Abstract
Today global health problems such as increased risks of oxidative stress-related diseases and antibiotic resistance are issues of serious concern. Oxidative stress is considered to be the underlying cause of many contemporary pathological conditions such as neurological disorders, ischemia, cancer, etc. Antibiotic-resistant bacteria are a concerning issue in clinical practice, causing an increase in deadly infections. Bryophytes synthesize an outstanding number of secondary metabolites that have shown several potential therapeutic and nutraceutical applications. Research in the field has led to the isolation and characterization of several compounds (flavonoids, terpenoids, and bibenzyls). Some of these compounds have shown promising in vitro antibacterial activities and antioxidant potential comparable to known natural antioxidants such as ascorbic acid and α-tocopherol. However, the process of developing new drugs from naturally occurring molecules is often an impervious path. In this paper, the current state of research of bryophytic antioxidant and antibacterial applications is discussed.
1. Introduction
Bryophytes lack anatomical features in order to avoid both abiotic and biotic stresses. As a consequence, they have to adapt to counteract environmental stresses with a high degree of chemical diversity [1]. A large number of chemical entities (ca. 3000) have been isolated from bryophytes: aromatic compounds such as phenolic compounds, polyphenols, bibenzyls, (bis)bibenzyls, and terpenoids [2]. Extensive research has been carried out to screen the biological activity of bryophytic molecules. Several molecules and extracts from bryophytes have shown a wide range of biological activities (among them, antimicrobic and antioxidant activities) [3]. In contemporary days, two issues of global concern arise, namely the increased risk of oxidative stress-related diseases and antibiotic resistance [4,5].
Aerobic organisms physiologically produce reactive oxygen species (ROS). ROS are produced in low to moderate concentrations during cellular metabolism and serve a wide number of significant cellular functions such as gene activation, cell growth, signalling molecules, and physiological processes such as inflammation [5]. To balance ROS production, aerobic organisms have developed both enzymatic and non-enzymatic antioxidant systems capable of maintaining adequate balance of oxidants/antioxidants [6]. However, exposure to oxidative stress-inducing agents (e.g., ionizing radiations, heavy metals, etc.), may lead to the disturbance of such homeostasis. Oxidative stress arises when antioxidant systems cannot cope with ROS production and therefore the balance shifts in favour of oxidants [6]. Increased ROS concentrations cause damage to nuclear DNA, mitochondrial DNA, membranes, and proteins. The disturbances in the cellular redox homeostasis participate in the onset of several increasing pathological conditions such as ischemia, neurological disorders, cancer, diabetes, atherosclerosis, etc. [7,8,9]. For example, ROS are implied in several processes related to tumorigeneses such as cell motility, tumour proliferation, and metastasis [10]. Furthermore, oxidative damage to mitochondrial DNA might cause dysfunctions in the mitochondrial respiratory chain causing further ROS generation and, ultimately, oncogenicity [11]. As a consequence of the increased risks of diseases related to oxidative stress, extensive research has been conducted on non-toxic natural antioxidants that can help to cope with oxidative stress [12].
Antioxidant compounds can act directly and indirectly on the redox balance of cells (Figure 1 and Figure 2) [13]. Some compounds are capable of directly antagonizing and reducing ROS. For example, compounds having phenolic groups in their structures’ phenolic groups (e.g., flavonoids, phenols) act as hydrogen donors to free radicals, stabilizing the excess electron on the aromatic ring by resonance. Other compounds such as flavonols act indirectly by chelating metal ions that can alter the redox balance in cells (e.g., zinc, copper) [14]. Furthermore, it is known that the Keap1/Nrf2/ARE system is involved in the activation of antioxidant responses in cells [15]. Compounds such as polyphenols, bibenzyls and terpenoids are inducers of Nrf2-ARE system, enhancing the expression of the antioxidants cytoprotective proteins (e.g., gluthatione-S-transferse; glutathione reductase; gamma-glutamylcisteine; NAD(P)H:quinone oxidoreductase; superoxide dismutase; catalase), granting long-term protection from oxidative stress [16]. Due to its central role in redox homeostasis, dysregulations of the Keap/Nrf2/ARE system have been linked to oxidative stress-related diseases [17].
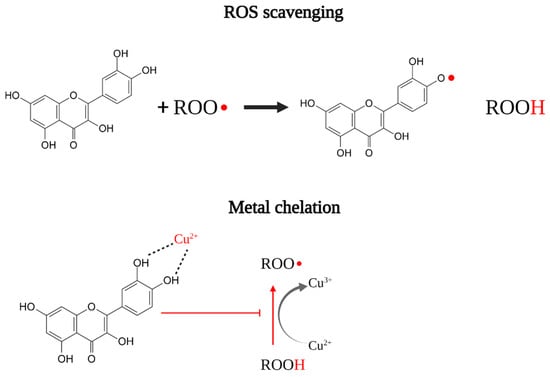
Figure 1.
Schematization of direct antioxidant activity through ROS scavenging and metal chelation.
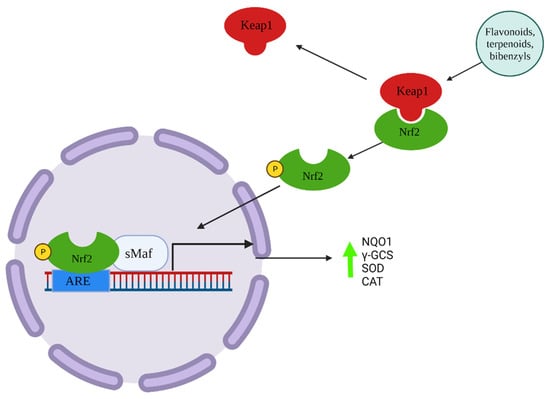
Figure 2.
Schematization of indirect induction of cytoprotective proteins through Keap1/Nrf2/ARE system by antioxidant compounds.
Furthermore, for decades antibiotics have been employed both therapeutically and prophylactically against human diseases as well as in agriculture and for animals [18,19]. As a consequence, several antibiotic-resistant strains have begun to spread, and bacterial infections have again become a threat [20]. Bryophytes, being rich in secondary metabolites that show several biological activities [3], might be a valuable source to discover novel drugs that aid coping with both prevention of oxidative stress-related diseases and antibiotic resistance issues. The present review is intended to revise the research state-of-the-art of antioxidant and antibacterial compounds found in bryophytes and to outline future investigation needed in the field of applications of bryophytes in nutraceuticals and pharmaceutics.
