Abstract
To combat the threat of antimicrobial resistance, it is important to discover innovative and effective alternative antibacterial agents. Garlic has been recommended as a medicinal plant with antibacterial qualities. Hence, we conducted this study to evaluate the antibacterial activity of ultrasonicated garlic extract against Escherichia coli, Staphylococcus aureus sub. aureus, Streptococcus mutans, and Poryphyromonas gingivalis. Aqueous ultrasonicated garlic extract was tested against these strains, and their antibacterial activity quantified using both agar disk diffusion and agar well diffusion methods; the plate count technique was used to estimate the total viable count. Moreover, Fourier-transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), and microplate spectrophotometry were used to characterize garlic nanoparticles. The results confirmed that all tested bacteria were sensitive to both sonicated and non-sonicated garlic extracts. Streptococcus mutans was the most susceptible bacteria; on the other hand, Escherichia coli was the most resistant bacteria. Furthermore, characterization of the prepared garlic nanoparticles, showed the presence of organosulfur and phenolic compounds, carboxyl groups, and protein particles. Based on the obtained results, ultrasonicated garlic extract is a potent antibacterial agent. It can come in handy while developing novel antibiotics against bacteria that have developed resistance.
1. Introduction
The increasing mortality rate of infectious diseases is one the most challenging public health problems faced by different countries worldwide. This compromises and poses a threat to human health. Numerous synthetic antibiotic agents have always been used for the management of infectious diseases. Looking at the figures from 2000 to 2015, the statistics confirm that global antibiotics consumption levels have reached 65%, including the use of strong antibiotic agents like colistin and carbapenem [1]. Unfortunately, the wide use of antibiotics has increased the development of bacterial resistant strains to antibiotics [2], which has resulted in a reduction in the effectiveness of some of the antibacterial agents, leading to high mortality rates. Antibiotic resistance is considered one of the world’s most urgent public health problems [3,4].
A literature review has provided guidelines to minimize the increase of antibiotic resistance in the management of infections. Antibiotic resistance can be reduced by making an appropriate, timely diagnosis before doing any treatment planning; proper prescription and use of antibiotics by physicians as well as patients; effective implementation of strategies to prevent the transmission of infectious diseases; and discovering new antibacterial agents might help to control microbial drug resistance [5]. Of these, invention of new antimicrobial agents has been given more attention compared to other strategies [6].
Medicinal plants have been used for many years in the treatment of a vast number of human diseases by the community, specifically in traditional medicine. They are considered the main source of new, natural, and safe drugs to be utilized in managing diseases as an effective and harmless alternative medicine [7,8], because they are not expensive and pose minimum side effects to humans. According to a report published in 2002 by the World Health Organization (WHO) in Geneva, medicinal plants have significant value and could be considered as the best source of complementary and alternative natural medicines [9].
Garlic, scientifically known as Allium sativum, is one of the oldest plants used as a spice in food and also used as a medicine because of its many benefits to human health and wellbeing [10,11]. Findings have shown that garlic can be used in the management of various diseases such as cardiovascular disease and hyperglycemia. Additionallygarlic has been approved to reduce the risk of cancer, boosting the immune system and protecting against inflammation as well as infectious diseases [12,13,14].
Results from different studies have shown that garlic extracts have the capacity to inhibit the growth of some pathogenic microorganisms [15,16]. Their antimicrobial activity has been linked to the presence of sulphur compounds [17], specifically, allicin which is the compound produced by the alliin lyase enzyme, after crushing or bruising a garlic bulb [12]. Many medical bacteria, including gram-positive and gram-negative strains, are sensitive to garlic extracts [18,19], indicating that that garlic has a reliable broad-spectrum of activity related to its chemical composition [20]. However, the amounts and types of antimicrobial substances extracted depend on diverse extraction methods. Ultrasound-assisted extraction is considered as one of the best green extraction methods for extracting bioactive compounds from various spices, including garlic. The advantages include low-temperature extraction, easy operation, less cost, time, and energy requirement, and reduced use of toxic chemicals [21,22]. Several studies have conducted ultrasonic-assisted extraction of bioactive antimicrobial substances from garlic such as allicin, essential oil, flavonoids, polyphenols, and sulfur compounds [23,24,25,26,27]. However, despite numerous studies done on garlic extract particles against bacteria, the antibacterial activity of probe ultrasonicated garlic extract against Staphylococcus aureus sub. aureus, Streptococcus mutans, Escherichia coli, and Poryphyromonas gingivalis are still not certain. This present study evaluates the antibacterial activity of probe ultrasonicated garlic extract against the four listed bacteria so that the results of this study can be utilized for the future development of novel antibacterial agents for replacing the existing antibacterial agents, against which the tested bacteria have developed resistance.
2. Materials and Methods
2.1. Source of Bacteria Strains
The tested microorganisms, Streptococcus mutans 11823 (ATCC 25175), Escherichia coli (ATCC 11234), Staphylococcus aureus sub. aureus (KCT 1928), and Poryphyromonas gingivalis (KCT 5352) were purchased from the Korean Culture Center for Microorganisms.
2.2. Culture Preparation
All tested bacteria were activated by re-culturing them on their specific agar growth media, E. coli was re-cultured on trypticase soy agar (TSA), S. mutans, P. gingivalis, and S. aureus sub. aureus were re-cultured on brain heart infusion (BHI) agar, and the agar plates were incubated for 24 h at 37 degrees Celsius in an inverted position. After 24 h, bacteria were picked from each agar plate as single colonies and then sub-cultured into their specific broth media. Using the spectrophotometer (Optizen 2120UV plus), the turbidity of the broth culture was standardized at an optical density (OD) of 0.05 at 600 nm, before being tested against a garlic extract.
