 b | 90 a | 95 a |
| 22 | M-S | No | 5.86 × 10−12 | 100 a | 95 a | 100 a |
| 23 | M-M | No | 1.23 × 10−11 | 100 a | 100 a | 95 a |
| 24 | M-M | Yes | 1 | 95 a | 100 a | 100 a |
| 25 | M-S | No | 1 | 100 a | 100 a | 100 a |
| 26 | M-M | No | 7.71 × 10−12 | 100 a | 100 a | 100 a |
| 27 | M-S | No | 8.52 × 10−11 | 81 b | 71 b | 71 a |
| 28 | M-S | No | 4.18 × 10−8 | 95 a | 100 a | 100 a |
| 29 | M-S | No | 1 | 81 b | 86 b | 86 b |
| Colonization Baits (%) | Pearson’s Correlation | ||
|---|---|---|---|
| Fusarium graminearum | Fusarium verticillioides | Stenocarpella maydis | |
| Fusarium graminearum | 1 * | 0.86 | 0.80 |
| Fusarium verticillioides | 0.86 | 1 | 0.78 |
| Stenocarpella maydis | 0.80 | 0.78 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, F.A.M.F.; Porto, V.B.C.; Guimarães, R.A.; Siqueira, C.d.S.; Faria, M.R.d.; Machado, J.d.C.; Medeiros, H.N.; Silva, D.D.d.; Santos Neto, H.; Pozza, E.A.; et al. Detection and Factors That Induce Stenocarpella spp. Survival in Maize Stubble and Soil Suppressiveness under Tropical Conditions. Appl. Sci. 2022, 12, 4974. https://doi.org/10.3390/app12104974
Pinto FAMF, Porto VBC, Guimarães RA, Siqueira CdS, Faria MRd, Machado JdC, Medeiros HN, Silva DDd, Santos Neto H, Pozza EA, et al. Detection and Factors That Induce Stenocarpella spp. Survival in Maize Stubble and Soil Suppressiveness under Tropical Conditions. Applied Sciences. 2022; 12(10):4974. https://doi.org/10.3390/app12104974
Chicago/Turabian StylePinto, Felipe Augusto Moretti Ferreira, Victor Biazzotto Correia Porto, Rafaela Araújo Guimarães, Carolina da Silva Siqueira, Mirian Rabelo de Faria, José da Cruz Machado, Henrique Novaes Medeiros, Dagma Dionísia da Silva, Helon Santos Neto, Edson Ampelio Pozza, and et al. 2022. "Detection and Factors That Induce Stenocarpella spp. Survival in Maize Stubble and Soil Suppressiveness under Tropical Conditions" Applied Sciences 12, no. 10: 4974. https://doi.org/10.3390/app12104974
APA StylePinto, F. A. M. F., Porto, V. B. C., Guimarães, R. A., Siqueira, C. d. S., Faria, M. R. d., Machado, J. d. C., Medeiros, H. N., Silva, D. D. d., Santos Neto, H., Pozza, E. A., & Medeiros, F. H. V. d. (2022). Detection and Factors That Induce Stenocarpella spp. Survival in Maize Stubble and Soil Suppressiveness under Tropical Conditions. Applied Sciences, 12(10), 4974. https://doi.org/10.3390/app12104974






