Abstract
The effect of three doses of UV-C radiation (1, 3 and 6 kJ m−2) on conservation potential after harvest of the Deglet-Nour date for five months of storage at 10 °C was studied. Contents of water, total sugar, carotenoids, proteins, total polyphenols, flavonoids and condensed tannins, as well as browning index, enzyme activities of polyphenoloxidase and peroxidase and antioxidant capacity of samples were monitored during storage using standard methods. Doses 1 and 6 kJ m−2 significantly slowed the water loss of samples until the second month of storage, with 17.68% and 16.02% of loss compared to control (31.45%). In the second month of storage, a significant increase in carotenoids was also observed for doses 1 and 6 kJ m−2, with values of 4.17 and 4.02 mg kg−1 versus the control (3.45 mg kg−1), which resulted in deceleration in carotenoid degradation. A gradual decrease in total sugar content was noted for all samples; it was slower within irradiated ones at the second month, where the slowing down of sugar consumption was significantly favored in the samples irradiated at 1 and 6 kJ m−2, which was marked by decreases of 4.98% and 4.57% versus 8.96% in the control. Protein content of irradiated samples (3 and 6 kJ m−2) increased at the third month, giving 1.70 and 2.41 g kg−1 compared to 1.29 g kg−1 for the control. An important decrease in enzymatic activity of polyphenoloxidase was detected, in addition to a fluctuation in peroxidase during storage. The browning index was lower in the irradiated sample until the fourth month of storage, where the result was more significant. An increase in the content of condensed tannins was detected, especially during the two first months, and while the significant increase in the content of flavonoids was read at the last month, it was detected from the first month for polyphenols. This was more significant for the highest dose, were the content reached 0.537 g kg−1 versus 0.288 g kg−1 in control at the first month. A dose-dependent increase in antiradical activity was noted during the last months of storage, while the increase in iron-reducing power was detected at the first month. UV-C delayed installation of Deglet-Nour browning and enriched it with antioxidants.
1. Introduction
Phoenix dactylifera L. is a vital plant for the desert region in Algeria, where it plays a very important ecological and socio-economic role for the population living in this region. Indeed, the fruit is the subject of important commercial activity, in particular the famous variety ‘Deglet-Nour’, which is a worldwide commercial variety of choice because it is flavorful, mellow and has an exquisite taste. Deglet-Nour derives its vernacular name from the expression finger of light, it is the most popular cultivar in all the palm groves of southeastern Algeria, and it reaches maturity in October–November.
During its maturation, it goes through four essential stages called Kimri, Khalal, Rutab and Tamar, where the fruit gradually loses its water and intensifies in color. In the final Tamar stage, Deglet-Nour undergoes a decrease in astringency, and it is light red in color with yellowish tints. Polyphenols are micronutrients, particularly abundant in fruit, grains and vegetables [1]. Their involvement in biological activities of dates was confirmed [2,3]. Degradation of fruit phenolics alters their nutritional quality, especially since these molecules characterize the sensorial properties of fruits and vegetables [4]. Their preservation within fruit and vegetables is a great necessity, since their oxidation by polyphenoloxidase (PPO) and peroxidase (POD) actions leads to installation of fruit browning [5], which drops their market value. The first experiments of food irradiation were carried out to avoid germination of fruits and vegetables. Moreover, food irradiation can control insect infestations and delay ripening of fresh fruit and vegetables [6]. Otherwise, it should be noted that UV-C radiation has not been tested to slow browning and degradation of related molecules, phenolics, in fruit and vegetables.
The main objective of this work was to study the effect of UV-C radiation on the quality of the Deglet-Nour date by monitoring changes in some of its organoleptic and pharmacological properties during five months of storage after irradiation. The color alteration was targeted by study of fruit browning, in addition to other quality criteria such as changes in antioxidant activity and content of major components.
2. Materials and Methods
2.1. Chemical, Reagents and Materials
All solvents and reagents used in the present study were purchased from Sigma Aldrich, Steinheim, Germany. This includes: acetone, methanol, β-carotene, phenol, sulfuric acid, glucose, polyvinylpyrrolidone, Bradford reagent, bovine serum albumin (BSA), 4-methyl-catechol, guaiacol, sodium fluoride (NaF), BHT, aluminum trichloride (AlCl3), quercetin, vanillin, catechin, gallic acid, Folin–Ciocalteu reagent, 2,2-diphenyl 1-picrylhydrazyl (DPPH), potassium ferricyanide (K3[Fe(CN)6]), sodium carbonate (Na2CO3), KH2PO4, K2HPO4, trichloroacetic acid (TCA), ferric chloride (FeCl3).
The spectrophotometer used in quantification of molecules and monitoring of enzymatic and antioxidant activities was anUV/VIS-7220G, Shenzhen ThreeNH Technology, Shenzhen, China; the centrifuge was an EBA 200 model, Hettich, Montévrain, France.
2.2. Plant Material and Experimental Design
A semi-soft date fruit, ‘Deglet-Nour’, was used in this study. Samples were harvested at full maturity, at Tamar stage of ripening in November 2017. They were from a young 10-year-old orchard in the southeast of Algeria. Dates were sorted to keep only the fruits with homogeneity of color and ripeness degree. Fruits were divided into five batches, three of them each received a different dose of UV-C ray (1 kJ m−2, 3 kJ m−2 and 6 kJ m−2); the fourth was used as control sample while the fifth was used to determine all experiment parameters before irradiation by D0 analysis. The UV-C apparatus, used to irradiate samples, consisted of two lamps emitting quasi-monochromatic UV radiation at 254 nm and having a normal output power of 60 W. All the samples were placed in perforated low density polyethylene bags and stored at 10 °C until the time of analysis. Sample storage was up to five months. In addition to D0 analysis, other analyses were carried out each month to ensure the monitoring of evolution of contents of water, carotenoids, total sugars, proteins, total polyphenols, flavonoids and condensed tannins, as well as browning index, enzyme activities of polyphenoloxidase and peroxidase, in addition to the antiradical activity and reducing power.
