Ultra-Scratch-Resistant, Hydrophobic and Transparent Organosilicon-Epoxy-Resin Coating with a Double Cross-Link Structure
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of Coatings
2.2.1. Preparation of the DETA-Organosilicon-Epoxy-Resin (DETA-OSER) Coating and MXDA-Organosilicon-Epoxy-Resin (MXDA-OSER) Coating
2.2.2. Preparation of the NH3-Organosilicon-Epoxy-Resin (NH3-OSER) Coating
2.2.3. Preparation of the TEOS/MTES (TM) Coatings
2.3. Characterization
3. Results and Discussion
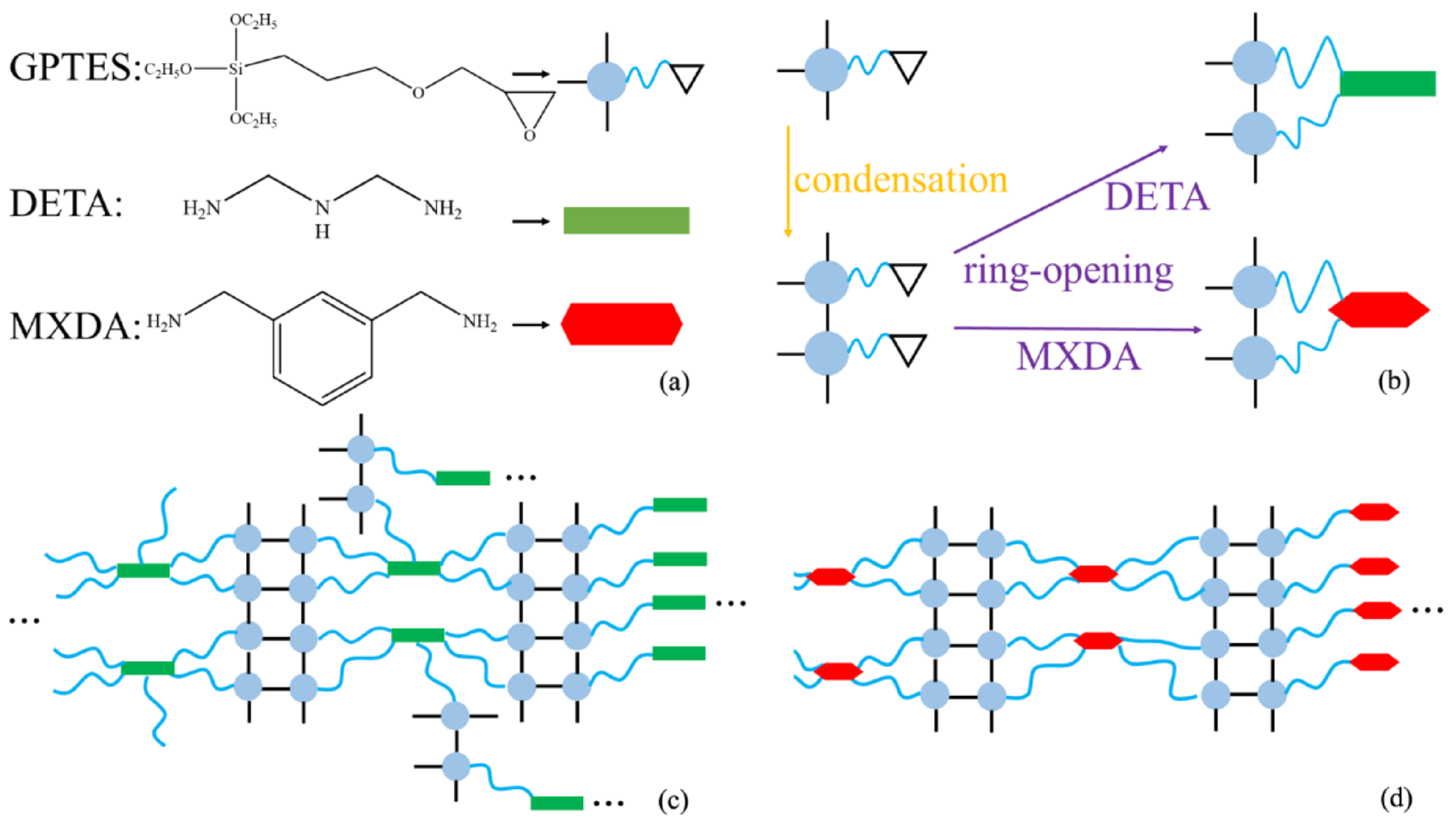
3.1. Formation Mechanism of the Double-Cross-Link Structure
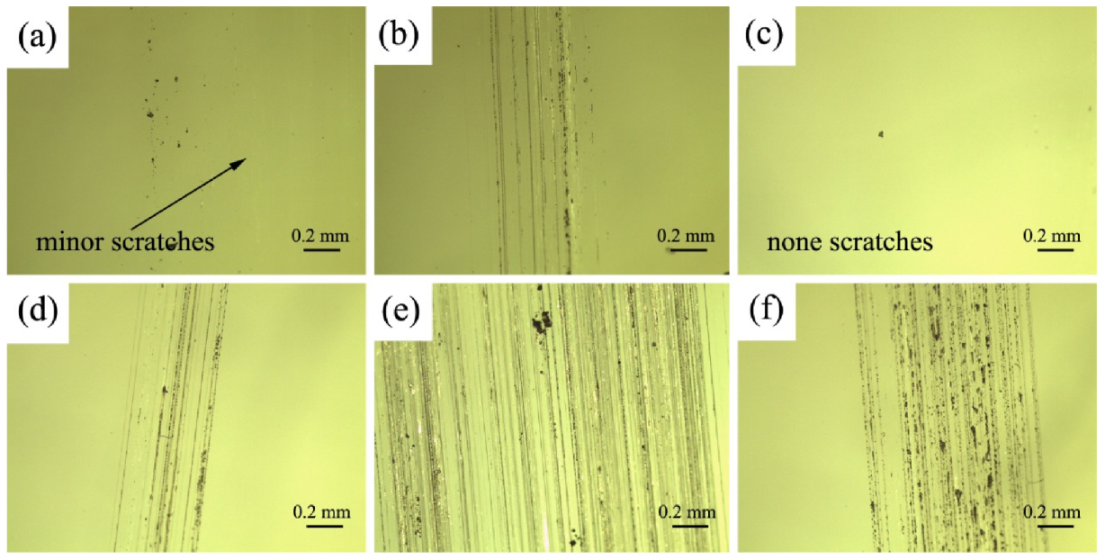
3.2. Mechanical Property of Coatings
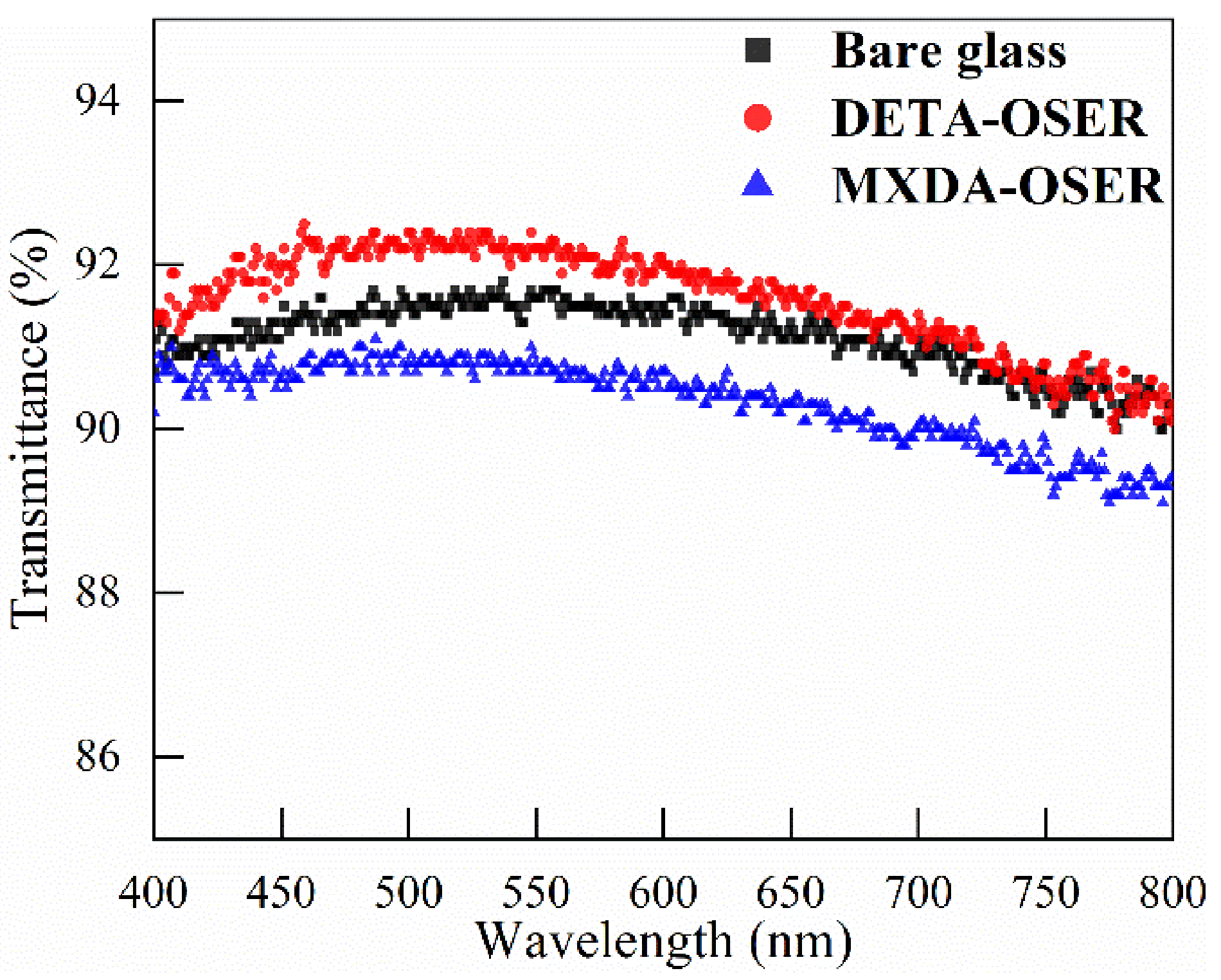
3.3. Morphology and Optical Property of Coatings
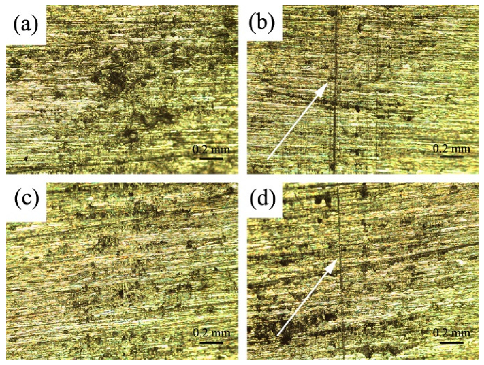
3.4. Durability in Different Environments of Coatings
3.5. The Coating on Different Substrates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Müllen, K.; Li, C.; Butt, H.-J.; Vollmer, D. Transparent, Thermally Stable and Mechanically Robust Superhydrophobic Surfaces Made from Porous Silica Capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cheng, Y.; Huang, J.; Xiong, J.; Ge, M.; Mao, J.; Liu, Z.; Dong, X.; Chen, Z.; Lai, Y. A transparent superhydrophobic coating with mechanochemical robustness for anti-icing, photocatalysis and self-cleaning. Chem. Eng. J. 2020, 399, 125746. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.L.; Zhang, H.L.; Yan, H.W.; Lv, H.B.; Jiang, B. Sol–Gel Preparation of Hydrophobic Silica Antireflective Coatings with Low Refractive Index by Base/Acid Two-Step Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 11470–11475. [Google Scholar] [CrossRef]
- Chevallier, P.; Turgeon, S.; Sarra-Bournet, C.; Turcotte, R.; Laroche, G. Characterization of Multilayer Anti-Fog Coatings. ACS Appl. Mater. Interfaces 2011, 3, 750–758. [Google Scholar] [CrossRef]
