Abstract
Due to the low density of hydrogen gas under ambient temperature and atmospheric pressure conditions, the high-pressure gaseous hydrogen storage method is widely employed. With high-pressure characteristics of hydrogen storage, rigorous safety precautions are required, such as filling of compressed gas in a hydrogen tank to achieve reliable operational solutions. Especially for the large-sized tanks (above 150 L), safety operation of hydrogen storage should be considered. In the present study, the compressed hydrogen gas behavior in a large hydrogen tank of 175 L is investigated for its filling. To validate the numerical approach used in this study, numerical models for the adaptation of the gas and turbulence models are examined. Numerical parametric studies on hydrogen filling for the large hydrogen tank of 175 L are conducted to estimate the hydrogen gas behavior in the hydrogen tank under various conditions of state of charge of pressure and ambient temperature. From the parametric studies, the relationship between the initial SOC pressure condition and the maximum temperature rise of hydrogen gas was shown. That is, the maximum temperature rise increases as the ambient temperature decreases, and the rise increases as the SOC decreases.
1. Introduction
Global warming is one of the most debated topics among environmentalists and politicians because of its implications on human welfare [1,2]. Fossil fuels, such as global oil, gas, and coal reserves, are steadily facing a depletion threat because they are finite resources. With these trends, alternative energy is receiving much attention worldwide, and hydrogen is considered as a potential primary energy source to replace traditional fuels [3,4].
In the development of hydrogen technology, hydrogen storage methods need to be implemented to meet the increasing demand for hydrogen [5]. Compressed-gas, cryogenic-liquid, and metal-hydrate storage methods are commonly employed for hydrogen [5]. For the transport system, the compressed-gas storage method is more common than other methods owing to its technical simplicity, high reliability, acceptable efficiency, and affordability [6,7].
In transportation systems, vehicles usually use fuel cell to incorporate high-pressure cylinders to store hydrogen on board. At present, vehicles with hydrogen tanks are diverse, with various volumes of tanks ranging from 50 L to 170 L. To address these issues, several researchers have conducted studies on the prediction of the compressed hydrogen gas (CGH2) behavior for its filling and fueling in a hydrogen tank for hydrogen vehicles. With these trends, Chung et al. [8] conducted a feasibility study applying the boiling model to analyze the multiphase-thermal flow in the pipe considering the phase change. Johnson et al. [9] developed thermal models for the filling of high-pressure hydrogen tanks with experimental validation using two models. They conducted numerical studies using the validated numerical model. Zhao et al. [10] investigated a CFD model, including modified standard k-epsilon turbulence and real gas model to predict the fast filling process of a type III (with metal liner) hydrogen vehicle cylinder with 74 L. Thermodynamic responses of fast filling under different pressure-rise patterns and filling times have been analyzed. From the above study, it is worth considering what happens to the tank in the case of fast filling in terms of heat and fluid flow.
Suryan et al. [11] conducted a numerical simulation of the filling process of hydrogen tanks considering real gas effects. The effect of changes in ambient temperature and initial and inlet gas temperatures is studied. However, a large-capacity hydrogen tank is required for long-distance transportation, such as that involving trains and ships. Additionally, the increasing demand for long-distance transportation and hydrogen vehicles using CGH2 has been increased considering the system operations and controls. Therefore, research on large-capacity hydrogen storage tanks is required, especially for its charging phenomenon considering hydrogen gas temperature and pressure variations. Jun Liu et al. [12] examined the fast filling (3, 5 min) and 10 min holding process of 150 L on-bus type III and type IV gaseous hydrogen storage cylinder using a two-dimensional (2D) axisymmetric model. T. Bourgeois et al. [13,14] studied hydrogen vehicle refueling requirements for the temperature limits to the tank, specification of the average delivery temperature. They developed new models of various types for predicting both the gas and material temperatures inside a vessel during filling and defueling. Li et al. [15] studied the effect of hydrogen temperature rise in 120 L type IV hydrogen cylinder to predict the influence on temperature with different ratios of length to diameter, different inlet diameters of cylinders, and different types of mass flow rate. Numerical studies should be based on the validated gas and turbulence model. Although many studies have been conducted for the hydrogen gas phenomenon during filling and refueling process, there are limited studies on numerical approaches for examining CGH2 for large-capacity hydrogen tanks during filling for over 150 L tank.