2. Methodology
The relevant literature was searched through Scopus and Web of Science using “article, abstract, and keywords” as the search field. Literature concerning the antibacterial activity was searched with both “antibacterial” and “antimicrobial” keywords since the two terms are used interchangeably. To investigate in greater detail the literature relating to compounds with known antibacterial activity, words such as “bibenzyls”, “flavonoids”, “terpenes”, and “terpenoids” (from now on specific compound classes) were inserted. The literature on antibiotic-resistant bacteria research was searched typing “bacteria species” AND “bryophytes”, “extracts” OR “specific compound class”. The bacterial strains considered for this review were those listed as “serious concern” and gathered from [10]. The same search was carried out to find literature on antioxidant activity by searching terms related to antioxidant compounds: “antioxidant activity” AND “bryophytes” OR “specific compound class”. For literature concerning the current state of in vivo research “bryophytes” AND “extracts” AND “animal models” OR “mice” OR “rats” OR “in vivo” were inserted. Regarding toxicological screening, “bryophytes” AND “extracts” OR “toxicity”, “genotoxicity” OR “toxicological screening” were inserted.
3. Antioxidant Activity
Secondary metabolites compounds function as a nonenzymatic defence that protects bryophytes against various environmental stresses [1,21]. In general, plants are known to produce several secondary metabolites that can act as antioxidant scavengers (e.g., phenolic compound; flavonoids; terpenes). Bryophytes have evolved the capacity to synthesize antioxidative molecules as a mechanism to deal with the formation of free radicals (e.g., reactive oxygen species, hydrogen peroxide) derived from abiotic stresses (i.e., light, desiccation, pollution). Several studies indicated that secondary metabolites in bryophytes are synthesized in response to oxidative stress-inducing agents such as UV radiations [22,23,24] and cadmium [24]. This feature has prompted researchers to deepen knowledge about the antioxidant potential of bryophytes for therapeutic purposes.
The vast majority of the research has focused on the in vitro antioxidant activity of bryophytes extracts and pure isolated compounds through multiple chemical methods such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABST) assay, lipid peroxidation test (LPT), β-carotene assay (Table 1 and Table 2) (for in-depth methodological review read [25]) [26,27,28,29,30,31,32,33]. The extraction methods mostly rely on solvents such as ethanol, methanol, water, and acetone which are the most common solvents used for in vitro antioxidant testing [25]. Extracts, depending on the solvent used and the selected species contain variable concentrations of phenolics, polyphenols, bibenzyls and terpenoids which are responsible for the antioxidant activity (Table 1 and Table 2). A study of [31] that focused on the chemical composition of pure ethanol extracts, revealed the presence of several glycosidic flavonoids (e.g., apigenin hexoside, kaempferol 3-galactoside). Another study obtained similar results from water:ethanol extracts of several moss species [34]. Among the 3000 compounds isolated and characterized in bryophytes [35], only a few molecules have been tested for their antioxidant activity (Table 2) [30,34,36,37,38,39,40,41,42]. The majority of the pure tested compounds were isolated from liverworts, while only two compounds, Ohiesin G and D, were from the moss Polytrichastrum alpinum. Interestingly, some of these studies found that terpenoids, (bis)bibenzyls isolated from liverworts had higher or similar antioxidant activity with respect to natural reference antioxidant compounds (ascorbic acid, α-tocopherol) (Table 2). Specifically, [36] isolated two sesquiterpenoids identified as (-)-herbertenediol and (-)-mastigophorene C and D that were found to have a DPPH activity comparable to that of quercetin and higher than ascorbic acid (DPPH IC50 from 1.9 ± 0.6 to 2.7 ± 0.8 µg/mL). In a study by [37], researchers isolated a caffeate ester, named subulatin, from the liverworts Jungermannia subulata; Lophocolea heterophylla and Scapania parvitexta. The compound inhibited lipid peroxidation at percentages comparable to those of α-tocopherol in an erytrocite membrane ghost system. [40] carried out wide testing on several pure compounds: the macrocyclic (bis)bibenzyls marchantins A, B, D, E, riccardin C, isoricciardin C; the bisbibenzyl paleatin B; the prenylated bibenzyls radunalinnin H. The result evidenced an antioxidant activity ranging from 0.4 to 15.7 µmol/L on the arachidonic acid peroxidation test, with marchantin A and perrotettin D showing the highest activity (IC50 0.4 and 0.72 µmol/L). In a study from [41], researchers tested the antioxidant activity of marchantin H. They found a DPPH IC0.20 0.51 ± 0.03 µM respect to 3.80 ± 0.33 µM measured in α-tocopherol (Table 2).
Cell models are a valuable tool in the selection of compound bioactivities prior to clinical trials in animals and humans [43]. Furthermore, in vivo testing of pure isolated compounds is a mandatory step in the drug discovery process, supporting in vitro and cell studies [44]. Currently, no research has focused on in vivo experimentations of bryophytic antioxidant compounds. At the present stage, only a few studies have investigated the antioxidant activity of bryophytes extracts and pure compounds in cell models (phagocytes; murine fibroblasts) [45,46,47,48,49,50]. Ielpo et al. [45] studied the antioxidant activity of acetonic extracts of Lunularia cruciata on phagocytes through the chemiluminescence inhibition test. In another study, Leptodictyum riparium acetonic extracts were used to treat whole blood phagocytes gathered from three healthy donors. Blood samples were treated with the ROS inducing agents opsonized zymosan (OZ) and phorbol myristate acetate (PMA). The authors found that the acetonic extracts of L. riparium inhibited luminol-dependent chemiluminescence in samples treated with OZ and PMA in a dose-dependent manner [46]. In a study by [47], the authors treated murine fibroblast NIH-3T3 cells with water extract from the moss Cryphaea heteromalla. NIH-3T3 cells exposed to 500 µM tert-butyl hydroperoxide (TBH) oxidative stress-inducing agent and treated with the water extract (0.5 µg/mL) showed a 50% inhibition of ROS generation for those treated only with TBH (500 µM) for 1 h.