- Brown, P.S.; Atkinson, O.D.L.A.; Badyal, J.P.S. Ultrafast Oleophobic-Hydrophilic Switching Surfaces for Antifogging, Self-Cleaning, and Oil-Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 7504–7511. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation Mechanism and Construction of Surfaces with Special Wettability for Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef]
- Mohammadkhani, R.; Ramezanzadeh, M.; Saadatmandi, S.; Ramezanzadeh, B. Designing a dual-functional epoxy composite system with self-healing/barrier anti-corrosion performance using graphene oxide nano-scale platforms decorated with zinc doped-conductive polypyrrole nanoparticles with great environmental stability and non-toxicity. Chem. Eng. J. 2019, 382, 122819. [Google Scholar]
- Cho, E.C.; Chang-Jian, C.W.; Chen, H.C.; Chuang, K.S.; Zheng, J.H.; Hsiao, Y.S.; Huang, J.H. Robust Multifunctional Superhydrophobic Coatings with Enhanced Water/Oil Separation, Self-Cleaning, Anti-Corrosion, and Anti-biological adhesion. Chem. Eng. J. 2017, 314, 347–357. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, T.; Liu, Z.; Pu, J.; Liu, W.; Wang, L. Superior corrosion resistance and self-healable epoxy coating pigmented with silanzied trianiline-intercalated graphene. Carbon 2018, 142, 164–176. [Google Scholar] [CrossRef]
- Siddique, R.H.; Gomard, G.; Hoelscher, H. The role of random nanostructures for the omnidirectional anti-reflection properties of the glasswing butterfly. Nat. Commun. 2015, 6, 6909. [Google Scholar] [CrossRef]
- Zhang, J.; Ai, L.; Lin, S.; Lan, P.; Lu, Y.; Dai, N.; Tan, R.; Fan, B.; Song, W. Preparation of humidity, abrasion, and dust resistant antireflection coatings for photovoltaic modules via dual precursor modification and hybridization of hollow silica nanospheres. Sol. Energy Mater. Sol. Cells 2019, 192, 188–196. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, Self-Healing Superamphiphobic Fabrics Prepared by Two-Step Coating of Fluoro-Containing Polymer, Fluoroalkyl Silane, and Modified Silica Nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- Dong, X.; Gao, S.; Huang, J.; Li, S.; Zhu, T.; Cheng, Y.; Zhao, Y.; Chen, Z.; Lai, Y. A self-roughened and biodegradable superhydrophobic coating with UV shielding, solar-induced self-healing and versatile oil-water separation ability. J. Mater. Chem. A 2019, 7, 2122–2128. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Z.; Jia, E.; Zhang, G.; Su, Z. Transparent and Scratch-Resistant Antifogging Coatings with Rapid Self-Healing Capability. ACS Appl. Mater. Interfaces 2019, 11, 30300–30307. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self-cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Huang, Y.; Jen, Y.; Ganguly, A.; Chen, K.-H.; Chen, L. Anti-reflecting and photonic nanostructures. Mater. Sci. Eng. R Rep. 2010, 69, 1–35. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Xia, B.-B.; Ye, H.-P.; Zhang, Y.-L.; Xiao, B.; Yan, L.-H.; Lv, H.-B.; Jiang, B. One-step sol–gel preparation of PDMS–silica ORMOSILs as environment-resistant and crack-free thick antireflective coatings. J. Mater. Chem. 2012, 22, 13132–13140. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Chi, F.; Liu, D.; Wu, H.; Lei, J. Mechanically robust and self-cleaning antireflection coatings from nanoscale binding of hydrophobic silica nanoparticles. Sol. Energy Mater. Sol. Cells 2019, 200, 109939. [Google Scholar] [CrossRef]
- Wang, Z.; van Andel, E.; Pujari, S.P.; Feng, H.; Dijksman, J.A.; Smulders, M.M.J.; Zuilhof, H. Water-repairable zwitterionic polymer coatings for anti-biofouling surfaces. J. Mater. Chem. B 2017, 5, 6728–6733. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042–1048. [Google Scholar] [CrossRef]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [Green Version]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [Green Version]
- Armaghani, D.J.; Mohamad, E.T.; Narayanasamy, M.S.; Narita, N.; Yagiz, S. Development of hybrid intelligent models for predicting TBM penetration rate in hard rock condition. Tunn. Undergr. Space Technol. 2017, 63, 29–43. [Google Scholar] [CrossRef]