The present study intends to conduct an investigation on large volume of the 175 L hydrogen storage tank, especially aimed to apply for railway vehicles. To the best of the author’s knowledge, the present study is the first attempt to quantitatively evaluate CGH2 behavior in a 175 L hydrogen storage tank. For that purpose, the CGH2 behavior in a hydrogen tank during filling was examined. To validate the numerical approach, numerical models for the adaptation of gas and turbulence models were investigated. Numerical parametric studies of hydrogen filling behavior were conducted to estimate the CGH2 behavior in a hydrogen tank under different ambient temperatures and initial state of charge (SOC) pressure conditions during filling.
2. Numerical Implementation
2.1. Governing Equation
The governing equations for the filling phenomenon of CGH2 are the equations for conservation of mass and momentum in unsteady relations. The governing equations are considered with the Reynolds density-averaged variables in Cartesian tensor notation [16]. The conservation equations of mass and momentum for the filling phenomenon of hydrogen gas are described as follows:
where , p, t, , E, , , T, and are the density, pressure, time, Kronecker delta tensor, total energy, thermal conductivity, specific heat, turbulence viscosity, turbulent Prandtl number, and deviatoric stress tensor respectively. Here total energy and deviatoric stress tensor are expressed as follows:
where represents the effective viscosity. The energy equation is coupled to the conservation of mass and momentum equation. The superscripts and—denote the turbulent fluctuating component and Reynolds time-averaged component, respectively.
2.2. Assumptions
- (1)
- Thermal properties of liner (aluminum) and laminate (CFRP) for the calculation materials were considered as constant with temperature.
- (2)
- Under high-speed jet and strong convection of hydrogen gas during the filling process, the influences of gravity are relatively small. Based on a two-dimensional axisymmetric model, the buoyancy effect is neglected.
- (3)
- Temperature of inlet gas is considered as a constant of pre-cooling temperature of 233 K. Additionally, the initial temperature of the hydrogen tank is considered as the ambient temperature.
2.3. Calculation Conditions
In the present study, a 175 L Type IV hydrogen tank was examined using a two-dimensional (2D) axisymmetric model. To verify the adaptability of the numerical models, a numerical validation study was conducted for the experimental results of Zheng et al. [6] with a Type III hydrogen tank. The specifications of the hydrogen tank examined are listed in Table 1. Mesh grid independence study was performed by successively decreasing the element size with a structured grid: a coarse grid with 100,000 meshes and a medium grid with 150,000 and a fine grid with 200,000 meshes. The mesh grid was selected for approximately 150,000 in the present study, as shown in Figure 1, until the solution becomes independent of the mesh density, as the number of mesh elements was increased gradually. The hydrogen gas flow was treated to be compressible and unsteady. Material properties considered for the present calculation are shown in Table 2. For filling condition, the condition of inlet of the cylinder was set to pressure inlet condition (initial SOC pressure conditions) with different temperature conditions shown in Table 3. An inflated layer of hexahedral elements was generated for the mesh treatment, and a structured and non-adaptive grid (hexahedral grid) was generated with nodes. The governing equations with the boundary conditions were solved using the finite difference method with the commercial computational fluid dynamics package (ANSYS Fluent, version 18) [16].

Table 1.
Specifications of hydrogen tank.
Figure 1.
The mesh grid for the numerical analysis (a) Type III (b) Type IV.

Table 2.
Material properties.

Table 3.
Calculation conditions.
3. Result and Discussion
3.1. Numerical Validation
The numerical validation was performed to verify the adaptability of the numerical models by comparing the obtained results with the experimental results of Zheng et al. [6] with a Type III hydrogen tank. Numerical conditions and details were considered with the experimental conditions in the work of Zheng et al. [6]. In our previous studies [3,16], the Redlich-Kwong gas model of hydrogen showed good adaptability for CGH2 filling behavior. For the adaptability of the turbulence model, the turbulence models of realizable k-epsilon [17], renormalization group (RNG) k-epsilon (RNG), shear stress transport (SST) models, and Reynolds stress model (RSM) were considered for the CGH2 behavior.
Figure 2 shows a comparison of the average gas pressure and temperature of hydrogen in the tank during the filling processes for 200 s obtained using the different turbulence models. Increasing trends in pressure and temperature distributions were observed. In the early stage of the filling process, the pressure and temperature deviations from the experimental results increased with maximum deviations of 11% at 40 s and 1% at 100 s, respectively. However, the deviations decreased with the filling time.