Other researchers have found dose-dependent Nrf2-ARE system induction by diterpenoids isolated from liverworts (Table 2) [48,49,50]. Nrf2 (nuclear factor erythroid 2- related factor) is a transcription factor responsive to cell redox status via Keap (Kelch-like ECH-associated protein 1), that bound the enhancer sequence ARE (antioxidant response elements). The ARE sequence is associated with several antioxidant cytoprotective proteins such as the NAD(P)H: quinone oxidoreductase 1 (NQO1) [13]. An investigation by [48] demonstrated that the diterpenoid frullanian D isolated from the liverwort Frullania hamatiloba stimulated the nuclear translocation of Nrf2, inducing Nrf2-related enzymes (NAD(P)H:quinone oxidoreductase 1, γ-glutamyl cysteine synthetase) in MOVAS cells. Furthermore, 18 h pretreatment with frullanian D ameliorated the H2O2 oxidative damage in the model cells. Another study found a NAD(P)H:quinone oxidoreductase 1 (NQO1) inducing activity in Diplophyllum taxifolium ethanolic extract. Researchers also isolated and characterized sixteen terpenoids and found that three of them, diplotaxifol A, diplotaxifol B, and atractynelonide III, induced NQO1 in a dose-dependent manner [49]. Similar results were obtained by [50] with the sacculatane diterpenoids epyphyllin A-D and pellianolactone B.
Therapeutic applications are also hampered by the need to collect large amounts of natural plant material, obtain pure material for high-value secondary metabolites, genetically stable populations and controlled growth conditions. As a result, in vitro cultures are the best way to develop large-scale production of bryophyte nutraceuticals [51]. At the current state of the research, methods to in vitro grow several bryophytes species have been well established [35]. Other studies have focused on the difference in antioxidant metabolites between in vitro grown and naturally grown bryophytes [30,33,37,52,53]. Interestingly, these studies have found no significant differences in antioxidant activity and metabolite composition in in vitro grown bryophytes.

Table 1.
List of extracts tested for in vitro antioxidant activity.
Table 1.
List of extracts tested for in vitro antioxidant activity.
| Extract | Species | Method | Activity | References | |
|---|---|---|---|---|---|
| Ethanol | Collected | Marchantia polymorpha | DPPH; ABTS; O2- | TFC: 4.62 mg/g DPPH | [28] |
| Water:ethanol (3:7) | Collected | Hypnum plumaeforme; Thuidium kanadae; Leucobryum juniperoideum | DPPH; ABTS; FRAP | TPC: 47.20 ± 11.20 to 119.87 ± 11.51 mg/GAE mg | [29] |
| Methanol | Collected | Marchantia pelacea | DPPH | DPPH: IC50 20 µg/mL | [32] |
| Water | Collected | Bryum moravicum | ABTS | TPC: 356.44 ± 9.56 µg/mg ABTS: 84.56 ± 7.93 µg ascorbic acid eq/mg | [26] |
| Methanol | Cultured | Lunularia cruciata | DPPH; ABTS | Scavenged DPPH: 48% (650 µg/mL) 35% (350 µg/mL) 22% (250 µg/mL) Scavenged ABST: 98% (2 mg/mL) | [30] |
| Acetone | Collected | Lunularia cruciata | Phagocytes chemiluminescence | See the reference | [45] |
| Acetone | Collected | Leptodictyum riparium | Whole blood chemiluminescence | See the reference | [46] |
| Water; methanol:water (8:2); ethanol:water (8:2) | Collected | Cryphaea heteromalla | NIH-3T3 murine fibroblast ROS production | See the reference | [47] |
| Ethanol | Collected | Thuidium tamariscellum | DPPH; H2O2 assay ABTS; FRAP | Total terpenoids: 25.95 mg/g DPPH: IC50 16 µg/mL H2O2: IC50 34.5 µg/mL ABTS: IC50 18.5 µg/mL FRAP: IC50 40 µg/mL | [54] |

Table 2.
List of pure isolated compounds tested for in vitro antioxidant activity.
Table 2.
List of pure isolated compounds tested for in vitro antioxidant activity.
| Compounds | Chemical Class | Species | Methods | Activity | References | |
|---|---|---|---|---|---|---|
| (-)-herbertenediol | Sesquiterpenoid | Collected | Mastigophora diclados | DPPH | IC50 1.9 ± 0.6 µg/mL | [36] |
| (-)-mastigophorene C | (Bis)bibenzyl | Collected | Mastigophora diclados | DPPH | IC50 2.7 ± 0.8 µg/mL | [36] |
| (-)-mastigophorene D | (Bis)bibenzyl | Collected | Mastigophora diclados | DPPH | IC50 2.0 ± 0.1 µg/mL | [36] |
| Subulatin | Caffeate ester | Cultured | Jungermannia subulata; Lophocolea heterophylla; Scapania parvitexta | Lipid peroxidation | See reference | [37] |
| Ohioesins F | Benzonaphthoxanthenones | Cultured | Polytrichastrum alpinum | DPPH; ABTS; FRAP; NO scavenging activity | DPPH: IC50 10 ± 0.16 µg/mL ABTS: IC50 14.3 ± 1.2 µg/mL NO assay: 63 ± 5.1 µg/mL FRAP: 9.8 ± 0.07 µg/mL | [38] |
| Ohioesin G | Benzonaphthoxanthenones | Cultured | Polytrichastrum alpinum | DPPH; ABTS; FRAP; NO scavenging activity | DPPH: IC50 10.1 ± 1.5 µg/mL ABTS: IC50 14.8 ± 1.5 µg/mL NO assay: 62.1 ± 5.0 µg/mL FRAP: 9.6 ± 1.2 µg/mL | [38] |
| Marchantin A | (Bis)bibenzyl | Collected | Marchantia ssp | DPPH | IC50: 20 µg/mL | [39] |
| Marchantin A | (Bis)bibenzyl | Arachidonic acid oxidation | IC50: 0.4 µmol/L | [40] | ||
| Marchantin B | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 0.4 µmol/L | [40] | |
| Marchantin D | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 5.6 µmol/L | [40] | |
| Marchantin E | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 2.7 µmol/L | [40] | |
| Marchantin H | (Bis)bibenzyl | Collected | Marchantia diptera | Lipid peroxidation; DPPH | DPPH: IC0.20 10.2 ± 0.2 µM | [41] |
| Isoriccardin C | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 5.3 µmol/L | [40] | |
| Riccardin C | (Bis)bibenzyl | Arachidonic acid oxidation | IC50: 12.7 µmol/L | [40] | ||
| Perrotettin D | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 0.72 µmol/L | [40] | |
| Peleatin B | (Bis)bibenzyl | Marchantia ssp | Arachidonic acid oxidation | IC50: 11.7 µmol/L | [40] | |
| Radulanin H | Bibenzyl | Arachidonic acid oxidation | IC50: 15.7 µmol/L | [40] | ||
| Plagiochin D | (Bis)bibenzyl | Collected | Plagiochila ovalifolia | DPPH | Unavailable | [42] |
| Frullanian D | Terpenoid | Frullania hamatiloba | MOVAS cells | Dose dependent NQO1 and γ-GCS induction; inhibition of H2O2-induced cytotoxicity | [48] | |
| Diplotaxifol A-B | Terpenoid | Diplophyllum taxifolium | Hepa 1c1c7 cells | Dose-dependent NQO1 induction | [49] | |
| Atractylenolide III | Terpenoid | Diplophyllum taxifolium | Hepa 1c1c7 cells | Dose-dependent NQO1 induction | [49] | |
| Epiphyllin A-D | Terpenoid | Pellia epiphylla | PC12 cells | NQO1 induction | [50] | |
| Pellianolactone B | Terpenoid | Pellia epiphylla | PC12 cells | Dose-dependent NQO1, γ-GCS induction, and inhibition of H2O2-induced cytotoxicity | [50] |
4. Antibacterial Activity
Bryophytes, like other organisms, have to deal with pathogens. As a consequence, these plants have adapted their biochemistry to synthesize several compounds to contrast the presence of pathogenic bacteria and fungi [1,21]. Ref. [2] reported several studies that have led to the identification and isolation of a large number of antibacterial compounds from bryophytes. Since then, the antibacterial activity of bryophytic extracts has been extensively researched.