2.3. Visual Analysis and Determination of Water Content
Monitoring of changes in the external color of the fruit was carried out by taking pictures just after irradiation and at the end of storage. The water content was determined by drying a known weight of the sample in an isothermal oven (Red LINE, Paris, France) at 80 °C ± 2 °C and at atmospheric pressure until obtaining a constant mass sample. The water content is equal to the loss of fruit mass in the measurement conditions.
2.4. Determination of Carotenoid Content
The extraction and quantification of carotenoids were carried out by macerating 1 g of pulp of each sample in 2 mL of acetone containing 0.05 mL of BHT (0.1%) for 24 h. the mixture was filtered, and the operation was repeated until a clear coloration of the extract was observed. Absorbance of the filtrate was determined at 450 nm against a blank (acetone). The concentrations of carotenoids were estimated by reference to the standard curve of β-carotene and the results were expressed in milligrams per kilogram of fruit dry weight (mg kg−1).
2.5. Determination of Total Sugar Content
The approach of Dubois et al. [7] was followed to determine total sugar using phenol and concentrated sulfuric acid. An amount of 0.2 g of date pulp for each sample was macerated in 3 mL of distilled water for 24 h, and the extract was centrifuged. An intake of 50 μL of the supernatant was diluted by addition of 10 mL of distilled water. An amount of 1 mL from solution of each sample, 0.05 mL of the phenol solution (80%) and 2 mL of concentrated sulfuric acid were homogenized and incubated at 30 °C for 15 min, and the reaction was stopped by a stream of cold water. Absorbance was measured at 490 nm.
The concentrations of total sugars in date extracts were calculated by reference to a calibration curve obtained using glucose; the results were expressed in grams glucose equivalent per kilogram dry weight.
2.6. Determination of Total Protein Content, PPO and POD Activities
Bradford method [8] was used to estimate total protein content in Deglet-Nour samples. Preparation of protein extracts was performed using the Lichanporn and Techavuthiporn [9] approach; 0.4 g of date paste was macerated in 4 mL of 0.05 M phosphate buffer pH 6.8 containing 0.08 g of polyvinylpyrrolidone. After 24 h, the mixture was centrifuged at 8000× g for 10 min. An amount of 250 μL of Bradford reagent was added to 1 mL of the supernatant, stirred and incubated for 30 min in the dark at ambient temperature and absorbance was measured at 595 nm. Protein concentrations were calculated by reference to the calibration curve obtained using bovine serum albumin (BSA) as standard. The results were expressed in grams of BSA equivalent per kilogram of dry weight (g kg−1).
Both enzymes’ activity was assayed using the protein extracts. PPO activity was assessed by measuring oxidation of 4-methyl-catechol as substrate at 410 nm, as described by Dassamiour et al. [4]. The increase in the absorbance at 410 nm at 30 °C was automatically recorded for 5 min. POD activity was determined using guaiacol as substrate, as previously reported by Daas Amiour and Hambaba [5]. One unit of enzyme activities was defined as the amount of the enzyme which caused a change of 0.01 in absorbance/min. Results were expressed in enzymatic unit per gram of protein (U g−1).
2.7. Determination of Browning Index and Contents of Flavonoids and Condensed Tannins
The browning index is a good indicator of color intensification of samples. It was determined by homogenizing 1 g of Deglet-Nour pulp in 2 mL of methanol containing NaF (4 mM). The homogenate was macerated for 30 min then filtered and centrifuged at 10,000× g for 15 min. The supernatant was used directly to measure absorbance at 430 nm, as a browning index per one gram of dry weight.
The extract was also used in flavonoid and tannin quantification; the first was carried out using the AlCl3 method as reported by Daas Amiour et al. [3]. Flavonoid concentration was calculated by reference to the calibration curve obtained using quercetin. The results were expressed in gram quercetin equivalent per kilogram of fruit pulp’s dry weight (g kg−1). The vanillin assay was carried out to determine condensed tannin content, which was calculated using a calibration curve of catechin.
2.8. Determination of Total Polyphenol Content
Date paste (0.5 g) of different irradiation doses was macerated in a mixture of solvents (30 mL H2O/30 mL methanol/40 mL acetone). After 24 h of maceration, the extract was centrifuged at 10,000× g for 10 min. Total phenolics content was assayed using Folin–Ciocalteu reagent, following the method described by Daas Amiour and Hambaba [5]. Absorbance was read at 760 nm and total phenolic concentrations were expressed in gram gallic acid equivalents per kilogram of dry weight (g kg−1).
2.9. Evaluation of Antioxidant Activity
Antioxidant activity was conducted on the extracts prepared for the determination of total phenolic compounds, using two tests.
2.9.1. Anti-Free-Radical Activity (DPPH Test)
Antiradical capacity was evaluated by the approach of Masuda et al. [10] using the extracts prepared for determination of phenol content. A volume of 2.45 mL of each extract was introduced into a test tube, to which 50 μL of 5 mM DPPH methanol solution was added and after 30 min of incubation at room temperature, the optical density was determined at 517 nm, against methanol as blank. The anti-free-radical activity was estimated in percentage using the following formula:
2.9.2. Ferric-Reducing Power Test
Oyaizu’s [11] method was used for this approach. A volume of 200 μL of different dose extracts was mixed with 200 μL of 0.2 M phosphate buffer solution (pH 6.6) and 200 μL of solution of K3Fe(CN)6 (1%). The whole was incubated at 50 °C for 30 min then 200 μL of 10% TCA were added to stop the reaction; preparations were centrifuged at 3750× g for 10 min. An amount of 400 μL of supernatant was combined with 400 μL of distilled water and 90 μL of aqueous solution of FeCl3 (0.1%) and after 1 h of incubation, the absorbance was read at 700 nm. Note that a stronger absorbance indicates increased reducing power.
2.10. Statistical Analysis
Each measurement was performed in triplicate for each sample starting from the extraction step. Data were analyzed by Graph pad prism using the One-way ANOVA test followed by Tukey’s post hoc test. Values are considered significant at p < 0.01. Correlation tests were performed using Excel.
