Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes
Abstract
1. Introduction
2. Methodology
3. Antioxidant Activity
| Extract | Species | Method | Activity | References | |
|---|---|---|---|---|---|
| Ethanol | Collected | Marchantia polymorpha | DPPH; ABTS; O2- | TFC: 4.62 mg/g DPPH | [28] |
| Water:ethanol (3:7) | Collected | Hypnum plumaeforme; Thuidium kanadae; Leucobryum juniperoideum | DPPH; ABTS; FRAP | TPC: 47.20 ± 11.20 to 119.87 ± 11.51 mg/GAE mg | [29] |
| Methanol | Collected | Marchantia pelacea | DPPH | DPPH: IC50 20 µg/mL | [32] |
| Water | Collected | Bryum moravicum | ABTS | TPC: 356.44 ± 9.56 µg/mg ABTS: 84.56 ± 7.93 µg ascorbic acid eq/mg | [26] |
| Methanol | Cultured | Lunularia cruciata | DPPH; ABTS | Scavenged DPPH: 48% (650 µg/mL) 35% (350 µg/mL) 22% (250 µg/mL) Scavenged ABST: 98% (2 mg/mL) | [30] |
| Acetone | Collected | Lunularia cruciata | Phagocytes chemiluminescence | See the reference | [45] |
| Acetone | Collected | Leptodictyum riparium | Whole blood chemiluminescence | See the reference | [46] |
| Water; methanol:water (8:2); ethanol:water (8:2) | Collected | Cryphaea heteromalla | NIH-3T3 murine fibroblast ROS production | See the reference | [47] |
| Ethanol | Collected | Thuidium tamariscellum | DPPH; H2O2 assay ABTS; FRAP | Total terpenoids: 25.95 mg/g DPPH: IC50 16 µg/mL H2O2: IC50 34.5 µg/mL ABTS: IC50 18.5 µg/mL FRAP: IC50 40 µg/mL | [54] |
| Compounds | Chemical Class | Species | Methods | Activity | References | |
|---|---|---|---|---|---|---|
| (-)-herbertenediol | Sesquiterpenoid | Collected | Mastigophora diclados | DPPH | IC50 1.9 ± 0.6 µg/mL | [36] |
| (-)-mastigophorene C | (Bis)bibenzyl | Collected | Mastigophora diclados | DPPH | IC50 2.7 ± 0.8 µg/mL | [36] |
| (-)-mastigophorene D | (Bis)bibenzyl | Collected | Mastigophora diclados | DPPH | IC50 2.0 ± 0.1 µg/mL | [36] |
| Subulatin | Caffeate ester | Cultured | Jungermannia subulata; Lophocolea heterophylla; Scapania parvitexta | Lipid peroxidation | See reference | [37] |
| Ohioesins F | Benzonaphthoxanthenones | Cultured | Polytrichastrum alpinum | DPPH; ABTS; FRAP; NO scavenging activity | DPPH: IC50 10 ± 0.16 µg/mL ABTS: IC50 14.3 ± 1.2 µg/mL NO assay: 63 ± 5.1 µg/mL FRAP: 9.8 ± 0.07 µg/mL | [38] |
| Ohioesin G | Benzonaphthoxanthenones | Cultured | Polytrichastrum alpinum | DPPH; ABTS; FRAP; NO scavenging activity | DPPH: IC50 10.1 ± 1.5 µg/mL ABTS: IC50 14.8 ± 1.5 µg/mL NO assay: 62.1 ± 5.0 µg/mL FRAP: 9.6 ± 1.2 µg/mL | [38] |
| Marchantin A | (Bis)bibenzyl | Collected | Marchantia ssp | DPPH | IC50: 20 µg/mL | [39] |
| Marchantin A | (Bis)bibenzyl | Arachidonic acid oxidation | IC50: 0.4 µmol/L | [40] | ||
| Marchantin B | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 0.4 µmol/L | [40] | |
| Marchantin D | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 5.6 µmol/L | [40] | |
| Marchantin E | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 2.7 µmol/L | [40] | |
| Marchantin H | (Bis)bibenzyl | Collected | Marchantia diptera | Lipid peroxidation; DPPH | DPPH: IC0.20 10.2 ± 0.2 µM | [41] |
| Isoriccardin C | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 5.3 µmol/L | [40] | |
| Riccardin C | (Bis)bibenzyl | Arachidonic acid oxidation | IC50: 12.7 µmol/L | [40] | ||
| Perrotettin D | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 0.72 µmol/L | [40] | |
| Peleatin B | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 11.7 µmol/L | [40] | |
| Radulanin H | Bibenzyl | Arachidonic acid oxidation | IC50: 15.7 µmol/L | [40] | ||
| Plagiochin D | (Bis)bibenzyl | Collected | Plagiochila ovalifolia | DPPH | Unavailable | [42] |
| Frullanian D | Terpenoid | Frullania hamatiloba | MOVAS cells | Dose dependent NQO1 and γ-GCS induction; inhibition of H2O2-induced cytotoxicity | [48] | |