The main techniques used to test antimicrobial chemicals from bryophytes are the disk diffusion test and the broth dilution test. Disk diffusion is a simple and reliable test in which a bacterial inoculum is applied to a culture agar plate [55]. Before the incubation (16–24 h at 35 °C), disks imbued with fixed concentrations of tested compounds are applied on the inoculated agar. After the incubation, the zone of inhibition (i.e., millimetres around disks with no bacteria growth) is measured. Broth dilution consists of two-fold serial dilution of the tested compound in a liquid culture medium (e.g., 4, 8, 16 µg/mL). Standardized bacterial suspensions (1–5 × 105 CFU/mL) are inoculated in antimicrobial containing tubes. After the incubation (16–24 h at 35 °C), tubes are examined to spot evidence of bacterial growth (i.e., medium turbidity). The lowest concentration at which bacterial growth is not evidenced represents the minimal inhibitory concentration (MIC) [55].
A wide number of studies have explored the in vitro antibacterial activity of various solvent extracts such as water, ethanol, methanol, chloroform, butanol [31,56,57,58,59,60,61,62,63,64]. Differences in the activity depend on the type of extract used, the tested species, the extraction procedure, and the bacteria strain. Other researchers, who have examined the chemical composition of the extracts and have found that, depending on the solvent used, bryophytic extracts are rich in flavonoids; terpenes; terpenoids; phenols; bibenzyls; and sterols [33,34,65,66,67]. These compounds are known to exert an antibacterial activity [68,69].
Some researchers achieved the isolation of compounds responsible for antibacterial activity in bryophytes: (a) the macrocyclic (bis)bibenzyls marchantin A [70] and perrottetin A, B, C, D, F [71,72]; (b) the bibenzyl lunularin [73]; (c) the glycosilate flavonoids luteolin-7-O-neohesperoside, apigenin-7-O-triglycoside, saponarin, (d) the flavonoids apigenin, lucenin-2, bartramiaflavone [74], (e) the sesquiterpenoids α-herbertenol; β-herbertenol; herbertene-1,2-diol; mastigophorene C, α-formyl herbertenol, and lepidozenolide [75,76]. These compounds have shown a weak to strong activity against several human pathogenic bacteria [70,71,73,74,75,76,77,78].
Gram-negative bacteria are particularly worrisome since they are becoming resistant to nearly all the antibiotic drug options available [79]. Among pathogenic bacteria, resistant strains of Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus ssp, Pseudomonas aeruginosa, Escherichia coli, Klebesiella pneumoniae are of serious concern [18,79]. Methicillin-resistant Staphylococcus aureus (MRSA) is a major threat in health care structures, being the most resistant S.aureus strain [18,80]. Several researches have focused on the efficacy of bryophyte extracts against S. aureus (Table 3). The vast majority of these studies have evidenced the antibacterial activity of several pure compounds against normal strains of S. aureus: bibenzyls [71,72,81,82]; sesquiterpenoids [75]; extracts [56,58,61,83,84]. Agar disk diffusion against S. aureus of several sesquiterpenoids isolated from Mastigophora diclados methanolic extracts results in a zone of inhibition ranging from 13 to 17 mm. The sesquiterpenoid mastigophorene C showed the highest activity (17 mm) yet lower to reference antibiotics chloramphenicol (22 mm) and kanamycin (23 mm). Marchantin A purified from methanolic extracts was found to be effective against S. aureus (MIC 3.13 µg/mL) [81]. Another study found a slightly lower MIC (0.062 mg/mL) and a disk diffusion inhibition zone of 11 mm [82]. Interestingly, in some studies, bryophyte extracts and pure compounds have shown anti-MRSA activity [76,83]. Ref. [85] have attempted to synthetize three geometrical isomers of the bis(bibenzyl) isoplagiochin D and tested the derivatives molecules on MRSA. They found that two out of three derivatives had a potent anti-MSRA activity (MIC 0.5 µg/mL and 2 µg/mL). MDR (Multi-Drug Resistant) Pseudomonas aeruginosa cause 6000 of 51,000 infections and about 400 deaths every year in the U.S. [79]. Interestingly, the inhibitory activities against Pseudomonas aeruginosa have been proved also by other studies both in organic solvent extracts [84,86,87,88] and bibenzyls and flavonoids [73,74]. Depending on the solvent and the selected species, MIC ranged from 3.91 µg/mL to 110 µg/mL. A study from [74] evaluated the antibacterial activity of seven flavonoids purified from methanol:acetone (8:2) extracts of five mosses species: apigenin, apigenin-7-O-triglycoside, vitexin, saponarine, lucenin-2, bartramiaflavone, luteolin-7-O-neohesperoside. bartramiaflavone; lucenin-2; saponarin; apigenin; and apigenin-7-O-triglycoside were found to be effective against normal strains Pseudomonas aeruginosa (MIC 8 µg/mL to 256 µg/mL), with saponarin, apigenin, and lucenin showing the highest activity. However, no data on the effectiveness on MDR P.aeruginosa strains have been yet produced.