3. Results and Discussion
3.1. Changes in External Color and in Carotenoid Content
The visual appearance of all samples, just after irradiation and at the end of every month of storage, is given in Figure 1 as photos taken in those times. Indeed, it appears clearly that browning was less pronounced on irradiated samples. This result can be explained by the inactivation, by ultraviolet radiation, of the polyphenoloxidase located in the surface tissue of the treated fruits. The intensification of the brown coloration in the irradiated samples is not visually distinguishable between the three doses in this case, but the results which follow have made it possible to demonstrate the difference.

Figure 1.
Changes in color appearance of date, control and UV-C treated samples, during 5 months of storage at 10 °C (the number at the left corner of each photo indicates the storage month no.).
Changes in carotenoid levels revealed a moderate elevation in treated samples during the first two months of storage versus a slow decline in all samples during the remainder of the period (Table 1), indicating progressive degradation of these pigments. In the second month the increase was significant; it was observed for doses 1 and 6 kJ m−2 with the values of 4.17 and 4.02 mg kg−1 versus the control (3.45 mg kg−1). Similar results were observed in fresh-cut carrot [12]. Carotenoids are subject, indeed, to isomerization and oxidation during processing and storage of foods because they are highly unsaturated. Degradation of carotenoids increases with length and severity of the processing conditions, temperature, storage time, transmission of light and exposition to O2 [13]. Moreover, the increase in their level may be due to their biosynthesis in response to stress caused by UV-C. It has been confirmed in several studies that the level of carotenoids increases following exposure to abiotic stress, such as drought, light, etc. The most important carotenoids identified in this cultivar of date are ß-carotene and lutein [14].

Table 1.
Changes in carotenoid contents of Deglet-Nour irradiated samples and control during five months of storage at 10 °C.
However, Freitas et al. [15] found that UV-C radiation significantly increased the content of carotenoids in pineapple core, which is agreement with the obtained result.
3.2. Changes in Moisture, Total Sugar and Protein Contents
In order to note the irradiation effect on water retention capacity, the variation in humidity rate within the fruit was measured, as it is a quality criterion of dates. The changes in water content of Deglet-Nour samples were obtained during storage period (Figure 2).
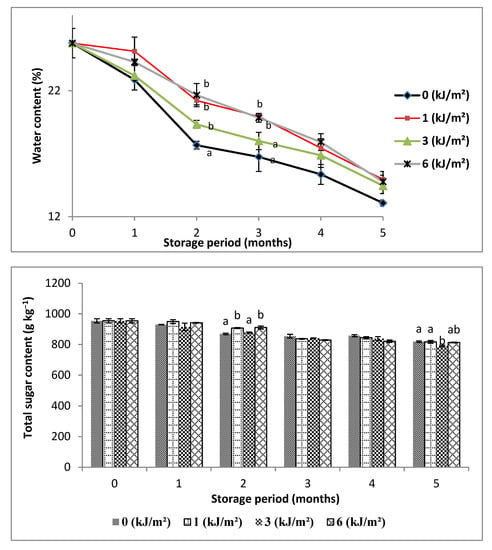
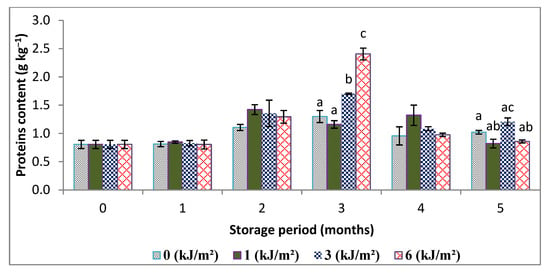
Figure 2.
Variations in water, total sugar and total protein contents of Deglet-Nour samples, during five months of storage at 10 °C. Vertical bars represent standard error (n = 3), different letters indicate a significant difference between the four samples at each storage period (p < 0.01), while the absence of the letters indicates the absence of difference.
Water content decreased in all treated samples and the control. Expressed in percentage, it ranged from 25.13 in the first month to 12.42% at the end of the storage period. Some authors have explained the decrease in moisture by evaporation of date water following the destruction of the cellulose walls and the pecto-cellulosic substances [16], probably during postharvest handling. The decrease in moisture content is less pronounced in the case of irradiated samples when referring to control, especially for doses 1 and 6 kJ m−2 during the first two months of storage, which can be explained by water retention and reduction in fruit sweating after irradiation. After one month of storage, the decreases were 2.52%, 10% and 5.82%, corresponding to doses of 1, 3 and 6 kJ m−2, respectively, versus 11.28% in the control. Doses 1 and 6 kJ·m−2 significantly slowed water loss of samples until the second month of storage, with 17.68% and 16.02% of loss compared to control (31.45%). It is certain that dehydrated Deglet-Nour loses its market value, as it normally has a soft to semi-soft consistency, and the slowing down of the water loss by this treatment participates in preserving the fruit quality. Radiation’s effect on water content found in this study is in perfect agreement with results obtained by Jemni et al. [17], who noted that water content was more important in the irradiated samples with a decrease rate between 174 and 130 g kg−1 of fresh weight for the same date variety.
The evolution of sugar content obtained for control and irradiated samples of the Deglet-Nour date, during five months of storage, is shown in Figure 2. The decrease in total sugars was slight but more pronounced in the case of the control until the second month of storage, where the difference appears significant. The diminution in total sugar content can be explained by the consumption of sugars as respiratory substrates [4] to ensure the energy supply necessary for the metabolism of the date fruit during postharvest storage.
Until the second month, the slowing down of sugar consumption was significantly favored in the samples irradiated at 1 and 6 kJ m−2, which was marked by decreases of 4.98% and 4.57% versus 8.96% in the control.
The obtained results of total sugar contents are in agreement with those of Lemoine and collaborators [18] on broccoli and those of Pan et al. [19] on strawberry. In fact, they found that the total sugar content decreased slightly immediately after treatment with 4.1 kJ m−2 of UV-C, and also during storage when referring to control group.