- Momeni, E.; Armaghani, D.J.; Hajihassani, M.; Amin, M.F.M. Prediction of uniaxial compressive strength of rock samples using hybrid particle swarm optimization-based artificial neural networks. Measurement 2015, 60, 50–63. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Guan, Z. Olefin Metathesis for Effective Polymer Healing via Dynamic Exchange of Strong Carbon–Carbon Double Bonds. J. Am. Chem. Soc. 2012, 134, 14226–14231. [Google Scholar] [CrossRef]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-Healing of Covalently Cross-Linked Polymers by Reshuffling Thiuram Disulfide Moieties in Air under Visible Light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef]
- Ji, S.; Cao, W.; Yu, Y.; Xu, H. Visible-Light-Induced Self-Healing Diselenide-Containing Polyurethane Elastomer. Adv. Mater. 2015, 27, 7740–7745. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Bapat, A.P.; Sumerlin, B.S. Room-Temperature Self-Healing Polymers Based on Dynamic-Covalent Boronic Esters. Macromolecules 2015, 48, 2098–2106. [Google Scholar] [CrossRef]
- Hasanipanah, M.; Monjezi, M.; Shahnazar, A.; Jahed Armaghani, D.; Farazmand, A. Feasibility of indirect determination of blast induced ground vibration based on support vector machine. Measurement 2015, 75, 289–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Wang, P.; Ye, L.; Luo, J.; Jiang, B. A convenient sol–gel approach to the preparation of nano-porous silica coatings with very low refractive indices. Chem. Commun. 2014, 50, 13813–13816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, P.; Wang, P.; Luo, J.; Jiang, B. Spherical-chain silica with super-hydrophobic surface and ultra-low refractive index for multi-functional broadband antireflective coatings. Sol. Energy 2020, 207, 1222–1230. [Google Scholar] [CrossRef]