Table 3.
List of the selected bacterial species and related antibacterial extracts and compounds.
Resistant strains of Escherichia coli and Klebsiella pneumoniae (i.e., Carbapenem-resistant Enterobacteriaceae) have become worrisome in clinical practice, due to the presence of NDM-1 (New Delhi metallo-beta-lactamase), which make them resistant to all beta-lactam antibiotics [89]. Each year, CRE E. coli and K. pneumoniae caused approximately 600 deaths [18]. Several studies have demonstrated the efficacy of bryophytes extracts against non-resistant Klebsiella pneumoniae [62,87,90], and extracts [61,91,92] and flavonoids against non-resistant E. coli [74]. Water, pure ethanol and water:ethanol extract were found to be effective against E. coli (MIC from 1.57 µg/mL to 3.91 µg/mL) [61,91]. On the other hand, flavonoids isolated by [74] showed weaker activity (64 µg/mL to 128 µg/mL) (Table 2). Other studies have demonstrated a strong antibacterial activity of the flavonoids bartramiaflavone, lucenin-2, saponarin, and apigenin (4 µg/mL to 8 µg/mL) and marchantin A against Enterococcus ssp [70,74].
In summary, the review literature has shown that, apart from a few studies on MRSA, no data have been produced on the drug-resistant strains of the other mentioned bacteria. Thus, part of the research should also consider Moreover, the totality of the studies has been addressed to test the in vitro antibacterial activity.
5. Conclusions and Future Perspective
Bryophytes synthetize unique compounds that have shown a wide range of biological activities such as antimicrobial, antiviral, antifungal, anticarcinogenic, insecticidal, neurotrophic, muscle relaxing, cardiotonic, and anti-obesity activities [2]. To our knowledge, bryophytes extracts/pure compounds have only been in vivo tested for their anti-inflammatory and antinociceptive [93], wound healing [94], anticarcinogenic [95,96], nanoparticle pharmacokinetics [97], and antilipidemic activities [29]. Although extensive research has been conducted on in vitro antioxidant activity, no investigations have examined the antioxidant activity of bryophytic extracts and pure compounds in in vivo models. Antioxidant activity should not be concluded based on single or multiple in vitro tests [25]. Physiological processes such as absorption, distribution, metabolism, and excretion can affect the effectiveness of certain compounds [98]. Bryophytes’ antibacterial compounds have shown promising activities against some human alarming pathogens. However, very little has been attempted in testing bryophytes against the most concerning pathogenic strains, apart from a few studies against MRSA. Furthermore, as for antioxidants, most of the data concerning the antibacterial activity were gathered from in vitro studies. In vitro antibacterial testing is based on two main techniques, namely the disk diffusion test and broth dilution test [99]. Both techniques involve the direct contact of the tested molecules with the bacterial cells, and therefore do not consider the pharmacokinetics and pharmacodynamics of the tested molecules [55]. Moreover, at the present stage only two studies have looked at the toxicity of pure bryophytic compounds and extracts [100,101]. Toxicity screening is an essential step in the drug development process [102] and should be carried out first with respect to in vitro and in vivo experimentations. Future research should be focused on the in vivo testing of antioxidant and antibacterial bryophytic compounds. This would help to circumscribe the huge number of molecules that have been discovered. Moreover, further experimentations should focus on the efficacy of bryophytic antibacterial against antibiotic-resistant strains. In conclusion, bryophytes can be exploited as a source of antioxidants and antibacterial compounds in perspective pharmaceutical and nutraceutical applications. Bibenzyls, terpenoids, phenols, and polyphenols from bryophytes are promising chemicals for the future development of novel antioxidant and antibiotic drugs. However, at the current stage, knowledge sustaining concrete applications of antioxidants and antibacterial from bryophytes is still fragmentary, and more in-depth multidisciplinary research is needed to select the safest and most effective compounds.
Author Contributions
Conceptualization, A.B. and P.C; methodology, P.C.; literature search, P.C., V.M. and S.S.; writing-original draft preparation, A.B., P.C., V.M. and S.S.; writing-review and editing, A.B. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xie, C.-F.; Lou, H.-X. Secondary Metabolites in Bryophytes: An Ecological Aspect. Chem. Biodivers. 2009, 6, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. Biologically Active Compounds from Bryophytes. Pure Appl. Chem. 2007, 79, 557–580. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, e1245049. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An Assessment of Oxidative Damage to Proteins, Lipids, and DNA in Brain from Patients with Alzheimer’s Disease. J. Neurochem. 1997, 68, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Oxidative Stress and Parkinson’s Disease. In Handbook of Clinical Neurology; Parkinson’s Disease and Related Disorders, Part I.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 83, pp. 507–520. [Google Scholar]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of Oxidative Stress in Cardiovascular Diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic Res. 2010, 44, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial Mutations and Mitoepigenetics: Focus on Regulation of Oxidative Stress-Induced Responses in Breast Cancers. Semin Cancer Biol. 2020, in press. [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and Indirect Antioxidant Properties of Inducers of Cytoprotective Proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef] [PubMed]