| Diplotaxifol A-B | Terpenoid | Diplophyllum taxifolium | Hepa 1c1c7 cells | Dose-dependent NQO1 induction | [49] | |
| Atractylenolide III | Terpenoid | Diplophyllum taxifolium | Hepa 1c1c7 cells | Dose-dependent NQO1 induction | [49] | |
| Epiphyllin A-D | Terpenoid | Pellia epiphylla | PC12 cells | NQO1 induction | [50] | |
| Pellianolactone B | Terpenoid | Pellia epiphylla | PC12 cells | Dose-dependent NQO1, γ-GCS induction, and inhibition of H2O2-induced cytotoxicity | [50] |
4. Antibacterial Activity
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, C.-F.; Lou, H.-X. Secondary Metabolites in Bryophytes: An Ecological Aspect. Chem. Biodivers. 2009, 6, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. Biologically Active Compounds from Bryophytes. Pure Appl. Chem. 2007, 79, 557–580. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, e1245049. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An Assessment of Oxidative Damage to Proteins, Lipids, and DNA in Brain from Patients with Alzheimer’s Disease. J. Neurochem. 1997, 68, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Oxidative Stress and Parkinson’s Disease. In Handbook of Clinical Neurology; Parkinson’s Disease and Related Disorders, Part I.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 83, pp. 507–520. [Google Scholar]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of Oxidative Stress in Cardiovascular Diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic Res. 2010, 44, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial Mutations and Mitoepigenetics: Focus on Regulation of Oxidative Stress-Induced Responses in Breast Cancers. Semin Cancer Biol. 2020, in press. [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and Indirect Antioxidant Properties of Inducers of Cytoprotective Proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef] [PubMed]
- Balasaheb Nimse, S.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Yao, J.-W.; Liu, J.; Kong, X.-Z.; Zhang, S.-G.; Wang, X.-H.; Yu, M.; Zhan, Y.-Q.; Li, W.; Xu, W.-X.; Tang, L.-J.; et al. Induction of Activation of the Antioxidant Response Element and Stabilization of Nrf2 by 3-(3-Pyridylmethylidene)-2-Indolinone (PMID) Confers Protection against Oxidative Stress-Induced Cell Death. Toxicol. Appl. Pharm. 2012, 259, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, e9372182. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Spellberg, B.; Gilbert, D.N. The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect Dis. 2014, 59 (Suppl. 2), S71–S75. [Google Scholar] [CrossRef]
- Shaw, A.J.; Goffinet, B. Bryophyte Biology; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-66794-4. [Google Scholar]
- Soriano, G.; Del-Castillo-Alonso, M.-Á.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Phenolic Compounds from Different Bryophyte Species and Cell Compartments Respond Specifically to Ultraviolet Radiation, but Not Particularly Quickly. Plant Physiol. Biochem. 2019, 134, 137–144. [Google Scholar] [CrossRef]
- Núñez-Olivera, E.; Otero, S.; Tomás, R.; Martínez-Abaigar, J. Seasonal Variations in UV-Absorbing Compounds and Physiological Characteristics in the Aquatic Liverwort Jungermannia Exsertifolia Subsp. Cordifolia over a 3-Year Period. Physiol. Plant. 2009, 136, 73–85. [Google Scholar] [CrossRef]
- Otero, S.; Núñez-Olivera, E.; Martínez-Abaigar, J.; Tomás, R.; Arróniz-Crespo, M.; Beaucourt, N. Effects of Cadmium and Enhanced UV Radiation on the Physiology and the Concentration of UV-Absorbing Compounds of the Aquatic Liverwort Jungermannia Exsertifolia Subsp. Cordifolia. Photochem. Photobiol. Sci. 2006, 5, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Bogdanovic-Pristov, J.; Pejin, I.; Sabovljevic, M. Potential Antioxidant Activity of the Moss Bryum Moravicum. Nat. Prod. Res. 2013, 27, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Glamočlija, J.; Ćirić, A.; Kien-Thai, Y.; Soković, M. The Moss Rhodobryum Ontariense Tea, a Good Source of Natural Antifungals Against Candida Albicans. Rev. Chim. 2013, 64, 5–554. [Google Scholar]