- Mousavi, H.; Jilavi, M.H.; Koch, M.; Arzt, E.; De Oliveira, P.W. Development of a Transparent Scratch Resistant Coating through Direct Oxidation of Al-Coated Glass. Adv. Eng. Mater. 2017, 19, 1600617. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Wang, X.; Lin, T. Fluoroalkyl Silane Modified Silicone Rubber/Nanoparticle Composite: A Super Durable, Robust Superhydrophobic Fabric Coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.; Rashid, M.A.; Chen, L.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W.; Qiu, Y. Vanillin-Based Epoxy Vitrimer with High Performance and Closed-Loop Recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Shen, L.; Cui, Z.; Kou, L.; Cheng, J.; Zhang, J. A renewable resveratrol-based epoxy resin with high Tg, excellent mechanical properties and low flammability. Chem. Eng. J. 2020, 383, 123124. [Google Scholar] [CrossRef]
- Zhi, J.; Zhang, L.-Z. Durable superhydrophobic surface with highly antireflective and self-cleaning properties for the glass covers of solar cells. Appl. Surf. Sci. 2018, 454, 239–248. [Google Scholar] [CrossRef]
- Kesmez, Ö. Preparation of hybrid nanocomposite coatings via sol-gel method for hydrophobic and self-cleaning properties. J. Mol. Struct. 2020, 1205, 127572. [Google Scholar] [CrossRef]
- Davis, S.R.; Brough, A.R.; Atkinson, A. Formation of silica/epoxy hybrid network polymers. J. Non-Cryst. Solids 2003, 315, 197–205. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Colleoni, C.; Guido, E.; Botteri, L.; Rosace, G. Thermal behaviour and flame retardancy of monoethanolamine-doped sol-gel coatings of cotton fabric. Prog. Org. Coat. 2017, 103, 174–181. [Google Scholar] [CrossRef]
- Nazir, T.; Afzal, A.; Siddiqi, H.M.; Saeed, S.; Dumon, M. The influence of temperature and interface strength on the microstructure and performance of sol–gel silica–epoxy nanocomposites. Polym. Bull. 2011, 67, 1539–1551. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Ye, H.; Xiao, B.; Yan, L.; Jiang, B. A simple route to prepare crack-free thick antireflective silica coatings with improved antireflective stability. Mater. Lett. 2012, 69, 86–88. [Google Scholar] [CrossRef]