- Balasaheb Nimse, S.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Yao, J.-W.; Liu, J.; Kong, X.-Z.; Zhang, S.-G.; Wang, X.-H.; Yu, M.; Zhan, Y.-Q.; Li, W.; Xu, W.-X.; Tang, L.-J.; et al. Induction of Activation of the Antioxidant Response Element and Stabilization of Nrf2 by 3-(3-Pyridylmethylidene)-2-Indolinone (PMID) Confers Protection against Oxidative Stress-Induced Cell Death. Toxicol. Appl. Pharm. 2012, 259, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, e9372182. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Spellberg, B.; Gilbert, D.N. The Future of Antibiotics and Resistance: A Tribute to a Career of Leadership by John Bartlett. Clin. Infect Dis. 2014, 59 (Suppl. 2), S71–S75. [Google Scholar] [CrossRef]
- Shaw, A.J.; Goffinet, B. Bryophyte Biology; Cambridge University Press: Cambridge, UK, 2000; ISBN 978-0-521-66794-4. [Google Scholar]
- Soriano, G.; Del-Castillo-Alonso, M.-Á.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Phenolic Compounds from Different Bryophyte Species and Cell Compartments Respond Specifically to Ultraviolet Radiation, but Not Particularly Quickly. Plant Physiol. Biochem. 2019, 134, 137–144. [Google Scholar] [CrossRef]
- Núñez-Olivera, E.; Otero, S.; Tomás, R.; Martínez-Abaigar, J. Seasonal Variations in UV-Absorbing Compounds and Physiological Characteristics in the Aquatic Liverwort Jungermannia Exsertifolia Subsp. Cordifolia over a 3-Year Period. Physiol. Plant. 2009, 136, 73–85. [Google Scholar] [CrossRef]
- Otero, S.; Núñez-Olivera, E.; Martínez-Abaigar, J.; Tomás, R.; Arróniz-Crespo, M.; Beaucourt, N. Effects of Cadmium and Enhanced UV Radiation on the Physiology and the Concentration of UV-Absorbing Compounds of the Aquatic Liverwort Jungermannia Exsertifolia Subsp. Cordifolia. Photochem. Photobiol. Sci. 2006, 5, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Bogdanovic-Pristov, J.; Pejin, I.; Sabovljevic, M. Potential Antioxidant Activity of the Moss Bryum Moravicum. Nat. Prod. Res. 2013, 27, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Glamočlija, J.; Ćirić, A.; Kien-Thai, Y.; Soković, M. The Moss Rhodobryum Ontariense Tea, a Good Source of Natural Antifungals Against Candida Albicans. Rev. Chim. 2013, 64, 5–554. [Google Scholar]
- Wang, X.; Cao, J.; Wu, Y.; Wang, Q.; Xiao, J. Flavonoids, Antioxidant Potential, and Acetylcholinesterase Inhibition Activity of the Extracts from the Gametophyte and Archegoniophore of Marchantia Polymorpha L. Molecules 2016, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-O.; Choi, K.-S.; Kim, Y.-H. In Vitro Antioxidative Activity of Moss Extract, and Effect of Moss on Serum Lipid Level of Mice Fed with High-Fat Diet. Trop. J. Pharm. Res. 2016, 15, 1215–1224. [Google Scholar] [CrossRef][Green Version]
- Mukhia, S.; Mandal, P.; Singh, D.K.; Singh, D. Comparison of Pharmacological Properties and Phytochemical Constituents of In Vitro Propagated and Naturally Occurring Liverwort Lunularia Cruciata. BMC Complementary Altern. Med. 2019, 19, 181. [Google Scholar] [CrossRef]
- Wolski, G.J.; Sadowska, B.; Fol, M.; Podsędek, A.; Kajszczak, D.; Kobylińska, A. Cytotoxicity, Antimicrobial and Antioxidant Activities of Mosses Obtained from Open Habitats. PLoS ONE 2021, 16, e0257479. [Google Scholar] [CrossRef]
- Siregar, E.S.; Pasaribu, N.; Sofyan, M.Z. Antioxidant Activity of Liverworts Marchantia Paleacea Bertol. From North Sumatra Indonesia. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 713. [Google Scholar]
- Mandić, M.R.; Oalđe, M.M.; Lunić, T.M.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Dj Božić, B.; Dj. Božić Nedeljković, B. Chemical Characterization and in Vitro Immunomodulatory Effects of Different Extracts of Moss Hedwigia Ciliata (Hedw.) P. Beauv. From the Vršačke Planine Mts., Serbia. PLoS ONE 2021, 16, e0246810. [Google Scholar] [CrossRef]
- Klavina, L.; Springe, G.; Nikolajeva, V.; Martsinkevich, I.; Nakurte, I.; Dzabijeva, D.; Steinberga, I. Chemical Composition Analysis, Antimicrobial Activity and Cytotoxicity Screening of Moss Extracts (Moss Phytochemistry). Molecules 2015, 20, 17221–17243. [Google Scholar] [CrossRef]
- Horn, A.; Pascal, A.; Lončarević, I.; Volpatto Marques, R.; Lu, Y.; Miguel, S.; Bourgaud, F.; Thorsteinsdóttir, M.; Cronberg, N.; Becker, J.D.; et al. Natural Products from Bryophytes: From Basic Biology to Biotechnological Applications. Crit. Rev. Plant Sci. 2021, 40, 191–217. [Google Scholar] [CrossRef]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Asakawa, Y. Cytotoxic, Radical Scavenging and Antimicrobial Activities of Sesquiterpenoids from the Tahitian Liverwort Mastigophora Diclados (Brid.) Nees (Mastigophoraceae). J. Nat. Med. 2010, 64, 417–422. [Google Scholar] [CrossRef]
- Tazaki, H.; Ito, M.; Miyoshi, M.; Kawabata, J.; Fukushi, E.; Fujita, T.; Motouri, M.; Furuki, T.; Nabeta, K. Subulatin, an Antioxidic Caffeic Acid Derivative Isolated from the in Vitro Cultured Liverworts, Jungermannia Subulata, Lophocolea Heterophylla, and Scapania Parvitexta. Biosci. Biotechnol. Biochem. 2002, 66, 255–261. [Google Scholar] [CrossRef][Green Version]
- Bhattarai, H.D.; Paudel, B.; Lee, H.K.; Oh, H.; Yim, J.H. In Vitro Antioxidant Capacities of Two Benzonaphthoxanthenones: Ohioensins F and G, Isolated from the Antarctic Moss Polytrichastrum Alpinum. Z. Naturforsch. C. J. Biosci. 2009, 64, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Wu, C.-L.; Lin, C.-W.; Chi, L.-L.; Chen, P.-Y.; Chiu, C.-J.; Huang, C.-Y.; Chen, C.-N. Marchantin A, a Cyclic Bis(Bibenzyl Ether), Isolated from the Liverwort Marchantia Emarginata Subsp. Tosana Induces Apoptosis in Human MCF-7 Breast Cancer Cells. Cancer Lett. 2010, 291, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Schwartner, C.; Bors, W.; Michel, C.; Franck, U.; Müller-Jakic, B.; Nenninger, A.; Asakawa, Y.; Wagner, H. Effect of Marchantins and Related Compounds on 5-Lipoxygenase and Cyclooxygenase and Their Antioxidant Properties: A Structure Activity Relationship Study. Phytomedicine 1995, 2, 113–117. [Google Scholar] [CrossRef]
- Hsiao, G.; Teng, C.-M.; Wu, C.-L.; Ko, F.-N. Marchantin H as a Natural Antioxidant and Free Radical Scavenger. Arch. Biochem. Biophys. 1996, 334, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sadamori, M. Studies on the New Biologically Active Substances of Tahitian and Tokushima’s Plagiochila Genus. Master’s Thesis, Tokushima Bunri University, Tokushima, Japan.