2.3. Preparation of Ultrasonicated Aqueous Garlic Extract
Fresh bulbs of garlic (Allium sativum, obtained from a local market, Gwangjin-gu, Seoul, Korea) were washed using tap water, peeled, cut into small pieces, and then dried in the oven at 55 degrees Celsius for seven days. The dried garlic was grounded using an electric blender, and 20 g of powder was measured and mixed with 100 mL of distilled water (DW) in a conical flask by the stirring machine. Four samples of 10 mL each were taken from the aqueous garlic mix, placed in a plastic tube, sonicated (Sonopuls HD 2200 probe ultrasonicator, Bandelin GmbH and Co. KG, Berlin, Germany) at 20 kHz frequency for 10 min at 100 power (W), and placed in a shaking incubator for 24 h at 300 rpm. After incubation, all sample solutions were separated from impurities by centrifugation at 10,000× g for 5 min, the supernatants were collected, and the pellets were discarded (ultrasonicated supernatant extract). Different concentrations of 100, 80, 60, 40, 20, 10, and 5 mg/mL were prepared from the ultrasonicated extract by dilution with distilled water (DW) and then tested against all four tested bacteria. Two more samples of 40 mg/mL were prepared from the sonicated aqueous garlic mix. The ultrasonicated samples were either filtrated by using Whatman No. 1 paper filter (ultrasonicated extract without centrifugation) or centrifuged (ultrasonicated supernatant). Another sample of the same concentration was prepared without sonication (extract without sonication or unsonicated extract).
2.4. Antibacterial Activity by Agar Disc Diffusion
The agar disk diffusion method [28] was used to determine the antibacterial effect of garlic extract against E. coli, S. mutans, P. gingivalis, and S. aureus sub. aureus. Prepared agar plates of different nutrient agar media were inoculated with 0.1 mL of a broth culture of tested bacteria. Using a sterile L-shape spreader; the inoculums were spread over the agar surface of the plates and kept aside. Sterile paper disks of 10 mm of diameter were saturated with 0.1 mL of different concentrations (100,80,60,40,20,10, and 5 mg/mL) of ultrasonicated garlic extract or 40 mg/mL of garlic extract sonicated without centrifugation, sonicated supernatant, or without sonication to be laid onto the seeded plates. Another disk was impregnated with 25 mg/mL of streptomycin (standard) and used as a positive control. Petri dishes with disks (saturated with garlic extract and control) were incubated overnight at 37 degrees Celsius in an inverted position. After incubation, the diameters of the zone of inhibition for each respective bacteria for every prepared concentration was measured around each disk using a ruler in millimeters [29]. Each assay was repeated in triplicate.
2.5. Antibacterial Activity by Agar Well Diffusion Method
The Agar well diffusion method is a well known method that is used frequently to determine the antibacterial effect of plant extracts. Using this method, prepared sterile agar plates were seeded with 0.1 mL of standardized bacterial inoculum on agar surfaces by an L-shaped spreader, and a sterile 9 mm cork borer was used to create eight uniform wells (holes) into the agar. Using the micropipette 100 µL of each concentration (100, 80, 60, 40, 20, 10, and 5 mg/mL) of ultrasonicated garlic extract or 40 mg/mL of garlic extract sonicated without centrifugation, sonicated supernatant, or without sonication were introduced into different wells. Moreover, well number eight or four was used as a positive control, and was inoculated with streptomycin (25 mg/mL). Plates were kept on a clean bench for 1 h to facilitate full penetration of garlic extracts in seeded agar petri dishes, then all plates were incubated for 24 h at 37 degrees Celsius and then the diameter of the inhibition zone around each well was measured in millimeter [29]. Each assay was repeated in triplicate, and the mean inhibition zone was calculated and recorded as the final inhibition zone for each set.
2.6. Post Interaction Antibacterial Activity
The antibacterial effect of garlic extract was determined by using the plate count technique [18], which indicates the number of bacteria that survived (total viable count) after overnight interaction between the garlic extract and tested bacteria. Total viable count (TVC) was calculated by mixing 5 mL of the selected serially diluted bacterial broth with 150 µL of each concentration of garlic extract in a test tube. Then, the tubes were incubated for 24 h in a shaking incubator at 37 degrees Celsius at 120 rpm. After incubation,100 µL of each interacted sample was poured out of the tubes and seeded on agar plates for overnight incubation at 37 degrees Celsius. Then, the total viable count was calculated on each plate and represented as colony-forming units per milliliter (CFU/mL) [30]. Each assay was done in triplicate to minimize errors.
2.7. Characterization of Garlic Nanoparticles
Characterization of garlic nanoparticles was done using different methods. First the garlic extract was characterized using a microplate spectrophotometer (SPECTRAmax PLUS 384). Briefly, four samples of 10 mL each were taken from the mother solution and placed in a plastic tube, then sonicated using variable frequencies for differing amounts of time and at different power as indicated below: sample 1 was sonicated at 20 kHz for 5 min at 100 power (W) ultrasonic, sample 2 was sonicated at 20 kHz for 5 min at 200 power (W), sample 3 was sonicated at 20 kHz for 10 min at 100 Power (W), sample 4 at 20 kHz for 10 min at 200 power (W), and then all the samples were placed in shaking incubator for 24 h at 300 rpm. After incubation, all sample solutions were separated from impurities by centrifugation at 10,000× g for 5 min, and the supernatants were collected, and their absorbance was scanned from 200 nm to 700 nm wavelength. Three replicates for each analysis were used, and the mean value of absorbance was obtained and recorded for graphic representation.