During the first month of storage, the difference between total sugar content of irradiated samples and that of control does not appear to be significant, probably because water loss was greater in control and thus respiration lower, since an increase in fruit breathing rates has been observed with higher moisture content [4,20]. Indeed, it was reported that Deglet-Nour fruit with 27% moisture had a respiration rate five times higher than that of fruit at 20–22% of moisture [21]. Slight decreases in Deglet-Nour sugar content do not alter its nutritional value, as it contains large amounts at the full mature stage Tamar, most of which are glucose and fructose.
It is also interesting to report that the increase in total sugar was observed in UV-C-treated sweet oranges [22], and that supports our obtained results of irradiated samples when compared to control.
Proteins belong to one of the most important classes of molecules present in all living organisms. They provide the essential functions of the cell. Variation in total protein content in control and irradiated samples of the Deglet-Nour date during storage are shown in Figure 2.
Analysis of the results shows that there is an increase in the total protein levels during storage compared to the control. This increase in protein content is more marked in the case of the irradiated sample at 6 kJ m−2 in the third month of storage. Indeed, samples of 3 and 6 kJ·m−2 gave 1.70 and 2.41 g·kg−1 versus 1.29 g kg−1 of the control. The increase may be due to the activation of biosynthesis of enzymes involved in synthesis of defense molecules against stress caused by UV-C. Most likely, the increase is concomitant with the slowing down of protein degradation, in particular by inhibition of the action of proteases, which is associated with the ripening and senescence of fruit, as has been reported in many works [23]. Therefore, it appears that the degree of slowing of protein decomposition is higher in the irradiated samples than in the control.
Other hypotheses cited by Pickering and Davies [24] indicate that protein synthesis or protection against degradation can be linked to maintaining the activity of antioxidant enzymes. Furthermore, Costa et al. and Lemoine et al. [18,25] confirmed that the protein content of broccoli is increased after irradiation at 8 kJ m−2 for 21 d of storage at 4 °C but at a rate of about 5.05 g kg−1 of fresh weight, which is greater than our result (2.40 g kg−1), recalling that the date generally contains small amounts of proteins.
Another study conducted by Xie et al. [26] on strawberry reported that the group of fruit which was treated with UV-C was marked by a significant decrease in the levels of simple sugars, namely glucose, fructose and sucrose, but also in the levels of organic acids, especially those of citric and ascorbic acids, while a slight decrease was observed for in malic acid. The researchers also noted that the growth, physicochemical quality, firmness and moisture of UV-C-treated samples were preserved when compared to control. The concentrations of nine types of essential amino acids were preserved in coconut water samples which were irradiated by UV-C [27].
3.3. Enzymatic Activity of Polyphenoloxidase, Peroxidase and Browning Index
The presence of PPO and POD enzymes in the Deglet-Nour date has been already confirmed via the demonstration and the determination of their catalytic activity within this fruit [4,5]. A significant decrease in PPO activity of 6 kJ m−2 irradiated samples was noted throughout the whole storage period (Figure 3); it was affected by UV-C treatment. It may be that the expression of PPO gene was inhibited; indeed, the decrease in PPO activity in certain tissues of Agaricus bisporus has been correlated with the inhibition of expression of the PPO gene in these tissues [28]. The actual results are in agreement with those of Mohammadi et al. [29]. By applying doses of 0.25, 0.5 and 0.75 kJ m−2 to strawberry fruit, these authors observed a decrease in PPO activity, and they obtained the lowest activity after 5 d of storage at 10 °C using 0.75 kJ m−2. Indeed, irradiation has been used to control the adverse browning phenomenon by inhibition of PPO activity [30].

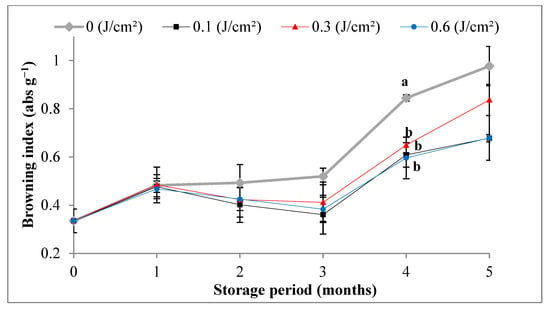
Figure 3.
Variations in PPO and POD activities and in the browning index values of Deglet-Nour samples during five months of storage at 10 °C. Vertical bars represent standard error (n = 3), different letters indicate a significant difference between the four samples at each storage period (p < 0.01), while the absence of the letters indicates the absence of difference.
The obtained results showed, for kJ m−2, a decrease in POD activity versus control from the second month until the end of storage (Figure 3). However, 1 kJ m−2 and 3 kJ m−2 induced a slowdown of POD activity for the last three months of storage. POD activity increased in irradiated samples during the first month, as generally peroxidase activity is stimulated by the readily available phenolic substrates, wounding and physiological stress, which were induced in this case by radiation and manipulation of samples.
No statistical significance was observed between the browning index values of treated and control dates during the first three months of storage, although the indexes of irradiated samples appeared to be lower (Figure 3). It was only in the fourth month that this difference was more significant in favor of a considerable reduction in browning indexes of irradiated samples, irrespective of the dose, in comparison with the control. Noting that, the analysis of results showed increased values of the browning index of all samples during storage, compared with the results of D0 as initial analysis. However, this increase was more pronounced in the case of control samples, proving that UV-C slowed browning of Deglet-Nour dates, which resulted in a longer shelf-life during storage. Indeed, ascorbic acid is a highly effective inhibitor of enzymatic browning, especially since generally, its rate increases within stored Deglet-Nour dates following its exposure to abiotic stress such as storage under modified atmospheres [4]. The result is consistent with that of Gonzalez-Aguilar et al. [31], who found that browning was significantly reduced during 21 d of storage at 5 °C of peaches (Prunus persica cv. Jefferson) following exposure to 3, 5 and 10 min to UV-C radiation.
Another study using comparable doses of UV-C reported the effectiveness of the low dose (0.5 kJ m−2) to preserve grapefruit from decay development, while the untreated control sample was marked by severe rind browning and necrotic peel [30]. It is also interesting to underline that the combination of UV-A and UV-C rays could exert a noticeable inactivation effect on PPO activity in apple juice, and the reduction in this enzyme activity was estimated at 32.58% [32].