- Liu, R.H.; Finley, J. Potential Cell Culture Models for Antioxidant Research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Ortan, A.; Fierascu, I.C.; Fierascu, I. In Vitro and in Vivo Evaluation of Antioxidant Properties of Wild-Growing Plants. A Short Review. Curr. Opin. Food Sci. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Ielpo, M.T.L.; Basile, A.; Miranda, R.; Moscatiello, V.; Nappo, C.; Sorbo, S.; Laghi, E.; Ricciardi, M.M.; Ricciardi, L.; Vuotto, M.L. Immunopharmacological Properties of Flavonoids. Fitoterapia 2000, 71, S101–S109. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Golia, B.; Montanari, S.; Castaldo Cobianchi, R.; Esposito, S. Antioxidant Activity in Extracts from Leptodictyum Riparium (Bryophyta), Stressed by Heavy Metals, Heat Shock, and Salinity. Plant. Biosyst. 2011, 145, 77–80. [Google Scholar] [CrossRef]
- Provenzano, F.; Sánchez, J.L.; Rao, E.; Santonocito, R.; Ditta, L.A.; Borrás Linares, I.; Passantino, R.; Campisi, P.; Dia, M.G.; Costa, M.A.; et al. Water Extract of Cryphaea Heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds. Int. J. Mol. Sci. 2019, 20, 5560. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-N.; Sun, Y.; Shen, T.; Zhang, J.-Z.; Zhou, J.-C.; Li, Y.; Chen, W.; Ren, Z.-J.; Li, Y.-L.; Wang, X.; et al. Diterpenoids from the Chinese Liverwort Frullania Hamatiloba and Their Nrf2 Inducing Activities. Phytochemistry 2019, 158, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.-Z.; Zhou, J.-C.; Shen, T.; Lou, H.-X. Terpenoids from Diplophyllum Taxifolium with Quinone Reductase-Inducing Activity. Fitoterapia 2016, 109, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Zhu, M.; Zhu, R.; Zhang, J.; Zhou, J.; Wang, T.; Qiao, Y.; Lou, H. Sacculatane Diterpenoids from the Chinese Liverwort Pellia Epiphylla with Protection against H2O2-Induced Apoptosis of PC12 cells. Phytochemistry 2019, 162, 173–182. [Google Scholar] [CrossRef]
- Becker, H. Secondary metabolites from bryophytes in vitro cultures. J. Hattori Bot. Lab. 1994, 76, 283–291. [Google Scholar] [CrossRef]
- Sahu, V.; Niranjan, A.; Asthana, A.K. In-Vitro Propagation and Identification of Phenol Compounds of Potential Medicinal Value in the Moss Oxystegus Stenophyllus (Mitt.) Gangulee. J. Bryol. 2014, 36, 325–327. [Google Scholar] [CrossRef]
- Sauerwein, M.; Becker, H. Growth, Terpenoid Production and Antibacterial Activity of an in Vitro Culture of the Liverwort Fossombronia Pusilla. Planta Med. 1990, 56, 364–367. [Google Scholar] [CrossRef]
- Mohandas, G.G.; Kumaraswamy, M. Antioxidant Activities of Terpenoids from Thuidium Tamariscellum (C. Muell.) Bosch. and Sande-Lac. a Moss. Pharmacogn. J. 2018, 10, 645–649. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility Test Methods. In Manual of Clinical Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1253–1273. ISBN 978-1-68367-280-7. [Google Scholar]
- Bodade, R.G.; Borkar, P.S.; Saiful Arfeen, M.; Khobragade, C.N. In Vitro Screening of Bryophytes for Antimicrobial Activity. J. Med. Plants 2008, 7, 23–28. [Google Scholar]
- Negi, K.; Chaturvedi, P. In Vitro Antimicrobial Efficacy of Rhynchostegium Vagans A. Jaeger (Moss) against Commonly Occurring Pathogenic Microbes of Indian Sub-Tropics. Asian Pac. J. Trop. Dis. 2016, 6, 10–14. [Google Scholar] [CrossRef]
- Negi, K.; Tewari, S.D.; Chaturvedi, P. Antibacterial Activity of Marchantia Papillata Raddi Subsp. Grossibarba (Steph.) Bischl. Against Staphylococcus Aureus. Indian J. Tradit. Knowl. 2018, 17, 763–769. [Google Scholar]
- Canli, K.; Altuner, E.M.; Akata, I. Antimicrobial Screening of Mnium Stellare. Bangladesh J. Pharmacol. 2015, 10, 321–325. [Google Scholar] [CrossRef]
- Canli, K.; Cetin, B.; Altuner, E.M.; Türkmen, Y.; Uzek, U.; Dursun, H. In Vitro Antimicrobial Screening of Hedwigia Ciliata Var. Leucophaea and Determination of the Ethanol Extract Composition by Gas Chromatography/Mass Spectrometry (GC/MS). J. Pure Appl. Microbiol. 2014, 8, 2987–2998. [Google Scholar]
- Saxena, K.; Yadav, U. In Vitro Assessment of Antimicrobial Activity of Aqueous and Alcoholic Extracts of Moss Atrichum Undulatum (Hedw.) P. Beauv. Physiol. Mol. Biol. Plants 2018, 24, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Manoj, G.S.; Murugan, K. Phenolic Profiles, Antimicrobial and Antioxidant Potentiality of Methanolic Extract of a Liverwort, Plagiochila Beddomei Steph. Indian J. Nat. Prod. Resour. 2012, 3, 173–183. [Google Scholar]
- Zhu, R.-L.; Wang, D.; Xu, L.; Shi, R.-P.; Wang, J.; Zheng, M. Antibacterial Activity in Extracts of Some Bryophytes from China and Mongolia. J. Hattori Bot. Lab. 2006, 100, 603–615. [Google Scholar]
- Basile, A.; Giordano, S.; Sorbo, S.; Vuotto, M.L.; Ielpo, M.T.L.; Castaldo Cobianchi, R. Antibiotic Effects of Lunularia Cruciata (Bryophyta) Extract. Pharm. Biol. 1998, 36, 25–28. [Google Scholar] [CrossRef]