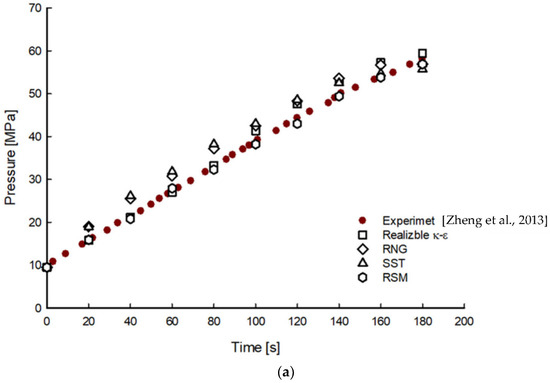
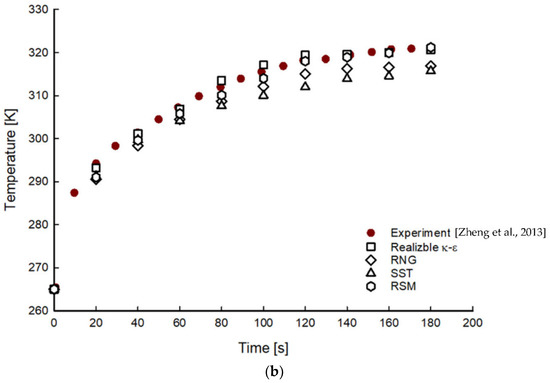
Figure 2.
Time histories of pressure (a) and temperature (b) distributions with different turbulence models during filling processes [6].
Among the different turbulence models, results obtained using the realizable k-epsilon and RSM models showed the closest agreement with the experimental values, while those from the RNG and SST models showed the largest deviation. These results are similar to those reported by Suryan et al. [18].
For the pressure distribution, the realizable k-epsilon and RSM models showed slight deviations of 2% and 1.6%, respectively. The realizable k-epsilon and RSM models showed minor deviations of 0.2% and 0.4%, respectively, in the temperature distribution. Between two turbulence models, reasonable solutions can be obtained in the RSM model. However, more computational effort and cost are required to solve six additional transport equations to calculate the Reynolds stress [19]. Therefore, the realizable k-epsilon model was found to be a suitable turbulence model considering the accuracy, convergence, and computational cost for the calculation of the CGH2 filling phenomenon.
3.2. CGH2 Behaviors
A certain quantity of hydrogen gas remains in the hydrogen tank, such that the fuel tank of the vehicle is refueled with residual fuel in it. The pressure of the remaining gas influences the maximum temperature rise during refueling. In addition, the ambient temperature can be influenced by the hydrogen state in the tank. Therefore, in the present study, numerical parametric studies of CGH2 behavior in a hydrogen tank during its filling for the 175 L tank were conducted with validated numerical method under different ambient temperatures and initial SOC pressure conditions, as shown in Table 3. The initial SOC pressure and ambient temperature conditions were 5~35 MPa (7~50%) and 243~313 K, respectively. Calculation of the filling process for the present simulation was proceeded until tank pressure reached the final pressure of 70 MPa.
The representative velocity vectors at 50 s under 5 MPa and 243 K from commencement of filling are illustrated in Figure 3. Gas velocity and velocity gradients are found to be highest in the vicinity of tank axis. It is observed that the incoming jet strikes the bottom of the tank, and flow turns along the surface toward the inlet, forming a secondary flow region on either side of inlet jet. Flow velocity gradually decreases with increase in pressure, as density of gas increases as the tank is filled up.
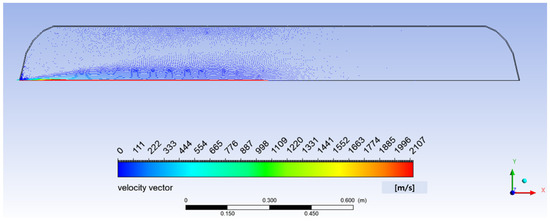
Figure 3.
Representative velocity vector (hydrogen filling at 50 s under 5 MPa and 243 K).
Figure 4 shows the representative temperature contour of hydrogen filling at 40 s under 5 MPa and 273 K, and Figure 5 shows the time histories of the hydrogen gas pressure and temperature in the tank for the 5 MPa SOC pressure condition under different ambient temperature conditions of the tank at 243–313 K. The rate of pressure rise is shown with a constant increase phase for 400 s, after which the pressure rise rate decreases.