The obtained garlic extract nanoparticles were characterized using the Fourier-transform infrared spectroscopy (FTIR) method. Four prepared samples of ultrasonicated garlic extract were dried in the oven for seven days, and then the precipitated pellets were analyzed using FTIR, and the results were recorded on an FTIR spectrometer in the range 4000–500 cm−1. An additional sample without sonication was used as a garlic control.
Furthermore, the size and morphology of the ultrasonicated garlic extract particles were characterized by utilizing transmission electron microscopy (TEM), where all four prepared samples were diluted from 1/100, 1/1000 to 1/1000 dilution factors, 2 µL of each sample were placed on the carbon-coated copper grid for overnight incubation, and then all prepared copper grids were analyzed using TEM.
2.8. Statistical Analysis
All treatments were repeated two times. Data were analyzed using a SAS program, Release 9.2. The significance of differences among the means was determined using analysis of variance and Duncan’s multiple range test (p ≤ 0.05).
3. Results and Discussion
3.1. Inhibition of Nanoparticles of Garlic Extract on the Different Bacteria
The antibacterial activity of the nanoparticles of the ultrasonicated extract was assessed against two strains of gram-positive bacteria (S. mutans and S. aureus sub. aureus) and two strains of gram-negative bacteria (E. coli and P. gingivalis) by using both the agar disk diffusion and agar well diffusion methods. The diameters of the inhibition zones were determined, and the results are illustrated in Figure 1 and Table 1, Table 2, Table 3 and Table 4. Garlic extracts have also been reported to be effective against both gram-positive and -negative bacteria such as Bacillus cereus, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Micrococcus flavus, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Salmonella typhimurium, and Staphylococcus aureus [31,32,33,34].
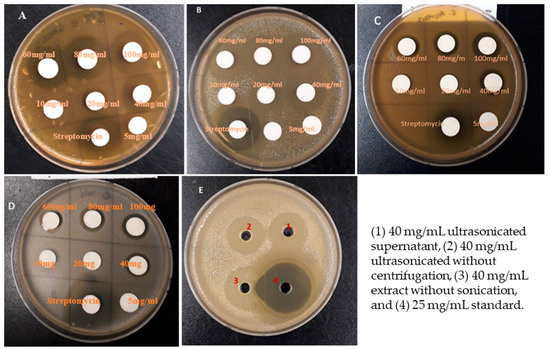
Figure 1.
Antibacterial activity of different concentrations of nanoparticles (supernatant) of garlic extract against S. mutans (A), P. gingivalis (B), S. aureus sub. aureus (C), and E. coli (D) by agar disk diffusion method. (E) represents the antibacterial effect of 40 mg/mL garlic extract treated in different conditions against P. gingivalis by the agar well diffusion method.

Table 1.
Antibacterial activity of nanoparticles (supernatant) of garlic extract against S. mutans, S. aureus sub. aureus, E. coli, and P. gingivalis by agar disk diffusion method.

Table 2.
Antibacterial activity of nanoparticles (supernatant) of garlic extract against S. mutans, S. aureus sub. Aureus, E. coli, and P. gingivalis by agar well diffusion method.

Table 3.
Antibacterial activity of 40 mg/mL garlic extracts treated in different conditions by agar disk diffusion method.

Table 4.
Antibacterial activity of 40 mg/mL garlic extracts treated in different conditions by agar well diffusion method.
The results show that different bacteria species exhibited different sensitivities against the ultrasonicated garlic extract at different concentrations of 5, 10, 20, 40, 80, and 100 mg/mL. The highest inhibition was observed against S. mutans at concentrations of 100 mg/mL of garlic nanoparticles and showed the zone of inhibition of 26.2 ± 0.8 mm by agar disk diffusion (Table 1) and 28.7 ± 0.9 mm by agar well diffusion method (Table 2), which is the greatest inhibition among all tested strains. P. gingivalis was ranked as the second most susceptible, followed by S. aureus sub. Aureus, and then E.coli was the bacteria to be least inhibited by nanoparticles from garlic extract in both the well and disk diffusion methods. Several studies have shown that garlic extracts possess an intense antibacterial activity against S. mutans [35]. The antibacterial activity of garlic is due to its phytochemicals such as allicin, flavonoids, polyphenols, and sulfur compounds [23,24,25,26,27].
Escherichia coli was the least susceptible and showed the minimum sensibility when tested against 40 mg/mL with a corresponding inhibition zone of 14.2 ± 0.8 mm and 16.3 ± 0.7 mm using the agar disk diffusion and agar well diffusion methods, respectively. The positive control result (streptomycin) confirmed that the bacteria were susceptible to streptomycin, the bacteria had various diameters of inhibition zones of 33.0, 31.9, 26.3, and 24.1 mm around the disk for S. mutans, P. gingivalis, S. aureus sub. Aureus, and E. coli, respectively, when tested by using agar disk diffusion (Table 1). Similarly, the antibacterial activity of garlic extracts was also found to be less effective against E. coli and S. aureus [36,37,38].