A study conducted on coconut water revealed the effectiveness of UV-C irradiation to reduce the activity of PPO and POD enzymes in the exposed samples by 94 and 93%, respectively [27].
The exposition of potato slices to a combination composed of ascorbic acid, calcium chloride and UV-C significantly reduced the activities of PPO and POD [33]. A negligible browning process was reported in fresh-cut lotus 5–10 min following it exposition to UV-C radiation, with a remarkable inactivation of the previously cited enzymes [34].
3.4. Total Polyphenol Content (TPC), Flavonoids and Condensed Tannins
During the storage period, total polyphenol content followed an increasing evolution for all irradiated and control samples compared to the initial analysis (D0) (Table 2). Total polyphenols in irradiated samples increased more than the control, regardless of dose, during the first month of storage. This increase is highly significant for the three doses but more considerable for the highest one. Indeed, this content reached 0.537 g kg−1 at the first month versus 0.288 g kg−1 in the control. This is similar to the result obtained by Winter and Rostàs [35], who observed that TPC content in soybean samples rapidly increased as a result of UV-C exposure. UV-C also induced the accumulation of phenolic compounds in tomatoes [36,37] and in grapes [38]. The increase in our case was followed by a decrease compared to the first month, but the levels remained higher than that of control for the remainder of the storage period. In comparison, Wu et al. [39] found a diminution of phenolic compounds after UV-C treatment of mushroom which led to its browning by, most likely, oxidation of these compounds. Perkins-Veazie et al. [40] reported that no change was observed in the phenolic content of blueberry samples irradiated at 2 and 4 kJ m−2. The nature of phenols, the constitution and the physiology of each plant, in addition to storage conditions, may be the source of this diversity of results.

Table 2.
Changes in contents of total phenols (TP), flavonoids and condensed tannins of Deglet-Nour irradiated samples and control during five months of storage at 10 °C.
The results illustrated in Table 2 show a significant increase, regardless of dose, in flavonoid contents of irradiated samples in the last two months of storage compared to values detected in the first three months, where there is no significant difference in flavonoid concentration between control and treated samples. This result is somewhat similar to those obtained by some researchers. According to Gonzalez-Aguilar et al. [41], an accumulation of phenols and total flavonoids is noted during storage, following the application of 2.46 and 4.93 kJ m−2 doses on Mangifera indica. In addition, exposure to a dose of 4.1 kJ m−2 induced an overproduction of total phenols in Fragaria × ananassa Duch. Cv. Toyonoka [42]. Flavonoids could play a protective role against pathogens and environmental stress, due, for example, to drought or ultraviolet radiation, as has been noted in several works. Generally, the increase in phenol levels following UV-C exposure is associated with stimulation of the expression of the phenylalanine ammonia lyase gene; this was also confirmed by Sheng and collaborators [38] when studying the effect of UV-C treatment on table grape in addition to other genes involved in the phenylpropanoid pathway. However, the application of increasing UV-C doses of 3.2, 9.6 and 19.2 kJ m−2 on Lycopersicom esculentum cv. Durita increased total phenol levels during storage [43]. In contrast, Costa et al. [23] observed a decrease in total phenol and flavonoid content in Brassica oleracea L. var. Italica, cv. Cicco following exposure to UV-C doses of 4, 7, 10 and 14 kJ m−2.
Contents of condensed tannins increased, varying considerably between the three doses of irradiation versus control, where the increase reached a maximum value of the order of 0.37 g kg−1 in the case of the 0.3 J cm−2 dose (Table 2). This augmentation is justified by a response to stress caused by UV-C light as a self-protecting function of the fruit. Several studies confirmed the biosynthesis of tannins by plants when exposed to abiotic stresses such as low cadmium stress [44] and arid conditions [45]. Compared to irradiated samples at the first two months of storage, a decrease in the content of condensed tannins was noticed towards the end of storage; it reached a minimum value of the order of 0.135 g kg−1 at the dose 0.6 J cm−2. However, the decrease relative to the control was not established. The decrease in tannin levels is probably due to the transformation of these compounds from the soluble form to the insoluble form, which leads to their precipitation, and consequently to the decrease in astringency, which is an index of advanced ripening in date fruit. This study confirms that tannins are capable of complexing proteins and other macromolecules such as nucleic acids, which can be involved in slowing down the enzymatic activity of POD and PPO during storage of samples. Indeed, the slowdown in these activities clearly appears from the third month; this coincides with the decrease in the content of tannins due to their combination with PPO and POD, which has limited their extractability and thus altered the PPO and POD function.
It is also interesting to note that the exposure of acerola to slight UV-C radiation during postharvest storage significantly decreased the rhythm of degradation of vitamin C and phenolic compounds but also preserved the nutraceutical quality of the fruit, unlike the control not exposed to UV-C [46]. These researchers suggested a possible retention of these elements in this fruit via a possible alteration of the metabolism process of these compounds, but also suggested an elevation in mitochondrial activity and an upregulation of the antioxidant system in order to correctly fight free radicals, especially ROS, a known agent for fruit rot [47].
Another study conducted by Park and Kim [48] reported that the exposition of peeled garlic cloves to low UV-C radiation considerably increased polyphenol and flavonoid content, especially apigenin and quercetin, considered as important antioxidant agents. This may explain the slight deterioration of this group marked by a significant decrease in the microbial population, while the control sample surprisingly developed a yellow color, indicating a possible acceleration in the maturation process [49]. A comparable elevation in total phenolic and flavonoid concentrations was also observed in lettuce exposed to UV-C [50].
3.5. Anti-Free-Radical Activity and Ferric-Reducing Power
The general reading of the results of the scavenging effect analysis of DPPH radical (Figure 4) showed an increased rate of DPPH scavenging from the fourth month in favor of samples irradiated at doses 0.3 and 0.6 J cm−2. At the end of the first three months of storage, no significant distinction could be drawn between the antiradical effect of irradiated samples and that of the control, despite the apparent increase in the case of treated samples.