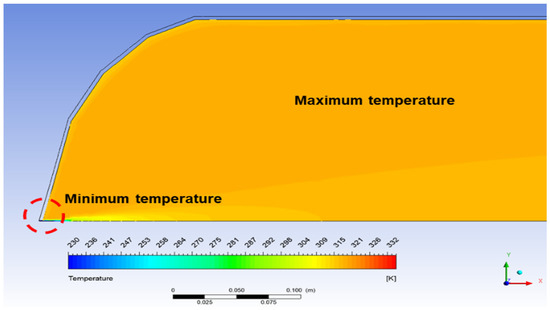
Figure 4.
Representative temperature contour of hydrogen filling at 40s under 5 MPa and 273 K.
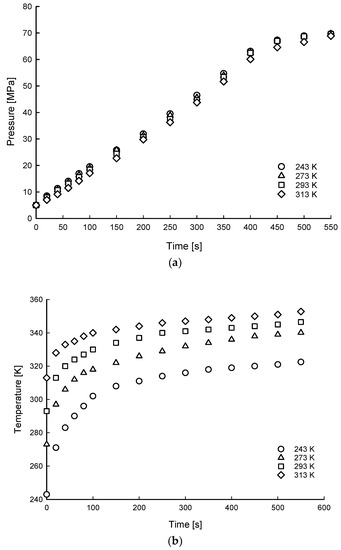
Figure 5.
Time histories of hydrogen of average gas pressure (a) and temperature (b) distributions of filling process for 5 MPa SOC pressure condition under different ambient temperature conditions.
The time to charge the hydrogen pressure to 70 MPa was from 586 s to 615 s under ambient temperature conditions of the tank from 243 K to 313 K. The gas temperature inside the tank increased dramatically at the beginning of charging. As the filling process continued, the rate of temperature rise decreased.
Figure 6, Figure 7 and Figure 8 show the time histories of hydrogen of average gas pressure and temperature in the tank for the 10, 20, and 35 MPa of SOC pressure conditions. The results of maximum temperature and time to fill up to 70 MPa under different ambient temperatures and initial SOC pressure conditions are summarized in Table 4. The average times to charge hydrogen up to 70 MPa were 600, 495, 191, and 148 s for 5, 10, 20, and 35 MPa, respectively. This means that the average charging time of hydrogen was from 600 s to 148 s for 7% to 50% SOC for the 175 L tank.
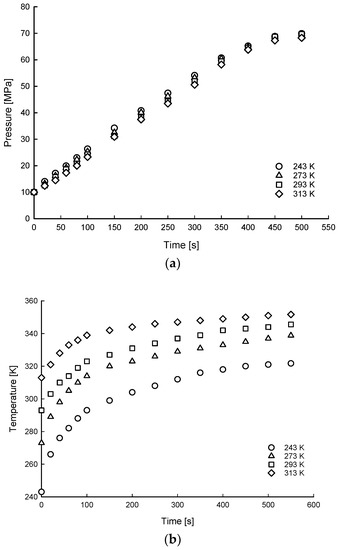
Figure 6.
Time histories of hydrogen of average gas pressure (a) and temperature (b) distributions of filling process for 10 MPa SOC pressure condition under different ambient temperature conditions.
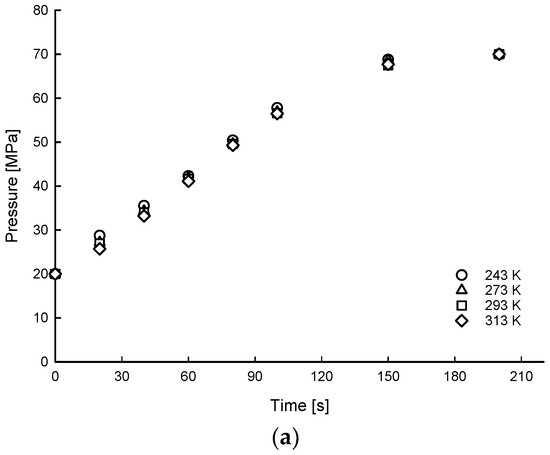
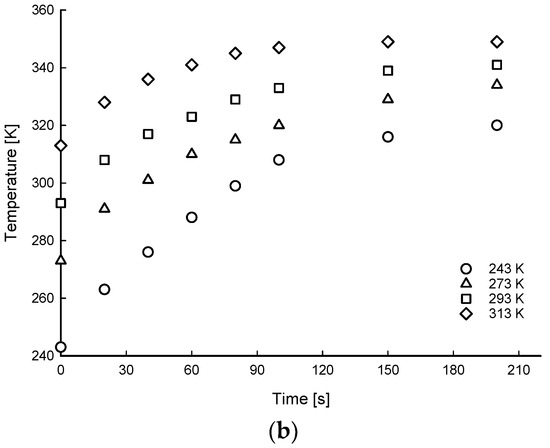
Figure 7.