On the other hand, the present study proved that all tested bacteria were resistant to 5 mg/mL and 10 mg/mL. Moreover, both E. coli and S. aureus sub. aureus were resistant specifically to 20 mg/mL. These results are in agreement with Khashan [32], who assessed the antibacterial activity of garlic extract against S. aureus and found that concentrations of garlic extract ranging from 10 to 20 mg/mL were unable to inhibit the growth of S. aureus. Moderate growth inhibition was observed at concentrations of garlic extract ranging from 40 to 60 mg/mL, while the concentrations of 80 to 100 mg/mL showed the strongest inhibition activity against S. aureus [32]. Contrary to E. coli and S. aureus sub. aureus, during our study S. mutans and P. gingivalis were sensitive to 20 mg/mL (Table 1 and Table 2).
In general, all four bacteria tested, whether gram-negative or gram-positive, were sensitive to nanoparticles of garlic extract, regardless of the concentration tested in this study. However, the growth inhibition depended on the bacterial species. These findings are consistent with previous research that looked at the antibacterial activity of south Indian spices [39], Greek garlic genotypes [33], and Chinese and Desi varieties [40] against Aeromonas hydrophila, B. cereus, E. coli, E. cloacae, Enterococcus faecalis, K. pneumonia, Listeria monocytogenes, M. flavus, P. mirabilis, P. aeruginosa, and Salmonella species.
The obtained results also show that all tested bacteria had higher inhibition zones when tested using the agar well diffusion method than when tested using the disk diffusion method. This could be due to the high absorption of garlic extract when introduced into the wells, whereas in the disk diffusion method, the disk is pressed on the agar surface and does not allow for the complete diffusion of garlic extract on the agar surface. Another supporting point is that when utilizing the disk diffusion approach, some extract’s active components may be held within the disk’s pores and limiting their ability to reach the inoculation media, preventing the extract from performing to its full potential [41].
In the present study, the diameter of the inhibition zone increased with the concentration of garlic extract; the more the concentration increased, the more the zone of inhibition increased (Table 1 and Table 2). The results of this study are in line with the findings of the research done by Fatemeh et al. [42], who investigated the antibacterial effect of garlic and Eucalyptus extracts on oral cariogenic bacteria, and reported that both Streptococcus mutans and Lactobacillus acidophilus were sensitive to garlic extract and the association between concentration of garlic extract and the inhibition zone was proved. As the concentration increased, the diameter of the inhibition zone gradually increased [42].
The results presented in Table 3 and Table 4 indicate the inhibition zones of the garlic extract on the four tested bacteria at a 40 mg/mL concentration under three different extraction conditions. The sonicated garlic extract treatment without centrifugation was the second strongest extract to inhibit the growth of all tested bacteria. Garlic extract without sonication showed the minimum inhibition zone compared to other extracts (Table 3). By using the agar well diffusion method, 40 mg/mL of the sonicated supernatant extract treatment showed high inhibition zones when compared with other types of garlic extracts. The inhibition zones of all tested bacteria were 21.9 ± 0.3, 17.2 ± 0.4, 16.4 ± 0.6, and 20.4 ± 0.7 mm on S. mutans, S. aureus sub. aureus, E. coli, and P. gingivalis respectively. The results were better than that of Garba et al. [43] and Liaqat et al. [44]. Inhibition zones of 24 mm and 23 mm were obtained against E. coli and S. aureus, respectively, when methanolic extract of garlic was used at 200 mg/mL [43]. On the other hand, methanolic garlic extract (200 mg/mL) exhibited higher activity against S. aureus (25.33 mm) followed by E. coli (22.33 mm) [44].
3.2. Antibacterial Activity of Nanoparticles of Garlic Extract on Tested Bacteria by Plate Count Method
Results showing the total viable counts of all tested bacteria in colony-forming units per millimeter (CFU/mL) post interaction with the garlic extract and bacteria broth culture are illustrated in Figure 2. The results proved that all tested bacteria were challenged by the garlic extract during overnight incubation and led to a high reduction in cell number of S. mutans at 100 mg/mL. S. mutans decreased from 1.77 × 104 CFU/mL (control) up to 0 CFU/mL, P. gingivalis showed a decline from 2.01 × 104 to 0 CFU/mL, S. aureus sub. aureus reduced from 2.05 × 105 CFU/mL to 0 CFU/mL, and the most resistant bacteria E. coli, decreased to 0 CFU/mL when tested with 100 mg/mL concentrations, compared to the control (2.03 × 105 CFU/mL).
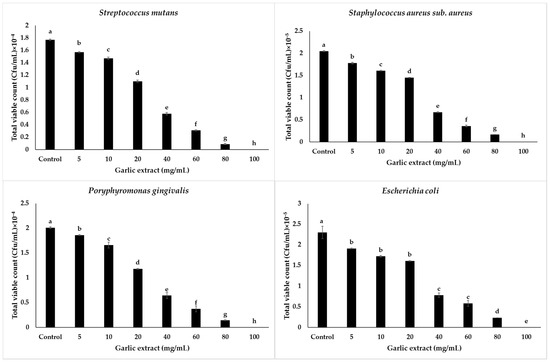
Figure 2.
Graphical presentation of antibacterial activity of nanoparticles (supernatant) of garlic extract in terms of total viable counts (CFU/mL) on tested bacteria. Values are means of determinations of three replicates, and bars represent standard deviations (S.Ds.) of the means. S.Ds. followed by different letters are significantly different at p ≤ 0.05 level by Duncan’s multiple range test.
In general, there was a reduction in total viable counts of all tested bacteria when bacteria interacted with the different concentrations of garlic extract from the lowest concentration of 5 mg/mL to the highest concentration of 100 mg/mL compared with the control bacteria cultures. In this study, it was discovered that increasing the concentration of garlic extract reduces the number of bacteria that survive in CFU/mL; these findings are consistent with those of Alwazni et al. [45], who compared the antibacterial effects of garlic and onion on E. coli, Salmonella typhi, Pseudomonas aeruginosa, and Klebsiella pneumonia.