- ASTM D3363; Standard Test Method for Film Hardness by Pencil Test. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.astm.org/Standards/D3363.htm (accessed on 15 September 2021).





| 0.5 | 1.0 | 1.5 | 2.0 | |
|---|---|---|---|---|
| DETA-OSER | 2H | 5H | 7H | 8H |
| MXDA-OSER | 3B | H | 6H | 8H |
| Refractive Index (±0.003) | Thickness | |
|---|---|---|
| DETA-OSER | 1.493 | 103 nm |
| Bare glass | 1.512 | 700 µm |
| MXDA-OSER | 1.525 | 114 nm |
| 1 Day | 3 Days | 5 Days | 7 Days | |
|---|---|---|---|---|
| pH = 2 | 8H | 8H | 8H | 7H |
| pH = 12 | 8H | <6B | <6B | <6B |
| Seawater | 8H | 8H | 8H | 6H |
| Acetone | 8H | 8H | 8H | 8H |
| 1 Day | 3 Days | 5 Days | 7 Days | |
|---|---|---|---|---|
| pH = 2 | 8H | 8H | 8H | 8H |
| pH = 12 | 8H | 3B | <6B | <6B |
| Seawater | 8H | 8H | 8H | 8H |
| Acetone | 8H | 8H | 8H | 8H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Lin, H.; Zeng, L.; Liang, Y.; Zeng, C.; Hong, R. Ultra-Scratch-Resistant, Hydrophobic and Transparent Organosilicon-Epoxy-Resin Coating with a Double Cross-Link Structure. Appl. Sci. 2022, 12, 4854. https://doi.org/10.3390/app12104854
Qiu Z, Lin H, Zeng L, Liang Y, Zeng C, Hong R. Ultra-Scratch-Resistant, Hydrophobic and Transparent Organosilicon-Epoxy-Resin Coating with a Double Cross-Link Structure. Applied Sciences. 2022; 12(10):4854. https://doi.org/10.3390/app12104854
Chicago/Turabian StyleQiu, Zeyu, Haofeng Lin, Longlong Zeng, Yunfeng Liang, Chunhong Zeng, and Ruijiang Hong. 2022. "Ultra-Scratch-Resistant, Hydrophobic and Transparent Organosilicon-Epoxy-Resin Coating with a Double Cross-Link Structure" Applied Sciences 12, no. 10: 4854. https://doi.org/10.3390/app12104854
APA StyleQiu, Z., Lin, H., Zeng, L., Liang, Y., Zeng, C., & Hong, R. (2022). Ultra-Scratch-Resistant, Hydrophobic and Transparent Organosilicon-Epoxy-Resin Coating with a Double Cross-Link Structure. Applied Sciences, 12(10), 4854. https://doi.org/10.3390/app12104854