Time histories of hydrogen of average gas pressure (a) and temperature (b) distributions of filling process for 20 MPa SOC pressure condition under different ambient temperature conditions.
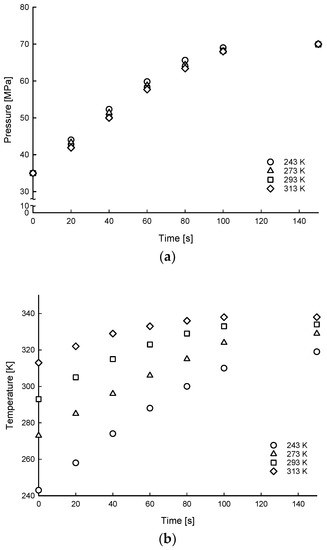
Figure 8.
Time histories of hydrogen of average gas pressure (a) and temperature (b) distributions of filling process for 35 MPa SOC pressure condition under different ambient temperature conditions.

Table 4.
Results of CGH2 filling phenomenon.
As for the different ambient temperature effects of the hydrogen tank, a relatively large pressure increase rate was observed with decreasing ambient temperature conditions. Therefore, a relatively shorter time to reach a maximum of 70 MPa was observed under decreased temperature conditions. This indicates that the charging time was different under ambient temperature conditions.
For the 5 MPa SOC pressure condition, the time to fully charge up to 70 MPa had a time difference of 29 s between ambient temperature conditions of 243 K and 313 K. A decrease in this time was observed with decreasing ambient temperature. Less time difference (17 s) was observed to fully charge hydrogen for a large SOC pressure condition of 35 MPa compared to the 29 s time difference with 5 MPa. With the different SOC conditions, the maximum temperature rise of the hydrogen tank when the pressure reached 70 MPa decreased as the initial SOC pressure increased. The temperature of hydrogen reached 313–353 K at an ambient temperature of 243–313 K for the 5 MPa SOC pressure condition. On the other hand, at 35 MPa of SOC pressure condition, the temperature of hydrogen reached 298–349 K at the same conditions. This is because the temperature rise occurred rapidly for the relatively smaller SOC pressure condition of 5 MPa compared with 35 MPa.
A relationship between the initial SOC pressure condition and the maximum temperature rise of hydrogen gas is observed in Figure 9 and Figure 10. The maximum temperature rise increased with decreasing ambient temperature, and the rise increased with decreasing SOC; similar results were reported by de Miguel, Nerea et al. [20]. In the small-initial-SOC condition of the tank, a relatively large quantity of hydrogen was injected into the tank at a low-density state. With hydrogen injection into the tank, heat was input to the tank and transferred; therefore, higher increases in pressure and temperature were observed under small-initial-SOC-pressure conditions.
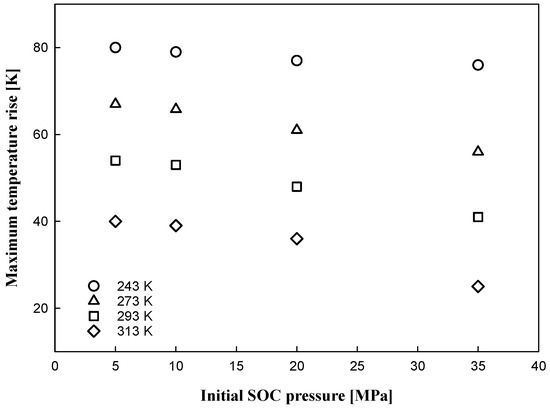
Figure 9.
Maximum temperature rise with initial SOC pressure condition.