3.3. Role of Sonication on Antibacterial Activity of Garlic Extract
The current findings show that 40 mg/mL of the sonicated supernatant garlic extract had the greatest inhibition on S. mutans. The counts reduced from 1.77 × 104 CFU/mL (control) to 0.58 × 104 CFU/mL, whereas 40 mg/mL the sonicated garlic extract without centrifugation reduced S. mutans colonies to 0.64 × 104 CFU/mL, and garlic extract without sonication did not show significant inhibition in the sonicated extracts. It reduced the bacterial counts to 0.88 × 104 CFU/mL. The second bacteria to be challenged with 40 mg/mL of the sonicated garlic extract were P. gingivalis; a decline from 2.01 × 104 CFU/mL to 0.64 × 104 CFU/mL was observed. The sonicated samples without centrifugation were effective against P. gingivalis up to 0.7 × 104 and the unsonicated garlic extract inhibited P. gingivalis up to 1.0 × 104 CFU/mL, Staphylococcus aureus followed, showing maximum effectivity at 40 mg/mL of the sonicated supernatant sample, bringing about a reduction in the bacterial count from 2.05 × 105 CFU/mL to 0.67 × 105 CFU/mL compared to 1.2 × 105 CFU/mL of the unsonicated sample (Figure 3).
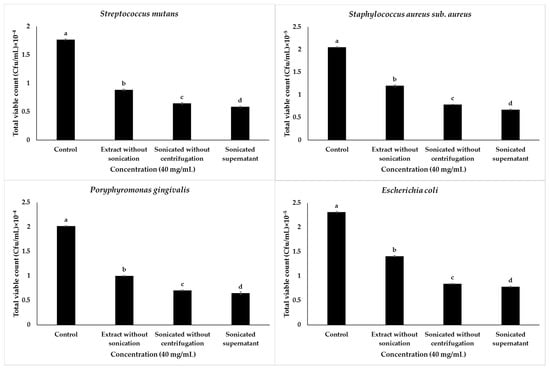
Figure 3.
Graphical presentation of the total viable count of 40 mg/mL of garlic extract treated in different conditions on tested bacteria. Values are means of determinations of three replicates, and bars represent standard deviations (S.Ds.) of the means. S.Ds. followed by different letters, are significantly different at p ≤ 0.05 level by Duncan’s multiple range test.
The least susceptible bacteria (E. coli) when treated at concentrations of 40 mg/mL showed a reduction in counts from 2.30 × 105 CFU/mL to 0.78 × 105 CFU/mL against the sonicated supernatant garlic extracts. The unsonicated garlic extracts without centrifugation decreased E. coli to 0.84 × 105 CFU/mL. The sample that showed the least bacterial inhibition on E. coli was garlic extract without sonication (1.4 × 105 CFU/mL). The above findings indicate the positive influence of sonication on increasing the capacity of garlic extract to inhibit the growth of all tested bacteria through increasing extraction of active compounds, mostly allicin, the biological compound of garlic that is responsible for many biological benefits of garlic extract, including its antibacterial property [46]. This is in accordance with another investigations based on the influence of ultrasound, microwaves, and other factors on synthetic allicin and showed that allicin production during ultrasonication extraction increases with sonication, through cavitation effects caused by ultrasound on the cell material by disrupting the cell wall structure, increasing the speed of diffusion, causing cell lysis, and ultimately releasing the cell contents as well, which indicates the high release of allicin during ultrasonication of garlic during extraction [47]. Effective extraction of garlic active compounds during sonication could be explained by the mechanism of the high solubility of garlic in water when ultrasonicated [21], leading to the breaking of garlic into nanoparticles that easily interact with biological systems.
Briefly, the effect of sonication on the antibacterial capacity of the garlic extract in the reduction of total viable counts when bacteria were tested against 40 mg/mL treated in different conditions was reported in Figure 3 and indicated that the sonicated supernatant garlic extract significantly reduced the total viable bacteria counts compared to other types of garlic extracts.
3.4. Characterization of Nanoparticles of Garlic Extract by Microplate Spectrophotometer
The UV-Vis spectrophotometric characterization was carried out using a microplate spectrophotometer, and the absorbance was scanned in the wavelength range from 200 nm to 750 nm. The spectrophotometer results showed that the maximum absorbance of nanoparticles of garlic extract was in the visible wavelength range of around 240–300 nm, which is typically the range of allicin [48], which is an organosulfur compound found in garlic and plays the vital role in the antibacterial property of garlic extract.
During this study, it was observed that the sonicated samples showed high absorbance compared to the unsonicated sample (control); however, no significant differences in the peak location or absorbance was observed among the four sonicated samples, prepared at various times and at different sonication power (Figure 4).
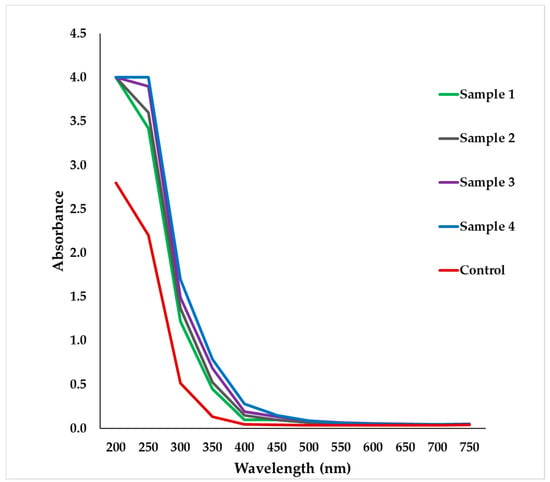
Figure 4.