- Wang, X.; Cao, J.; Wu, Y.; Wang, Q.; Xiao, J. Flavonoids, Antioxidant Potential, and Acetylcholinesterase Inhibition Activity of the Extracts from the Gametophyte and Archegoniophore of Marchantia Polymorpha L. Molecules 2016, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-O.; Choi, K.-S.; Kim, Y.-H. In Vitro Antioxidative Activity of Moss Extract, and Effect of Moss on Serum Lipid Level of Mice Fed with High-Fat Diet. Trop. J. Pharm. Res. 2016, 15, 1215–1224. [Google Scholar] [CrossRef][Green Version]
- Mukhia, S.; Mandal, P.; Singh, D.K.; Singh, D. Comparison of Pharmacological Properties and Phytochemical Constituents of In Vitro Propagated and Naturally Occurring Liverwort Lunularia Cruciata. BMC Complementary Altern. Med. 2019, 19, 181. [Google Scholar] [CrossRef]
- Wolski, G.J.; Sadowska, B.; Fol, M.; Podsędek, A.; Kajszczak, D.; Kobylińska, A. Cytotoxicity, Antimicrobial and Antioxidant Activities of Mosses Obtained from Open Habitats. PLoS ONE 2021, 16, e0257479. [Google Scholar] [CrossRef]
- Siregar, E.S.; Pasaribu, N.; Sofyan, M.Z. Antioxidant Activity of Liverworts Marchantia Paleacea Bertol. From North Sumatra Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 713. [Google Scholar]
- Mandić, M.R.; Oalđe, M.M.; Lunić, T.M.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Dj Božić, B.; Dj. Božić Nedeljković, B. Chemical Characterization and in Vitro Immunomodulatory Effects of Different Extracts of Moss Hedwigia Ciliata (Hedw.) P. Beauv. From the Vršačke Planine Mts., Serbia. PLoS ONE 2021, 16, e0246810. [Google Scholar] [CrossRef]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical Composition Analysis, Antimicrobial Activity and Cytotoxicity Screening of Moss Extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef]
- Horn, A.; Pascal, A.; Lončarević, I.; Volpatto Marques, R.; Lu, Y.; Miguel, S.; Bourgaud, F.; Thorsteinsdóttir, M.; Cronberg, N.; Becker, J.D.; et al. Natural Products from Bryophytes: From Basic Biology to Biotechnological Applications. Crit. Rev. Plant Sci. 2021, 40, 191–217. [Google Scholar] [CrossRef]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Asakawa, Y. Cytotoxic, Radical Scavenging and Antimicrobial Activities of Sesquiterpenoids from the Tahitian Liverwort Mastigophora Diclados (Brid.) Nees (Mastigophoraceae). J. Nat. Med. 2010, 64, 417–422. [Google Scholar] [CrossRef]
- Tazaki, H.; Ito, M.; Miyoshi, M.; Kawabata, J.; Fukushi, E.; Fujita, T.; Motouri, M.; Furuki, T.; Nabeta, K. Subulatin, an Antioxidic Caffeic Acid Derivative Isolated from the in Vitro Cultured Liverworts, Jungermannia Subulata, Lophocolea Heterophylla, and Scapania Parvitexta. Biosci. Biotechnol. Biochem. 2002, 66, 255–261. [Google Scholar] [CrossRef][Green Version]
- Bhattarai, H.D.; Paudel, B.; Lee, H.K.; Oh, H.; Yim, J.H. In Vitro Antioxidant Capacities of Two Benzonaphthoxanthenones: Ohioensins F and G, Isolated from the Antarctic Moss Polytrichastrum Alpinum. Z. Naturforsch. C. J. Biosci. 2009, 64, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Wu, C.-L.; Lin, C.-W.; Chi, L.-L.; Chen, P.-Y.; Chiu, C.-J.; Huang, C.-Y.; Chen, C.-N. Marchantin A, a Cyclic Bis(Bibenzyl Ether), Isolated from the Liverwort Marchantia Emarginata Subsp. Tosana Induces Apoptosis in Human MCF-7 Breast Cancer Cells. Cancer Lett. 2010, 291, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Schwartner, C.; Bors, W.; Michel, C.; Franck, U.; Müller-Jakic, B.; Nenninger, A.; Asakawa, Y.; Wagner, H. Effect of Marchantins and Related Compounds on 5-Lipoxygenase and Cyclooxygenase and Their Antioxidant Properties: A Structure Activity Relationship Study. Phytomedicine 1995, 2, 113–117. [Google Scholar] [CrossRef]