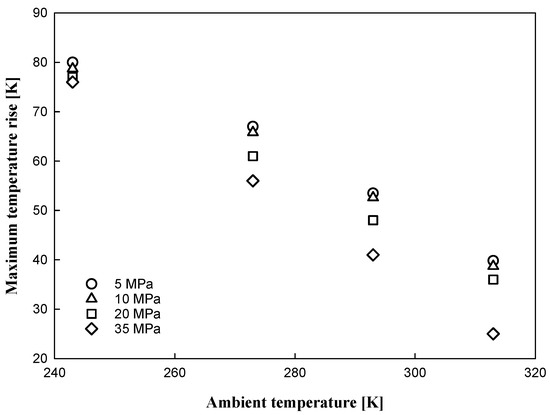
Figure 10.
Maximum temperature rise with ambient temperature condition under different SOC pressure conditions.
From a safety point of view, more careful operation is required for the 5 MPa SOC pressure condition at an ambient temperature of 313 K because it is possible to exceed the temperature limit while filling the tank with hydrogen. In rapid filling, regardless of the type of tank, it is desirable to fill the tank at an initial SOC pressure of 10% or greater.
4. Conclusions
In this study, CGH2 behavior in a hydrogen tank was investigated numerically during its filling. To validate the numerical approach, numerical models for the adaptation of gas and turbulence models were investigated. Numerical parametric studies of hydrogen filling behavior were conducted to estimate the CGH2 behavior in a hydrogen tank under different ambient temperatures and initial state of charge (SOC) pressure conditions during filling for the 175 L tank. The main conclusions of this study are as follows:
- From a numerical validation study for the comparison of experimental results of Zheng et al. [6] with a Type III hydrogen tank, gas model and turbulence model were validated. The Redlich–Kwong gas model and realizable k-epsilon and RSM models was found to be a suitable gas model and turbulence model, respectively. However, the realizable k-epsilon model was found to be a suitable turbulence model considering the accuracy, convergence, and computational cost for the calculation of the CGH2 filling phenomenon.
- The average times to charge hydrogen up to 70 MPa were 600, 495, 191, and 148 s for 5, 10, 20, and 35 MPa, respectively, indicating that the average charging time of hydrogen was from 600 s to 148 s for 7% to 50% SOC for the 175 L tank. The time when the pressure reached 70 MPa was influenced by the ambient temperature and SOC condition. For the 5 MPa SOC condition, the time to fully charge up to 70 MPa had a time difference of 29 s between ambient temperature conditions of 313 K and 243 K. Less time difference (17 s) was observed to fully charge hydrogen for a large SOC condition of 35 MPa compared with the 5 MPa case.
- With the different SOC conditions, the maximum temperature rise of the hydrogen tank when the pressure reached 70 MPa decreased as the initial SOC pressure increased because the temperature rise occurred rapidly for the relatively smaller SOC condition. The temperature rises of hydrogen reached 313–353 K at an ambient temperature of 243–313 K for the 5 MPa SOC condition. However, at 35 MPa of SOC, the temperature of hydrogen reached 298–349 K at an ambient temperature of 243–313 K.
- A relationship between the initial SOC pressure condition and the maximum temperature rise of hydrogen gas showed that the maximum temperature rise increased with decreasing ambient temperature, and the rise increased with decreasing SOC.
- The numerical approach for the applications presented in this paper could also be widely applied to design hydrogen facilities and systems. The present model will be compared with a three-dimensional model in a future study.