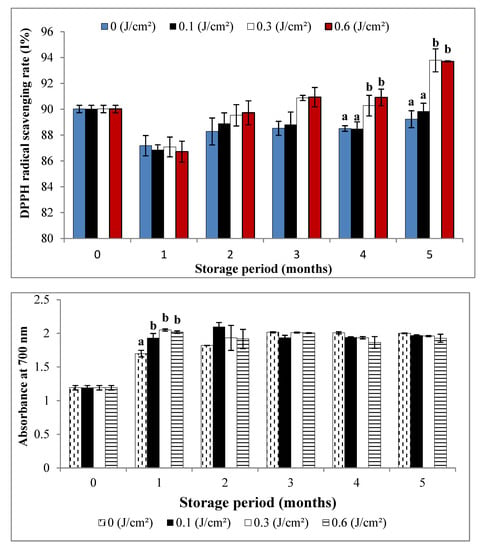
Figure 4.
Changes in DPPH scavenging activity and iron-(III)-reducing power of the of Deglet-Nour samples during five months of storage at 10 °C. Vertical bars represent standard error (n = 3); a, b: different letters indicate a significant difference between the four samples at each storage period (p < 0.01), while the absence of the letters indicates the absence of difference.
The increase in antioxidant activity during the storage of dates is probably due to biosynthesis and accumulation of phenolic compounds that play a very important role in free radical scavenging. The combination of these results with those of the total polyphenol (PPT) assays revealed a very significant linear correlation between the anti-free-radical capacity of the extracts and their PPT content (R2 = 0.80) from the third month of storage, which means that the antioxidant activity is most likely due to the phenol composition of Deglet-Nour extracts. Most of these compounds have antioxidant activities, due to the presence of many hydroxyls in their structures, which can react with free radicals.
The results of Kim et al. [43] on Citrus pomaces, as well as those of Lemoine et al. and Martínez-Hernández et al. [51,52] on broccoli and Vicente et al. [53] on sweet pepper, revealed that the increase in antioxidant capacity could be linked to the elevation in total phenols within UV irradiated samples, which is similar to ours. The rates of ferric reduction using Deglet-Nour samples, expressed as absorbance in 700 nm, are mentioned in Figure 4.
The analysis of the results makes it possible to observe a significant increase in absorbance of irradiated samples after one month of storage relative to the control. An increase in absorbance means an increase in the reducing power of tested extracts. The increase in iron (III) reductive activity in all samples compared to the initial (D0) analysis would be due to the concentration of antioxidant molecules in the extracts as a result of water loss that was much higher in the control samples. Linear correlation between ferric-reducing power and phenolic contents was medium (R2 = 0.5), thus involving part of these compounds. Generally, the reducing power of date extracts is due to the presence of substances having an ideal structure for free radical scavenging: possessing free hydroxyl groups and acting as hydrogen donors. Similar results have been observed by Gonzalez-Aguilar et al. [41] on mango, Perkins-Veazie et al. [40] on blueberries and Sheng et al. [38] on table grape, where the increase in PPT content led to increased antioxidant activity.
In addition to phenolics, involvement of selenium can be established, as date fruit is a good source of this element, 2.4–4 mg kg−1 [2]. Indeed, Molan et al. [54] found that the increase in the level of selenium in green tea leads to an increase in its antioxidant activities FRAP and DPPH. Otherwise, implication of carotenoids in these reductive activities cannot be considered, especially since the correlation between carotenoid content and antioxidant activity rate is low (R2 = 0.13; 0.01).
Moreover, the exposition of lettuce to UV-C radiation can significantly decrease DPPH scavenging activity, but also helps this plant to adapt and increase its tolerance to salinity stress [50].
In general, and from research studies that realized fruits and vegetables using UV-C radiation, results are encouraging, since quality was altered but improved in in most cases. Indeed, astudy conducted by Hosseini et al. [55] showed the effectiveness of low UV-C to preserve the physicochemical and sensory parameter of pistachio. Furthermore, low doses are sufficient for some fruits and vegetables, and indeed these researchers noted that the dose of 2.1 kJ m−2 was more active than 4.5 kJ m−2, and pistachio were in this case lighter, redder and less yellow than the highest tested dose.
Another study demonstrated that the exposition of a freshly extracted tomato juice to UV-C could be a good alternative to preserve the industrial quality of this preparation by slightly increasing some physicochemical properties such as total soluble solid, water activity, pH, color, titratable acidity and clarity [56]. The color parameter of orange juice was also preserved after UV-C exposition, and the researchers noted a non-negligible antimicrobial effect against Saccharomyces cerevisiae [57] which is very promising for future industrial application. Almost the same physicochemical results were observed for white grape juice, and this time the UV-C radiation was active against the Escherichia coli K-12 strain [58].
4. Conclusions
UV-C radiation low doses contributed to enrichment of date fruit with total polyphenols, flavonoids and tannins, which led to the increase in the antioxidant activity. The enrichment in component contents of irradiated samples reached a maximum of 0.537 g kg−1 versus the control (0.288 g kg−1) for PPT, and a maximum of 0.370 g kg−1 versus the control (0.131 g kg−1) of tannins during the first month of storage. On the other hand, a maximum of flavonoids of 0.050 g kg−1 versus the control (0.035 g kg−1) was obtained after 5 and 6 months of storage. This physical technique has delayed the enzymatic browning within this fruit. Indeed, the dose (0.6 J cm−2) resulted in the minimum browning index (0.59 abs g−1) after four months of storage, which was comparable to the other doses. UV-C radiation of Deglet-Nour fruit may have a positive impact on human health by increasing the levels of certain bio-compounds in addition to preserving its nutritional quality and extending its shelf-life. No distinguishable difference between the effects of the three doses can be confirmed, thus there is a need to test other doses and other parameters, to be able to pick out the most adequate for the preservation of nutritional quality and organoleptic properties of Deglet-Nour fruit.