- Hsiao, G.; Teng, C.-M.; Wu, C.-L.; Ko, F.-N. Marchantin H as a Natural Antioxidant and Free Radical Scavenger. Arch. Biochem. Biophys. 1996, 334, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sadamori, M. Studies on the New Biologically Active Substances of Tahitian and Tokushima’s Plagiochila Genus. Master’s Thesis, Tokushima Bunri University, Tokushima, Japan.
- Liu, R.H.; Finley, J. Potential Cell Culture Models for Antioxidant Research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Ortan, A.; Fierascu, I.C.; Fierascu, I. In Vitro and in Vivo Evaluation of Antioxidant Properties of Wild-Growing Plants. A Short Review. Curr. Opin. Food Sci. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Ielpo, M.T.L.; Basile, A.; Miranda, R.; Moscatiello, V.; Nappo, C.; Sorbo, S.; Laghi, E.; Ricciardi, M.M.; Ricciardi, L.; Vuotto, M.L. Immunopharmacological Properties of Flavonoids. Fitoterapia 2000, 71, S101–S109. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Golia, B.; Montanari, S.; Castaldo Cobianchi, R.; Esposito, S. Antioxidant Activity in Extracts from Leptodictyum Riparium (Bryophyta), Stressed by Heavy Metals, Heat Shock, and Salinity. Plant. Biosyst. 2011, 145, 77–80. [Google Scholar] [CrossRef]
- Provenzano, F.; Sánchez, J.L.; Rao, E.; Santonocito, R.; Ditta, L.A.; Borrás Linares, I.; Passantino, R.; Campisi, P.; Dia, M.G.; Costa, M.A.; et al. Water Extract of Cryphaea Heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds. Int. J. Mol. Sci. 2019, 20, 5560. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-N.; Sun, Y.; Shen, T.; Zhang, J.-Z.; Zhou, J.-C.; Li, Y.; Chen, W.; Ren, Z.-J.; Li, Y.-L.; Wang, X.; et al. Diterpenoids from the Chinese Liverwort Frullania Hamatiloba and Their Nrf2 Inducing Activities. Phytochemistry 2019, 158, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.-Z.; Zhou, J.-C.; Shen, T.; Lou, H.-X. Terpenoids from Diplophyllum Taxifolium with Quinone Reductase-Inducing Activity. Fitoterapia 2016, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Zhu, M.; Zhu, R.; Zhang, J.; Zhou, J.; Wang, T.; Qiao, Y.; Lou, H. Sacculatane Diterpenoids from the Chinese Liverwort Pellia Epiphylla with Protection against H2O2-Induced Apoptosis of PC12 cells. Phytochemistry 2019, 162, 173–182. [Google Scholar] [CrossRef]
- Becker, H. Secondary metabolites from bryophytes in vitro cultures. J. Hattori Bot. Lab. 1994, 76, 283–291. [Google Scholar] [CrossRef]
- Sahu, V.; Niranjan, A.; Asthana, A.K. In-Vitro Propagation and Identification of Phenol Compounds of Potential Medicinal Value in the Moss Oxystegus Stenophyllus (Mitt.) Gangulee. J. Bryol. 2014, 36, 325–327. [Google Scholar] [CrossRef]
- Sauerwein, M.; Becker, H. Growth, Terpenoid Production and Antibacterial Activity of an in Vitro Culture of the Liverwort Fossombronia Pusilla. Planta Med. 1990, 56, 364–367. [Google Scholar] [CrossRef]
- Mohandas, G.G.; Kumaraswamy, M. Antioxidant Activities of Terpenoids from Thuidium Tamariscellum (C. Muell.) Bosch. and Sande-Lac. a Moss. Pharmacogn. J. 2018, 10, 645–649. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility Test Methods. In Manual of Clinical Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1253–1273. ISBN 978-1-68367-280-7. [Google Scholar]
- Bodade, R.G.; Borkar, P.S.; Saiful Arfeen, M.; Khobragade, C.N. In Vitro Screening of Bryophytes for Antimicrobial Activity. J. Med. Plants 2008, 7, 23–28. [Google Scholar]