Microplate spectrophotometer absorption of nanoparticles of the garlic extract prepared using various sonication time and power (Sample 1, 2, 3, 4: sonicated samples, control: unsonicated sample).
A slight difference was observed in sonication time which indicated that as sonication time increased, the absorbance of the four sonicated samples slightly increased. The marginal differences might be due to high extraction efficiency when the sonication time increased; however, the power (Watt) did not show an impact on the peak location or absorbance.
3.5. Characterization of Nanoparticle of Garlic Extract by FTIR
FTIR spectroscopy was used to discover the successful extraction of garlic nanoparticles, and spectra were obtained in the wavenumber range of 500–3500 cm−1. The results of the FTIR spectrum of the ultra-sonicated garlic extract showed visible peaks at around 3345.8, 2934.1, 2360.5, 1635.6, 1417.5, 1130.7, 1025, 930.61, 815.4, and 591.5 cm−1 wavenumbers (Figure 5).
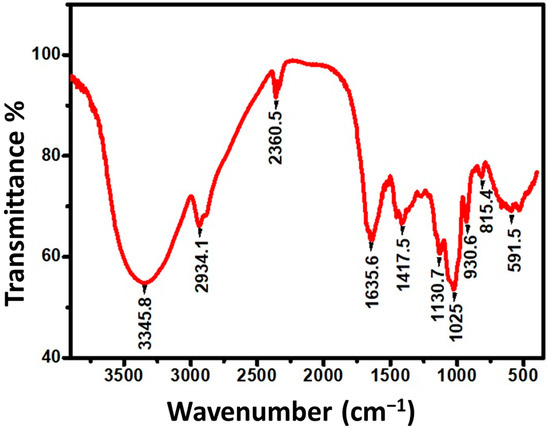
Figure 5.
FTIR spectrum of nanoparticles from garlic extract.
The wide peak at 3345.8 cm−1 can be assigned to the presence of the O–H stretching vibration in the hydroxyl group. These results also indicated that there is asymmetric stretching in the C–H bonds at 2934.1 cm−1, at 1635 cm−1 FTIR revealed the presence of carbonyl or carboxylic (C=O) stretching bands, the same results confirm the presence of the –O–H bend in carboxylic at 1417 cm−1, at 1130.7 cm−1 the results revealed the presence of an S=O bond, the presence of C–N stretching vibrations in primary amines was observed at 1025 cm−1, at 930.6 cm−1 FTIR showed the presence of a γ-C–H deformation in =CH2, at 815 cm−1 we observed the presence of an S–C bond which might indicate the absorption of allicin, an organosulfur compound present in the garlic extract [49], and finally these results recorded the presence of a C–H bend in the alkynes at 530 cm−1.
The current ultrasonicated garlic extract FTIR spectrum matches previous research on the presence of functional groups in aqueous garlic extracts [50,51]. Based on the spectrum mentioned above, we would say that phenolic, organosulfur compounds, amino acids, carboxylic groups, and proteins are the active groups that play a key part in the antibacterial activity of ultrasonicated garlic extract. This conclusion is supported by findings of several investigations that found the same major phytochemicals in garlic extract [52,53].
3.6. Characterization of Nanoparticles of Garlic Extract by TEM
In order to confirm the nature of the nanoparticles from the ultrasonicated garlic extract, TEM was used and it revealed that the garlic nanoparticles consisted of random sized particles, a few rods, and a few spherical particles (Figure 6), which were randomly dispersed, and had small sizes (less than 50 nm). The antibacterial property of garlic extract could be attributed to its bioactive particles’ small size and morphology since particle size and surface area influence the interaction between chemical compounds and biological systems. Reduction in the size of bioactive particles leads to an increased surface area coming in contact with microorganisms, enhancing their interaction and leading to antibacterial activities [54], this is in line with other studies that have been done on the antibacterial properties of nanoparticles and have indicated that small and formless particles are the most effective particles to inhibit the growth of bacteria [55,56]. Various writings have documented how nanoparticles act and highlighted that they inhibit bacterial growth by anchoring to and penetrating the bacterial cell wall. When the reach the inside of the bacteria, they start modulating cellular signaling by dephosphorylating putative key peptide substrates on tyrosine residues leading to the inhibition of bacteria growth [57].
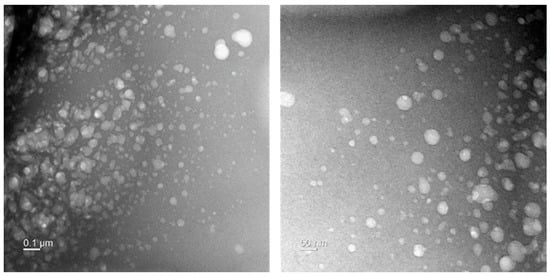
Figure 6.
Transmission electron microscope image of garlic nanoparticles.
4. Conclusions
In general, the results of this present study demonstrated that the ultrasonicated garlic extracts showed the antibacterial capacity to inhibit the growth of S. mutans, S. aureus sub. aureus, P. gingivalis, and E. coli bacteria. The efficiency of garlic against bacteria might be related to its phenolic, organosulfur compounds, amino acids, carboxylic groups, and protein contents. Furthermore, we strongly recommend further studies to evaluate the antibacterial activity of ultrasonicated garlic extract against viruses and fungi.