Author Contributions
Data curation, E.A.; Formal analysis, S.D. and A.H.A.; Funding acquisition, S.J.A.; Investigation, A.e.D.; Methodology, O.B., L.S. and M.S.B.; Supervision, R.S.; Visualization, H.A.; Writing—review & editing, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request from the corresponding author.
Acknowledgments
The author expresses thanks to the Algerian Ministry of Higher Education. Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2022R249), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Baliga, M.S.; Baliga, B.R.V.; Kandathil, S.M.; Bhat, H.P.; Vayalil, P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 2011, 44, 1812–1822. [Google Scholar] [CrossRef]
- Daas Amiour, S.; Alloui-Lombarkia, O.; Bouhdjila, F.; Ayachi, A.; Hambaba, L. Étude de l’implication des composés phénoliques des extraits de trois variétés de datte dans son activité antibactérienne. Phytothérapie 2014, 12, 135–142. [Google Scholar] [CrossRef]
- Dassamiour, S.; Vidal, V.; Laurent, S.; Sallanon, H.; Charles, F. Effect of gaseous pretreatment on enzymatic browning of mature date after cold storage. Fruits 2018, 73, 243–251. [Google Scholar] [CrossRef]
- Daas Amiour, S.; Hambaba, L. Effect of pH, temperature and some chemicals on polyphenoloxidase and peroxidase activities in harvested Deglet Nour and Ghars dates. Postharvest Biol. Technol. 2016, 111, 77–82. [Google Scholar] [CrossRef]
- Xuetong, F.; Sommers, C.H. Irradiation of Fresh and Fresh-Cut Fruits and Vegetables: Quality and Shelf Life. Ch. 15. In Food Irradiation Research and Technology, 2nd ed.; Blackwell Publishing and the Institute of Food Technologists: Hoboken, NJ, USA, 2012; pp. 271–293. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lichanporn, I.; Techavuthiporn, C. The effects of nitric oxide and nitrous oxide on enzymatic browning in longkong (Aglaia dookkoo Griff.). Postharvest Biol. Technol. 2013, 86, 62–65. [Google Scholar] [CrossRef]
- Masuda, T.; Yonemori, S.; Oyama, Y.; Takeda, Y.; Tanaka, T.; Andoh, T.; Nakata, M. Evaluation of the antioxidant activity of environmental plants: Activity of the leaf extracts from seashore plants. J. Agric. Food Chem. 1999, 47, 1749–1754. [Google Scholar] [CrossRef]
- Oyaizu, M. Antioxidative activities of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. Nippon Shokuhin Kogyo Gakkaishi J. Jpn. Soc. Food Sci. 1988, 35, 771–775. [Google Scholar] [CrossRef]
- Alegria, C.; Pinheiro, J.; Duthoit, M.; Gonçalves, E.M.; Moldão-Martins, M.; Abreu, M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT Food Sci. Technol. 2012, 48, 197–203. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Changes in carotenoids during processing and storage of foods. Arch. Latinoam. Nutr. 1999, 49, 38S–47S. [Google Scholar] [PubMed]
- Boudries, H.; Kefalas, P.; Hornero-Méndez, D. Carotenoid composition of Algerian date varieties (Phoenix dactylifera) at different edible maturation stages. Food Chem. 2007, 101, 1372–1377. [Google Scholar] [CrossRef]
- Freitas, A.; Moldão-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef]
- Mutlak, H.H.; Mann, J. Darkening of dates: Control by microwave heating. Date Palm J. 1984, 3, 303–316. [Google Scholar]
- Jemni, M.; Gómez, P.A.; Souza, M.; Chaira, N.; Ferchichi, A.; Otón, M.; Artés, F. Combined effect of UV-C, ozone and electrolyzed water for keeping overall quality of date palm. LWT Food Sci. Technol. 2014, 59, 649–655. [Google Scholar] [CrossRef]
- Lemoine, M.L.; Civello, P.M.; Martínez, G.A.; Chaves, A.R. Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. Italica). J. Sci. Food Agric. 2007, 87, 1132–1139. [Google Scholar] [CrossRef]
- Pan, J.; Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Combined use of UV-C irradiation and heat treatment to improve postharvest life of strawberry fruit. J. Sci. Food Agric. 2004, 84, 1831–1838. [Google Scholar] [CrossRef]
- Yahia, E.M.; Kader, A.A. Date (Phoenix dactylifera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits, 1st ed.; Woodhead Publishing: Sawston, UK, 2011. [Google Scholar] [CrossRef]
- Rygg, G.L. Date development, handling and packing in the United States. In Agricultural Handbook No. 482, 1st ed.; Agricultural Research Service, US Department of Agriculture Date Development: Washington, DC, USA, 1975. [Google Scholar]
- Hu, L.; Yang, C.; Zhang, L.; Feng, J.; Xi, W. Effect of light-emitting diodes and ultraviolet irradiation on the soluble sugar, organic acid, and carotenoid content of postharvest sweet oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef]
- Barka, E.A.; Kalantari, S.; Makhlouf, J.; Arul, J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J. Agric. Food Chem. 2000, 48, 667–671. [Google Scholar] [CrossRef]
- Pickering, A.M.; Davies, K.J. Degradation of damaged proteins: The main function of the 20S proteasome. Prog. Mol. Biol. Transl. Sci. 2012, 109, 227–248. [Google Scholar] [CrossRef]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, J.; Charles, M.T.; Charlebois, D.; Khanizadeh, S.; Rolland, D.; Roussel, D.; Zhang, Z. Preharvest ultraviolet-C irradiation: Influence on physicochemical parameters associated with strawberry fruit quality. Plant Physiol. Biochem. 2016, 108, 337–343. [Google Scholar] [CrossRef]
- Yannam, S.K.; Patras, A.; Pendyala, B.; Vergne, M.; Ravi, R.; Gopisetty, V.; Sasges, M. Effect of UV-C irradiation on the inactivation kinetics of oxidative enzymes, essential amino acids and sensory properties of coconut water. J. Food Sci. Technol. 2020, 57, 3564–3572. [Google Scholar] [CrossRef]