- Negi, K.; Chaturvedi, P. In Vitro Antimicrobial Efficacy of Rhynchostegium Vagans A. Jaeger (Moss) against Commonly Occurring Pathogenic Microbes of Indian Sub-Tropics. Asian Pac. J. Trop. Dis. 2016, 6, 10–14. [Google Scholar] [CrossRef]
- Negi, K.; Tewari, S.D.; Chaturvedi, P. Antibacterial Activity of Marchantia Papillata Raddi Subsp. Grossibarba (Steph.) Bischl. Against Staphylococcus Aureus. Indian J. Tradit. Knowl. 2018, 17, 763–769. [Google Scholar]
- Canli, K.; Altuner, E.M.; Akata, I. Antimicrobial Screening of Mnium Stellare. Bangladesh J. Pharmacol. 2015, 10, 321–325. [Google Scholar] [CrossRef]
- Canli, K.; Cetin, B.; Altuner, E.M.; Türkmen, Y.; Uzek, U.; Dursun, H. In Vitro Antimicrobial Screening of Hedwigia Ciliata Var. Leucophaea and Determination of the Ethanol Extract Composition by Gas Chromatography/Mass Spectrometry (GC/MS). J. Pure Appl. Microbiol. 2014, 8, 2987–2998. [Google Scholar]
- Saxena, K.; Yadav, U. In Vitro Assessment of Antimicrobial Activity of Aqueous and Alcoholic Extracts of Moss Atrichum Undulatum (Hedw.) P. Beauv. Physiol. Mol. Biol. Plants 2018, 24, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Manoj, G.S.; Murugan, K. Phenolic Profiles, Antimicrobial and Antioxidant Potentiality of Methanolic Extract of a Liverwort, Plagiochila Beddomei Steph. Indian J. Nat. Prod. Resour. 2012, 3, 173–183. [Google Scholar]
- Zhu, R.-L.; Wang, D.; Xu, L.; Shi, R.-P.; Wang, J.; Zheng, M. Antibacterial Activity in Extracts of Some Bryophytes from China and Mongolia. J. Hattori Bot. Lab. 2006, 100, 603–615. [Google Scholar]
- Basile, A.; Giordano, S.; Sorbo, S.; Vuotto, M.L.; Ielpo, M.T.L.; Castaldo Cobianchi, R. Antibiotic Effects of Lunularia Cruciata (Bryophyta) Extract. Pharm. Biol. 1998, 36, 25–28. [Google Scholar] [CrossRef]
- Lunić, T.M.; Oalđe, M.M.; Mandić, M.R.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Božić Nedeljković, B.D. Extracts Characterization and In Vitro Evaluation of Potential Immunomodulatory Activities of the Moss Hypnum Cupressiforme Hedw. Molecules 2020, 25, 3343. [Google Scholar] [CrossRef]
- Yucel, T.B. Chemical Composition and Antimicrobial and Antioxidant Activities of Essential Oils of Polytrichum Commune (Hedw.) and Antitrichia Curtipendula (Hedw.) Brid. Grown in Turkey. Int. J. Second. Metab. 2021, 8, 272. [Google Scholar] [CrossRef]
- Onder, A.; Yıldız, A.; Cinar, A.S.; Zengin, G.; Ak, G.; Ozenoğlu, H. The Comparison of the Phytochemical Composition, Antioxidant and Enzyme Inhibition Activity of Two Moss Species: Plagiomnium Ellipticum (Brid.) T. Kop. and Antitrichia Californica Sull., from Southwest Ecological Region in Turkey. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Zinsmeister, H.D.; Becker, H.; Eicher, T. Bryophytes, a Source of Biologically Active, Naturally Occurring Material? Angew. Chem. Int. Ed. Engl. 1991, 30, 130–147. [Google Scholar] [CrossRef]
- Nandy, S.; Dey, A. Bibenzyls and Bisbybenzyls of Bryophytic Origin as Promising Source of Novel Therapeutics: Pharmacology, Synthesis and Structure-Activity. Daru 2020, 28, 701–734. [Google Scholar] [CrossRef] [PubMed]
- Bukvicki, D.; Novaković, M.; Ilić Tomić, T.; Nikodinović-Runić, J.; Todorović, N.; Veljić, M.; Asakawa, Y. Biotransformation of Perrottetin F by Aspergillus Niger: New Bioactive Secondary Metabolites. Rec. Nat. Prod. 2021, 15, 281–292. [Google Scholar] [CrossRef]
- Lu, Z.-Q.; Fan, P.-H.; Ji, M.; Lou, H.-X. Terpenoids and Bisbibenzyls from Chinese Liverworts Conocephalum Conicum and Dumortiera Hirsuta. J. Asian Nat. Prod. Res. 2006, 8, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial Activity of Pure Flavonoids Isolated from Mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Asakawa, Y. Chemical Constituents of Malagasy Liverworts, Part II: Mastigophoric Acid Methyl Ester of Biogenetic Interest from Mastigophora Diclados (Lepicoleaceae Subf. Mastigophoroideae). Chem. Pharm. Bull. 2004, 52, 1382–1384. [Google Scholar] [CrossRef]