Author Contributions
Conceptualization: M.-S.K. and S.-W.C.; Methodology: H.-K.J. and J.-H.R.; Validation: M.-S.K. and S.-W.C.; Investigation: K.-W.L. and J.-H.R.; Data Curation: M.-S.K. and S.-W.C.; Writing, Review and Editing—Original Draft Preparation: M.-S.K.; Supervision, M.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) for the Materials/Parts Technology Development Program (20017575, Development of Applicability Evaluation Technology for Cryogenic Insulation Material and Storage Vessel considering Operating Condition of Hydrogen Commercial Vehicle).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was supported in part by the Materials/Parts Technology Development Program (20017575, Development of Applicability Evaluation Technology for Cryogenic Insulation Material and Storage Vessel considering Operating Condition of Hydrogen Commercial Vehicle) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea), and in part by the Railroad Technology Research Program through the Ministry of Land, Infrastructure and Transport of Korean Government under Grant 22RTRP-C146008-05.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. Available online: https://www.nature.com/articles/nature08823 (accessed on 7 December 2018). [CrossRef] [PubMed]
- Akansu, S. Internal combustion engines fueled by natural gas? hydrogen mixtures. Int. J. Hydrogen Energy 2004, 29, 1527–1539. [Google Scholar] [CrossRef]
- Kim, S.C.; Lee, S.H.; Yoon, K.B. Thermal characteristics during hydrogen fueling process of type IV cylinder. Int. J. Hydrogen Energy 2010, 35, 6830–6835. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Salimian, M.; Okhay, O.; Rajendran, S.; Rajkumar, A.; Sousa, J.M.G.; Ferreira, A.L.C.; Campos, J.; Gracio, J. Hydrogen Storage for Energy Application. In Hydrogen Storage; InTech Open: London, UK, 2012. [Google Scholar]
- Zheng, J.; Guo, J.; Yang, J.; Zhao, Y.; Zhao, L.; Pan, X.; Ma, J.; Zhang, L. Experimental and numerical study on temperature rise within a 70 MPa type III cylinder during fast refueling. Int. J. Hydrogen Energy 2013, 38, 10956–10962. [Google Scholar] [CrossRef]
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 4569–4574. [Google Scholar] [CrossRef]
- Chung, S.-M.; Seo, Y.-S.; Jeon, G.-M.; Kim, J.-W.; Park, J.-C. Parameter Study of Boiling Model for CFD Simulation of Multiphase-Thermal Flow in a Pipe. J. Ocean Eng. Technol. 2021, 35, 50–58. [Google Scholar] [CrossRef]
- Johnson, T.; Bozinoski, R.; Ye, J.; Sartor, G.; Zheng, J.; Yang, J. Thermal model development and validation for rapid filling of high pressure hydrogen tanks. Int. J. Hydrogen Energy 2015, 40, 9803–9814. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, G.; Liu, Y.; Zheng, J.; Chen, Y.; Zhao, L.; Guo, J.; He, Y. Numerical study on fast filling of 70 MPa type III cylinder for hydrogen vehicle. Int. J. Hydrogen Energy 2012, 37, 17517–17522. [Google Scholar] [CrossRef]
- Suryan, A.; Kim, H.D.; Setoguchi, T. Three dimensional numerical computations on the fast filling of a hydrogen tank under different conditions. Int. J. Hydrogen Energy 2012, 37, 7600–7611. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, S.; Zhang, Z.; Zheng, J.; Zhao, Y. Numerical study on the fast filling of on-bus gaseous hydrogen storage cylinder. Int. J. Hydrogen Energy 2020, 45, 9241–9251. [Google Scholar] [CrossRef]
- Bourgeois, T.; Brachmann, T.; Barth, F.; Ammouri, F.; Baraldi, D.; Melideo, D.; Acosta-Iborra, B.; Zaepffel, D.; Saury, D.; Lemonnier, D. Optimization of hydrogen vehicle refuelling requirements. Int. J. Hydrogen Energy 2017, 42, 13789–13809. [Google Scholar] [CrossRef]
- Bourgeois, T.; Ammouri, F.; Baraldi, D.; Moretto, P. The temperature evolution in compressed gas filling processes: A review. Int. J. Hydrogen Energy 2018, 43, 2268–2292. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, J.; Chang, Q.; Xing, W. Effects of geometry and inconstant mass flow rate on temperatures within a pressurized hydrogen cylinder during refueling. Int. J. Hydrogen Energy 2012, 37, 6043–6052. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ryu, J.-H.; Oh, S.-J.; Yang, J.-H.; Choi, S.-W. Numerical Investigation on Influence of Gas and Turbulence Model for Type III Hydrogen Tank under Discharge Condition. Energies 2020, 13, 6432. [Google Scholar] [CrossRef]
- Shih, T.-H. A Realizable Reynolds Stress Algebraic Equation Model; National Aeronautics and Space Administration: Washington, DC, USA, 1993; Volume 105993.
- Suryan, A.; Kim, H.; Setoguchi, T. Comparative study of turbulence models performance for refueling of compressed hydrogen tanks. Int. J. Hydrogen Energy 2013, 38, 9562–9569. [Google Scholar] [CrossRef]
- Prieler, R.; Demuth, M.; Spoljaric, D.; Hochenauer, C. Numerical investigation of the steady flamelet approach under different combustion environments. Fuel 2015, 140, 731–743. [Google Scholar] [CrossRef]
- De Miguel, N.; Acosta, B.; Baraldi, D.; Melideo, R.; Cebolla, R.O.; Moretto, P. The role of initial tank temperature on refuelling of on-board hydrogen tanks. Int. J. Hydrogen Energy 2016, 41, 8606–8615. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).