- Lei, J.; Li, B.; Zhang, N.; Yan, R.; Guan, W.; Brennan, C.S.; Peng, B. Effects of UV-C treatment on browning and the expression of polyphenol oxidase (PPO) genes in different tissues of Agaricusbisporus during cold storage. Postharvest Biol. Technol. 2018, 139, 99–105. [Google Scholar] [CrossRef]
- Mohammadi, N.; Mohammadi, S.; Abdossi, V.; Akbar-Boojar, M.A. Effect of UV-C radiation on antioxidant enzymes in strawberry fruit (Fragaria x ananassa cv. Camarosa). J. Agric. Biol. Sci. 2012, 7, 860–864. [Google Scholar]
- Kim, J.; Marshall, M.R.; Wei, C. Polyphenoloxidase. In Seafood Enzymes Utilization and Influence on Postharvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 271–315. [Google Scholar]
- Gonzalez-Aguilar, G.; Wang, C.Y.; Buta, G.J. UV-C irradiation reduces breakdown and chilling injury of peaches during cold storage. J. Sci. Food Agric. 2004, 84, 415–422. [Google Scholar] [CrossRef]
- Akgün, M.P.; Ünlütürk, S. Effects of ultraviolet light emitting diodes (LEDs) on microbial and enzyme inactivation of apple juice. Int. J. Food Microbiol. 2017, 260, 65–74. [Google Scholar] [CrossRef]
- Teoh, L.S.; Lasekan, O.; Adzahan, N.M.; Hashim, N. The effect of ultraviolet treatment on enzymatic activity and total phenolic content of minimally processed potato slices. J. Food Sci. Technol. 2016, 53, 3035–3042. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Ma, Y.; Zhang, M.; Zhao, Y.; Zhao, X. Effect of UV-C treatment on the quality of fresh-cut lotus (Nelumbo nucifera Gaertn.) root. Food Chem. 2019, 278, 659–664. [Google Scholar] [CrossRef]
- Winter, T.R.; Rostás, M. Ambient ultraviolet radiation induces protective responses in soybean but does not attenuate indirect defense. Environ. Pollut. 2008, 155, 290–297. [Google Scholar] [CrossRef]
- Jagadeesh, S.L.; Charles, M.T.; Gariepy, Y.; Goyette, B.; Raghavan, G.S.V.; Vigneault, C. Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioproc. Technol. 2011, 4, 1463–1472. [Google Scholar] [CrossRef]
- Bravo, S.; García-Alonso, J.; Martín-Pozuelo, G.; Gómez, V.; García-Valverde, V.; Navarro-González, I.; Periago, M.J. Effects of postharvest UV-C treatment on carotenoids and phenolic compounds of vine-ripe tomatoes. Int. J. Food Sci. 2013, 48, 1744–1749. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Wu, X.; Guan, W.; Yan, R.; Lei, J.; Xu, L.; Wang, Z. Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 2016, 118, 51–58. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry fruit response to postharvest application of ultraviolet radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Villegas-Ochoa, M.A.; Martínez-Téllez, M.A.; Gardea, A.A.; Ayala-Zavala, J.F. Improving Antioxidant Capacity of Fresh-Cut Mangoes Treated with UV-C. J. Food Sci. 2007, 72, S197–S202. [Google Scholar] [CrossRef]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Kubota, C.; Kroggel, M.; Choi, J.H. Quality of fresh-cut tomatoes as affected by salt content in irrigation water and post-processing ultraviolet-C treatment. J. Sci. Food Agric. 2008, 88, 1969–1974. [Google Scholar] [CrossRef]
- Qin, G.Q.; Yan, C.L.; Wei, L.L. Effect of cadmium stress on the contents of tannin, soluble sugar and proline in Kandeliacandel (L.) Druce seedlings. Acta Ecol. Sin. 2006, 36, 112–123. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shao, H.B.; Ye, G.F.; Lin, Y.M. Effects of fertilization and drought stress on tannin biosynthesis of Casuarina equisetifolia seedlings branchlets. Acta Physiol. Plant. 2012, 34, 1639–1649. [Google Scholar] [CrossRef][Green Version]
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; de Miranda, M.R.A.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 21972–21985. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778–8817788. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, J.G. Low-dose UV-C irradiation reduces the microbial population and preserves antioxidant levels in peeled garlic (Allium sativum L.) during storage. Postharvest Biol. Technol. 2015, 100, 109–112. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol. Biochem. 2014, 83, 126–133. [Google Scholar] [CrossRef]
- Lemoine, M.L.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. Influence of a combined hot air and UV-C treatment on quality parameters of fresh-cut broccoli florets at 0 °C. Int. J. Food Sci. 2010, 45, 1212–1218. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Gómez, P.A.; Pradas, I.; Artés, F.; Artés-Hernández, F. Moderate UV-C pretreatment as a quality enhancement tool in fresh-cut Bimi® broccoli. Postharvest Biol. Technol. 2011, 62, 327–337. [Google Scholar] [CrossRef]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P.J. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Akhavan, H.R.; Maghsoudi, H.; Hajimohammadi-Farimani, R.; Balvardi, M. Effects of a rotational UV-C irradiation system and packaging on the shelf life of fresh pistachio. J. Sci. Food Agric. 2019, 99, 5229–5238. [Google Scholar] [CrossRef]
- Bhat, R. Impact of ultraviolet radiation treatments on the quality of freshly prepared tomato (Solanum lycopersicum) juice. Food Chem. 2016, 213, 635–640. [Google Scholar] [CrossRef]
- Niu, L.; Wu, Z.; Yang, L.; Wang, Y.; Xiang, Q.; Bai, Y. Antimicrobial Effect of UVC Light-Emitting Diodes against Saccharomyces cerevisiae and Their Application in Orange Juice Decontamination. J. Food Prot. 2021, 84, 139–146. [Google Scholar] [CrossRef]
- Unluturk, S.; Atilgan, M.R. Microbial Safety and Shelf Life of UV-C Treated Freshly Squeezed White Grape Juice. J. Food Sci. 2015, 80, M1831–M1841. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).