- Shu, Y.-F.; Wei, H.-C.; Wu, C.-L. Sesquiterpenoids from Liverworts Lepidozia Vitrea and L. Fauriana. Phytochemistry 1994, 37, 773–776. [Google Scholar] [CrossRef]
- Perry, N.B.; Foster, L.M. Sesquiterpene/Quinol from a New Zealand Liverwort, Riccardia Crassa. J. Nat. Prod. 1995, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.M.; Burgess, E.J.; Lorimer, S.D.; Perry, N.B. A Cytotoxic Sesquiterpene and Unprecedented Sesquiterpene-Bisbibenzyl Compounds from the Liverwort Schistochila Glaucescens. Tetrahedron 2002, 58, 7875–7882. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the Antibiotic Resistance Crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Morita, D.; Sawada, H.; Ogawa, W.; Miyachi, H.; Kuroda, T. Riccardin C Derivatives Cause Cell Leakage in Staphylococcus Aureus. Biochim. Et Biophys. Acta-Biomembr. 2015, 1848, 2057–2064. [Google Scholar] [CrossRef][Green Version]
- Keserű, G.M.; Nógrádi, M. The Chemistry of Macrocyclic Bis(Bibenzyls). Nat. Prod. Rep. 1995, 12, 69–75. [Google Scholar] [CrossRef]
- Ivković, I.; Bukvički, D.; Novaković, M.; Ivanović, S.; Stanojević, O.; Nikolić, I.; Veljić, M. Antibacterial Properties of Thalloid Liverworts Marchantia Polymorpha L., Conocephalum Conicum (L.) Dum. and Pellia Endiviifolia (Dicks.) Dumort: Scientific Paper. J. Serb. Chem. Soc. 2021. [Google Scholar] [CrossRef]
- Vollár, M.; Gyovai, A.; Szucs, P.; Zupkó, I.; Marschall, M.; Csupor-Lffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and Antimicrobial Activities of Selected Bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Nath, V.; Sahu, V.; Rawat, A.K.S. Antibacterial Activity of Some Bryophytes Used Traditionally for the Treatment of Burn Infections. Pharm. Biol. 2011, 49, 526–530. [Google Scholar] [CrossRef]
- Onoda, K.; Sawada, H.; Morita, D.; Fujii, K.; Tokiwa, H.; Kuroda, T.; Miyachi, H. Anti-MRSA Activity of Isoplagiochin-Type Macrocyclic Bis(Bibenzyl)s Is Mediated through Cell Membrane Damage. Bioorganic Med. Chem. 2015, 23, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Castaldo-Cobianchi, R.; Giordano, S.; Basile, A.; Violante, U. Occurrence of Antibiotic Activity in Conocephalum Conicum, Mnium Undulatum and Leptodictyum Riparium (Bryophytes). Plant Biosyst. 1988, 122, 303–311. [Google Scholar] [CrossRef]
- Ilhan, S.; Savaroǧlu, F.; Çolak, F.; Işçen, C.F.; Erdemgil, F.Z. Antimicrobial Activity of Palustriella Commutata (Hedw.) Ochyra Extracts (Bryophyta). Turk. J. Biol. 2006, 30, 149–152. [Google Scholar]
- Rodríguez-Rodríguez, J.C.; Samudio-Echeverry, I.J.P.; Sequeda-Castañeda, L.G. Evaluation of the Antibacterial Activity of Four Ethanolic Extracts of Bryophytes and Ten Fruit Juices of Commercial Interest in Colombia against Four Pathogenic Bacteria. Acta Hortic. 2012, 964, 251–258. [Google Scholar] [CrossRef]
- Sengupta, S.; Chattopadhyay, M.; Grossart, H.-P. The Multifaceted Roles of Antibiotics and Antibiotic Resistance in Nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.A.S.; Sousa, E.O.; Rodrigues, F.F.G.; Campos, A.R.; Lacerda, S.R.; Costa, J.G.M. Phytochemical Screening and Synergistic Interactions between Aminoglycosides, Selected Antibiotics and Extracts from the Bryophyte Octoblepharum Albidum Hedw (Calymperaceae). Arch. Biol. Sci. 2012, 64, 465–470. [Google Scholar] [CrossRef]
- Kandpal, V.; Chaturvedi, P.; Negi, K.; Gupta, S.; Sharma, A. Evaluation of Antibiotic and Biochemical Potential of Bryophytes from Kumaun Hills and Tarai Belt of Himalayas. Int. J. Pharm. Pharm. Sci. 2016, 8, 65–69. [Google Scholar]