Author Contributions
Conceptualization, T.G. and S.C.C.; methodology, T.G., A.V., S.J.K. and I.S.; formal analysis, T.G., A.V. and S.C.C.; investigation, T.G. and S.C.C.; writing—original draft preparation, T.G., I.S., K.D.K. and S.C.C.; writing—review and editing, I.S. and S.C.C.; supervision, S.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CFU: Colony-forming unity; FTIR: Fourier-transform infrared spectroscopy; TEM: Transmission electron microscopy; TVC: Total viable count; WHO: World Health Organization.
References
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.I.; Hughes, D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 2011, 35, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassoum, O.; Sougou, N.M.; Diongue, M.; Lèye, M.M.M.; Mbodji, M.; Fall, D.; Seck, I.; Faye, A.; Tal-Dia, A. Assessment of General Public’s Knowledge and Opinions towards Antibiotic Use and Bacterial Resistance: A Cross-Sectional Study in an Urban Setting, Rufisque, Senegal. Pharmacy 2018, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Wennberg, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, B.; Kishor, A.; Singh, S.; Bhat, M.N.; Surmal, O.; Musarella, C.M. Exploring Plant-Based Ethnomedicine and Quantitative Ethnopharmacology: Medicinal Plants Utilized by the Population of Jasrota Hill in Western Himalaya. Sustainability 2020, 12, 7526. [Google Scholar] [CrossRef]
- WHO. Traditional Medicine Strategy 2002–2005; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Karuppiah, P.; Rajaram, S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple drug resistant chemical pathogens. Asian Pac. J. Trop. Biomed. 2012, 2, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Botas, J.; Fernandes, Â.; Barros, L.; Alves, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. A Comparative Study of Black and White Allium sativum L.: Nutritional Composition and Bioactive Properties. Molecules 2019, 24, 2194. [Google Scholar] [CrossRef] [Green Version]
- Chekki, R.Z.; Snoussi, A.; Hamrouni, I.; Bouzouita, N. Chemical composition, antibacterial and antioxidant activities of Tunisian garlic (Allium sativum) essential oil and ethanol extract. Med. J. Chem. 2014, 3, 947–956. [Google Scholar]
- Gam, D.-H.; Park, J.-H.; Kim, J.-H.; Beak, D.-H.; Kim, J.-W. Effects of Allium sativum Stem Extract on Growth and Migration in Melanoma Cells through Inhibition of VEGF, MMP-2, and MMP-9 Genes Expression. Molecules 2022, 27, 21. [Google Scholar] [CrossRef]
- Patiño-Morales, C.C.; Jaime-Cruz, R.; Sánchez-Gómez, C.; Corona, J.C.; Hernández-Cruz, E.Y.; Kalinova-Jelezova, I.; Pedraza-Chaverri, J.; Maldonado, P.D.; Silva-Islas, C.A.; Salazar-García, M. Antitumor Effects of Natural Compounds Derived from Allium sativum on Neuroblastoma: An Overview. Antioxidants 2022, 11, 48. [Google Scholar] [CrossRef]
- Yetgin, A.; Canli, K.; Altuner, E.M. Comparison of antimicrobial activity of Allium sativum cloves from China and Taşköprü, Turkey. Adv. Pharmacol. Sci. 2018, 2018, 9302840. [Google Scholar] [PubMed] [Green Version]
- Chen, C.; Liu, C.H.; Cai, J.; Zhang, W.; Qi, W.L.; Wang, Z.; Liu, Z.-B.; Yang, Y. Broad-spectrum antimicrobial activity, chemical composition and mechanism of action of garlic (Allium sativum) extracts. Food Control 2018, 86, 117–125. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Fufa, B. Anti-bacterial and anti-fungal properties of garlic extract (Allium sativum): A review. Microbiol. Res. J. Int. 2019, 28, 1–5. [Google Scholar] [CrossRef]
- Kshirsagar, M.M.; Dodamani, A.S.; Karibasappa, G.N.; Vishwakarma, P.K.; Vathar, J.B.; Sonawane, K.R.; Khobragade, V.R. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. Ayu 2018, 39, 165–172. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Brajer, M.; Voća, S.; Galić, A.; Radman, S.; Rimac-Brnčić, S.; Xia, Q.; Zhu, Z.; Grimi, N.; Barba, F.J.; et al. ultrasound as a promising tool for the green extraction of specialized metabolites from some culinary spices. Molecules 2021, 26, 1866. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlic, B.; Vladic, J.; Ramic, M.; Brindza, J.; Vidovic, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Mathialagan, R.; Mansor, N.; Shamsuddin, M.R.; Uemura, Y.; Majeed, Z. Optimisation of ultrasonic-assisted extraction (UAE) of allicin from garlic (Allium sativum L.). Chem. Eng. Trans. 2017, 56, 1747–1752. [Google Scholar]
- Ciric, A.R.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gupta, D.; Sen, D.; Bhattacharjee, C. Process intensification on the enhancement of allicin yield from Allium sativum through ultrasound attenuated nonionic micellar extraction. Chem. Eng. Process. Process Intensif. 2021, 169, 108610. [Google Scholar] [CrossRef]
- Salie, F.; Eagles, P.F.; Leng, H.M. Preliminary antimicrobial screening of four South African Asteraceae species. J. Ethnopharmacol. 1996, 52, 27–33. [Google Scholar] [CrossRef]
- Wikler, M.A. Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement; Part M2-A9. M100-S17; C.L.S.I. (Clinical and Laboratory Standard Institute): Pennsylvania, PA, USA, 2007. [Google Scholar]