- Semerjyan, I.; Semerjyan, G.; Semerjyan, H.; Trchounian, A. Antibacterial Properties and Flavonoids Content of Some Mosses Common in Armenia. Iran. J. Pharm. Sci. 2021, 16, 31–42. [Google Scholar] [CrossRef]
- Tosun, A.; Küpeli Akkol, E.; Süntar, I.; Özenoğlu Kiremit, H.; Asakawa, Y. Phytochemical Investigations and Bioactivity Evaluation of Liverworts as a Function of Anti-Inflammatory and Antinociceptive Properties in Animal Models. Pharm. Biol. 2013, 51, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Süntar, İ.; Keleş, H.; Özenoğlu Kiremit, H.; Asakawa, Y.; Küpeli Akkol, E. Wound Healing Potential of Selected Liverworts Growing in Turkey. Turk. J. Pharm. Sci. 2016, 13, 285–291. [Google Scholar] [CrossRef]
- Hu, Z.; Kong, F.; Si, M.; Tian, K.; Yu, L.X.; Young, C.Y.F.; Yuan, H.; Lou, H. Riccardin D Exerts Its Antitumor Activity by Inducing DNA Damage in PC-3 Prostate Cancer Cells In Vitro and In Vivo. PLoS ONE 2013, 8, e74387. [Google Scholar] [CrossRef]
- Niu, H.; Qian, L.; Sun, B.; Liu, W.; Wang, F.; Wang, Q.; Ji, X.; Luo, Y.; Nesa, E.U.; Lou, H.; et al. Inactivation of TFEB and NF-ΚB by Marchantin M Alleviates the Chemotherapy-Driven pro-Tumorigenic Senescent Secretion. Acta Pharm. Sin. B 2019, 9, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, D.; Jiao, Y.; Guo, H.; Zheng, D.; Jia, L.; Duan, C.; Liu, Y.; Tian, X.; Shen, J.; et al. In Vitro and in Vivo Evaluation of Riccardin D Nanosuspensions with Different Particle Size. Colloids Surf. B Biointerfaces 2013, 102, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Kinghorn, A.D. Drug Discovery from Medicinal Plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Cuevas, F.; Mojoli, A.; Maldonado, M.; Rojas de Arias, A.; Pandolfi, E.; Segovia-Corrales, A. In Vivo Analysis of the Genotoxic Potential of 14-Hydroxylunularin, a Molecule with Leishmanicidal Effect. Rev. Latinoam. Química 2011, 39, 100–106. [Google Scholar]
- Ozturk, S.; Yayintas, O.T. Investigation of Hepatotoxic Effect of Bryophytes (Homalothecium Sericeum (HEDW) Schimp.) on Rat Liver. Fresenius Environ. Bull. 2021, 30, 1134–1146. [Google Scholar]
- Parasuraman, S. Toxicological Screening. J. Pharmacol. Pharm. 2011, 2, 74–79. [Google Scholar] [CrossRef]
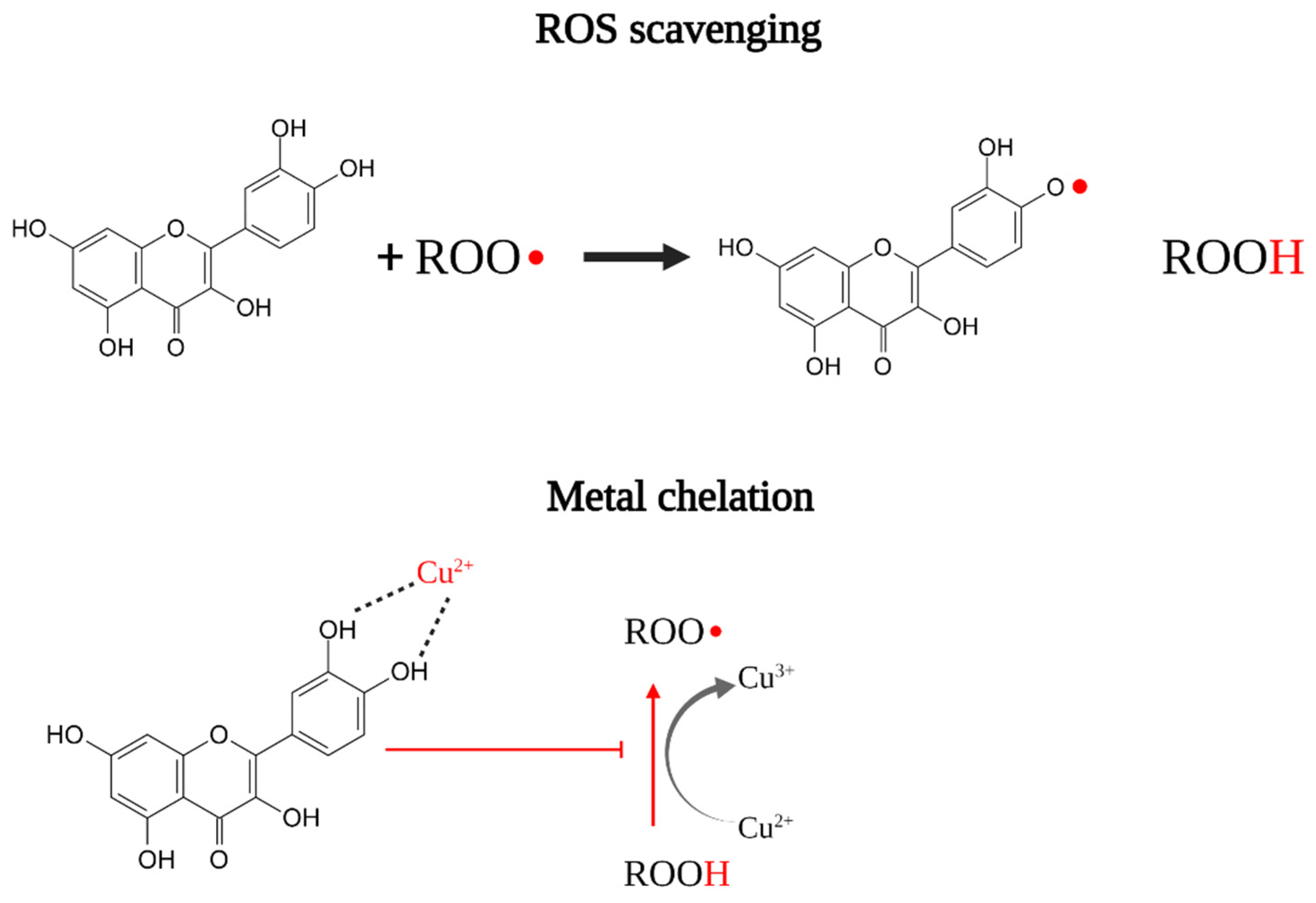
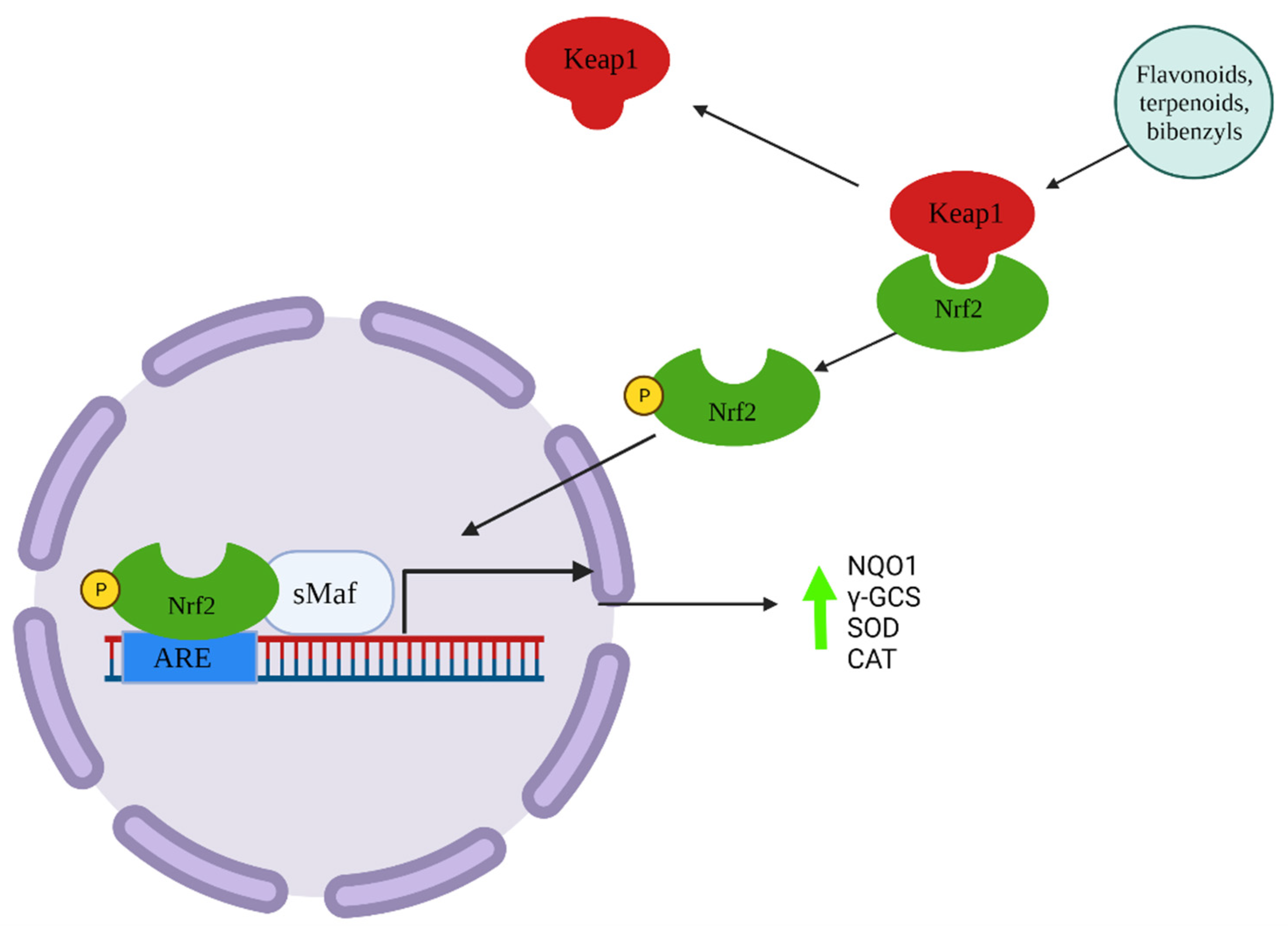
| Bacteria Species | Antibacterial Compound/Extract | Compound Class | Methods | MIC (µg/mL)/Inhibition Zone (mm) | Bryophytes Species | References |
|---|---|---|---|---|---|---|
| Staphylococcus aureus | Water | (1) Disk diffusion; (2) Broth dilution | (1) 8.86 ± 0.23 mm (2) 0.78 µg/mL | Atrichium undulatum | [61] | |
| Ethanol | (1) Disk diffusion; (2) Broth dilution | (1) 13.66 ± 0.57 mm (2) 2.40 µg/mL | Atrichium undulatum | [61] | ||
| Ethanol | Disk diffusion | 11 mm (50 µL) 14 mm (100 µL) 14 mm (150 µL) | Mnium stellare | [59] | ||
| Butanol | Broth dilution | 30 µg/mL | Mnium marginatum | [84] | ||
| (1) α-herbertenol; (2) β-herbertenol; (3) herbertene-1,2-diol; (4) mastigophorene C; (5) α-formyl herbertenol; | Sesquiterpenoids | Disk diffusion | (1) 15 mm (2) 16 mm (3) 13 mm (4) 17 mm (5) 16 mm | Mastigophora diclados | [75] | |
| Perrotettin A-D | (Bis)bibenzyl | Unknown | Unknown | Marchantia polymorpha | [71] | |
| Perrotettin F | (Bis)bibenzyl | Broth dilution | 100 µM | Lunularia cruciata | [72] | |
| Marchantin A | (Bis)bibenzyl | Broth dilution | 3.13 µg/mL | Marchantia polymorpha | [81] | |
| Marchantin A | (Bis)bibenzyl | Disk diffusion, Broth dilution | 11 mm 0.062 mg/mL | [82] | ||
| MR Staphylococcus aureus | Lepidozenolide | Sesquiterpenoids | Broth dilution | 100 µg/mL | Lepidozia faunaria | [76] |
| (1) Isop-2; (2) Isop-3 | (Bis)bibenzyl derivatives | Broth dilution | (1) 0.5 µg/mL (2) 2 µg/mL | Isoplagiochin D synthetic derivatives | [85] | |
| Methanol | Disk diffusion | See the reference | See the reference | [83] | ||
| Acetone | Disk diffusion | 9 mm | Palustriella commutata | [87] | ||
| Water:Ethanol | Broth dilution | 3.91 µg/mL | Marchantia palmata; Hydrogonium gracilantum | [91] | ||
| Ethanol | Disk diffusion | 2.9 mm/mg | Trichocolea tomentosa | [88] | ||
| Pseudomonas aeruginosa | (1) Acetone, (2) Ethanol, (3) Water | Disk diffusion | (1) 5 mm (2) 3 mm (3) 2 mm | Leptodictyumriparium | [86] | |
| (1) Acetone, (2) Ethanol, (3) Water | Disk diffusion | (1) 3 mm (2) 3 mm (3) 3 mm | Conocephalum conicum | [86] | ||
| Chloroform | Broth dilution | 110 µg/mL | Plagiochasma appendiculatum | [84] | ||
| Chloroform | Broth dilution | 20 µg/mL | Conocephalum conicum | [84] | ||
| Marchantin A | (Bis)bibenzyl | Unknown | Marchantia ssp | [70] | ||
| (1) Bartramiaflavone; (2) Lucenin-2; (3) Saponarin; (4) Apigenin; (5) Apigenin-7-O-triglycoside | Flavonoids | Broth dilution | (1) 256 µg/mL (2) 8 µg/mL (3) 8 µg/mL (4) 8 µg/mL (5) 256 µg/mL | Bartramia pomiformis; Hedwigia ciliata; Plagiomnium cuspidatum; Plagiomnium affine; Dicranum scopiarum | [74] | |
| Lunularin | Bibenzyl | Broth dilution | 64 µg/mL | Dumortiera hirsuta | [73] | |
| Perrotettin F | (Bis)bibenzyl | Broth dilution | 150 µM | Lunularia cruciata | [72] | |
| (1) Bartramiaflavone; (2) Lucenin-2; (3) Saponarin; (4) Apigenin | Flavonoids | Broth dilution | (1) 64 µg/mL (2) 64 µg/mL (3) 4 µg/mL (4) 128 µg/mL | Bartramia pomiformis; Hedwigia ciliata; Plagiomnium cuspidatum; Plagiomnium affine | [74] | |
| Klebsiella pneumoniae | Ethanol | Broth dilution | 512 µg/mL | Octoblepharum albidium | [90] | |
| Acetone | Disk diffusion | 11 mm | Palustriella commutata | [87] | ||
| Methanol | Disk diffusion | 7 mm | Palustriella commutata | [87] | ||
| Methanol | Broth dilution | 0.125 mg/mL | Plagiochila beddomei | [62] | ||
| Water | (1) Disk diffusion; (2) Broth dilution | (1) 6.83 ± 0.28 mm (2) 1.57 µg/mL | Atrichium undulatum | [61] | ||
| Ethanol | (1) Disk diffusion; (2) Broth dilution | (1) 14.66 ± 0.57 mm (2) 3.40 µg/mL | Atrichium undulatum | [61] | ||
| E. coli | Water:Ethanol | (1) Disk diffusion (2) Broth dilution | (1) see reference (2) 3.91 µg/mL | Hydrogonium gracilantum | [91] | |
| (1) Apigenin; (2) Vitexin; (3) Saponarin; (4) Lucenin-2; | Flavonoids | Broth dilution | (1) 128 µg/mL (2) 128 µg/mL (3) 128 µg/mL (4) 64 µg/mL | Plagiomnium affine, Dicranum scoparium; Plagiomnium cuspidatum; Hedwigia ciliata | [74] | |
| Marchantin A | (Bis)bibenzyl | Unknown | Marchantia ssp | [70] | ||
| Enterobacter ssp | (1) Bartramiaflavone; (2) Luteolin-7-O-neohesperoside; (3) Lucenin-2; (4) Saponarin; (5) Apigenin-7-O-triglycoside; (6) Apigenin | Flavonoids | Broth dilution | (1) 8 µg/mL (2) 256 µg/mL (3) 8 µg/mL (4) 4 µg/mL (5) 256 µg/mL (6) 4 µg/mL | Bartramia pomiformis; Hedwigia ciliata; Plagiomnium cuspidatum; Plagiomnium affine; Dicranum scopiarum | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianciullo, P.; Maresca, V.; Sorbo, S.; Basile, A. Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes. Appl. Sci. 2022, 12, 160. https://doi.org/10.3390/app12010160
Cianciullo P, Maresca V, Sorbo S, Basile A. Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes. Applied Sciences. 2022; 12(1):160. https://doi.org/10.3390/app12010160
Chicago/Turabian StyleCianciullo, Piergiorgio, Viviana Maresca, Sergio Sorbo, and Adriana Basile. 2022. "Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes" Applied Sciences 12, no. 1: 160. https://doi.org/10.3390/app12010160
APA StyleCianciullo, P., Maresca, V., Sorbo, S., & Basile, A. (2022). Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes. Applied Sciences, 12(1), 160. https://doi.org/10.3390/app12010160