- Gopal, J.; George, R.P.; Muraleedharan, P.; Khatak, H.S. Photocatalytic inhibition of microbial adhesion by anodized titanium. Biofouling 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Durairaj, S.; Srinivasan, S.; Lakshmana-perumalsamy, P. In vitro Antibacterial Activity and Stability of Garlic Extract at Different pH and Temperature. Electron. J. Biol. 2009, 5, 5–10. [Google Scholar]
- Khashan, A.A. Antibacterial activity of garlic extract (Allium sativum) against Staphylococcus aureus in vitro. GJBB 2014, 3, 346–348. [Google Scholar]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, R.M.; Saleh, A.H.; Ali, K.S. GC-MS analysis and antibacterial activity of garlic extract with antibiotic. J. Med. Plants Stud. 2020, 8, 26–30. [Google Scholar]
- Hoglund, K.B.; Barnett, B.K.; Watson, S.A.; Melgarejo, M.B.; Kang, Y. Activity of bioactive garlic compounds on the oral microbiome: A literature review. Gen. Dent. 2020, 68, 27–33. [Google Scholar]
- Rees, L.P.; Minney, S.F.; Plummer, N.T.; Slater, J.H.; Skyrme, D.A. Aquantitative assessment of the antimicrobial activity of garlic (Allium sativum). World J. Microbiol. Biotechnol. 1993, 9, 303–307. [Google Scholar] [CrossRef]
- Hughes, B.G.; Lawson, L.D. Antimicrobial effects of Allium sativum L. (garlic), Allium ampeloprasum L. (elephant garlic), and Allium cepa L. (onion), garlic compounds and commercial garlic supplement products. Phytother. Res. 1991, 5, 154–158. [Google Scholar] [CrossRef]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef]
- Indu, M.N.; Hatha, A.; Abirosh, C.; Harsha, U.; Vivekanandan, G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonas hydrophila. Braz. J. Microbiol. 2006, 37, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Naureen, I.; Riaz, M.; Anjum, F.; Fatima, H.; Rafiq, M.A. Biofilm Inhibition and Antibacterial Potential of Different Varieties of Garlic (Allium sativum) Against Sinusitis Isolates. Dose-Response 2021, 19, 15593258211050491. [Google Scholar] [CrossRef]
- Gangoué-Piéboji, J.; Eze, N.; Djintchui, A.N.; Ngameni, B.; Tsabang, N.; Pegnyemb, D.E.; Biyiti, L.; Ngassam, P.; Koulla-Shiro, S.; Galleni, M. The in vitro antimicrobial activity of some traditionally used medicinal plants against beta-lactam-resistant bacteria. J. Infect. Dev. Ctries 2009, 3, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Fatemeh, A.M.; Soraya, H.; Mohammad, Y.A.; Jalal, P.; Javad, S. Antibacterial effect of Eucalyptus globulus Labill) and garlic (Allium sativum) extracts on oral Cariogenic bacteria. J. Microbiol. Res. Rev. 2013, 1, 12–17. [Google Scholar]
- Garba, I.; Umar, A.I.; Abdulrahman, A.B.; Tijjani, M.B.; Aliyu, M.S.; Zango, U.U.; Muhammad, A. Phytochemical and antibacterial properties of garlic extracts. Bayero J. Pure Appl. Sci. 2013, 6, 45–48. [Google Scholar] [CrossRef]
- Liaqat, A.; Zahoor, T.; Atif, M.; Muhammad, R. Characterization and antimicrobial potential of bioactive components of sonicated extract from garlic (Allium sativum) against foodborne pathogens. J. Food Process. Preserv. 2019, 43, e13936. [Google Scholar] [CrossRef]
- Alwazni, D.S.; Drwesh, M.F. Comparative study of effect garlic and anion extract on the growth of some gram negative bacteria. Mag. Al-Kufa Univ. Biol. 2010, 2, 238–244. [Google Scholar]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Ilić, D.; Nikolić, V.; Stanković, M.; Nikolić, L.; Stanojević, L.; Mladenović-Ranisavljević, I.; Smelcerović, A. Transformation of synthetic allicin: The influence of ultrasound, microwaves, different solvents and temperatures, and the products isolation. Sci. World J. 2012, 2012, 561823. [Google Scholar] [CrossRef] [Green Version]
- Wanyika, H.; Gachanja, A.; Kenji, G.; Keriko, J.; Mwangi, A. A rapid method based on uv spectrophotometry for quantitative determination of allicin in aqueous garlic extracts. J. Agric. Sci. Technol. 2010, 12, 74–82. [Google Scholar]
- Rastogi, L.; Arunachalam, J. Green synthesis route for the size controlled synthesis of biocompatible gold nanoparticles using aqueous extract of garlic (Allium sativum). Adv. Mater. Lett. 2013, 4, 548–555. [Google Scholar] [CrossRef]
- Rastogi, L.; Arunachalam, J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nano particles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater. Chem. Phys. 2011, 129, 558–563. [Google Scholar] [CrossRef]
- Yulizar, Y.; Harits, A.A.; Abduracman, L. Green synthesis of gold nanoparticles using aqueous garlic (Allium sativum L.) Extract, and its interaction study with melamine. Bull. Chem. React. Eng. Catal. 2017, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montaño, A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Whitesides, G.M. The “right” size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Bouqellah, N.A.; Mohamed, M.M.; Ibrahim, Y. Synthesis of eco-friendly silver nanoparticles using Allium sp. and their antimicrobial potential on selected vaginal bacteria. Saudi J. Biol. Sci. 2019, 26, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).