- Lunić, T.M.; Oalđe, M.M.; Mandić, M.R.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Božić Nedeljković, B.D. Extracts Characterization and In Vitro Evaluation of Potential Immunomodulatory Activities of the Moss Hypnum Cupressiforme Hedw. Molecules 2020, 25, 3343. [Google Scholar] [CrossRef]
- Yucel, T.B. Chemical Composition and Antimicrobial and Antioxidant Activities of Essential Oils of Polytrichum Commune (Hedw.) and Antitrichia Curtipendula (Hedw.) Brid. Grown in Turkey. Int. J. Second. Metab. 2021, 8, 272. [Google Scholar] [CrossRef]
- Onder, A.; Yıldız, A.; Cinar, A.S.; Zengin, G.; Ak, G.; Ozenoğlu, H. The Comparison of the Phytochemical Composition, Antioxidant and Enzyme Inhibition Activity of Two Moss Species: Plagiomnium Ellipticum (Brid.) T. Kop. and Antitrichia Californica Sull., from Southwest Ecological Region in Turkey. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Zinsmeister, H.D.; Becker, H.; Eicher, T. Bryophytes, a Source of Biologically Active, Naturally Occurring Material? Angew. Chem. Int. Ed. Engl. 1991, 30, 130–147. [Google Scholar] [CrossRef]
- Nandy, S.; Dey, A. Bibenzyls and Bisbybenzyls of Bryophytic Origin as Promising Source of Novel Therapeutics: Pharmacology, Synthesis and Structure-Activity. Daru 2020, 28, 701–734. [Google Scholar] [CrossRef] [PubMed]
- Bukvicki, D.; Novaković, M.; Ilić Tomić, T.; Nikodinović-Runić, J.; Todorović, N.; Veljić, M.; Asakawa, Y. Biotransformation of Perrottetin F by Aspergillus Niger: New Bioactive Secondary Metabolites. Rec. Nat. Prod. 2021, 15, 281–292. [Google Scholar] [CrossRef]
- Lu, Z.-Q.; Fan, P.-H.; Ji, M.; Lou, H.-X. Terpenoids and Bisbibenzyls from Chinese Liverworts Conocephalum Conicum and Dumortiera Hirsuta. J. Asian Nat. Prod. Res. 2006, 8, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial Activity of Pure Flavonoids Isolated from Mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Asakawa, Y. Chemical Constituents of Malagasy Liverworts, Part II: Mastigophoric Acid Methyl Ester of Biogenetic Interest from Mastigophora Diclados (Lepicoleaceae Subf. Mastigophoroideae). Chem. Pharm. Bull. 2004, 52, 1382–1384. [Google Scholar] [CrossRef]
- Shu, Y.-F.; Wei, H.-C.; Wu, C.-L. Sesquiterpenoids from Liverworts Lepidozia Vitrea and L. Fauriana. Phytochemistry 1994, 37, 773–776. [Google Scholar] [CrossRef]
- Perry, N.B.; Foster, L.M. Sesquiterpene/Quinol from a New Zealand Liverwort, Riccardia Crassa. J. Nat. Prod. 1995, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.M.; Burgess, E.J.; Lorimer, S.D.; Perry, N.B. A Cytotoxic Sesquiterpene and Unprecedented Sesquiterpene-Bisbibenzyl Compounds from the Liverwort Schistochila Glaucescens. Tetrahedron 2002, 58, 7875–7882. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the Antibiotic Resistance Crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Morita, D.; Sawada, H.; Ogawa, W.; Miyachi, H.; Kuroda, T. Riccardin C Derivatives Cause Cell Leakage in Staphylococcus Aureus. Biochim. Et Biophys. Acta-Biomembr. 2015, 1848, 2057–2064. [Google Scholar] [CrossRef][Green Version]
- Keserű, G.M.; Nógrádi, M. The Chemistry of Macrocyclic Bis(Bibenzyls). Nat. Prod. Rep. 1995, 12, 69–75. [Google Scholar] [CrossRef]
- Ivković, I.; Bukvički, D.; Novaković, M.; Ivanović, S.; Stanojević, O.; Nikolić, I.; Veljić, M. Antibacterial Properties of Thalloid Liverworts Marchantia Polymorpha L., Conocephalum Conicum (L.) Dum. and Pellia Endiviifolia (Dicks.) Dumort: Scientific Paper. J. Serb. Chem. Soc. 2021. [Google Scholar] [CrossRef]
- Vollár, M.; Gyovai, A.; Szucs, P.; Zupkó, I.; Marschall, M.; Csupor-Lffler, B.; Bérdi, P.; Vecsernyés, A.; Csorba, A.; Liktor-Busa, E.; et al. Antiproliferative and Antimicrobial Activities of Selected Bryophytes. Molecules 2018, 23, 1520. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Nath, V.; Sahu, V.; Rawat, A.K.S. Antibacterial Activity of Some Bryophytes Used Traditionally for the Treatment of Burn Infections. Pharm. Biol. 2011, 49, 526–530. [Google Scholar] [CrossRef]
- Onoda, K.; Sawada, H.; Morita, D.; Fujii, K.; Tokiwa, H.; Kuroda, T.; Miyachi, H. Anti-MRSA Activity of Isoplagiochin-Type Macrocyclic Bis(Bibenzyl)s Is Mediated through Cell Membrane Damage. Bioorganic Med. Chem. 2015, 23, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Castaldo-Cobianchi, R.; Giordano, S.; Basile, A.; Violante, U. Occurrence of Antibiotic Activity in Conocephalum Conicum, Mnium Undulatum and Leptodictyum Riparium (Bryophytes). Plant Biosyst. 1988, 122, 303–311. [Google Scholar] [CrossRef]
- Ilhan, S.; Savaroǧlu, F.; Çolak, F.; Işçen, C.F.; Erdemgil, F.Z. Antimicrobial Activity of Palustriella Commutata (Hedw.) Ochyra Extracts (Bryophyta). Turk. J. Biol. 2006, 30, 149–152. [Google Scholar]
- Rodríguez-Rodríguez, J.C.; Samudio-Echeverry, I.J.P.; Sequeda-Castañeda, L.G. Evaluation of the Antibacterial Activity of Four Ethanolic Extracts of Bryophytes and Ten Fruit Juices of Commercial Interest in Colombia against Four Pathogenic Bacteria. Acta Hortic. 2012, 964, 251–258. [Google Scholar] [CrossRef]
- Sengupta, S.; Chattopadhyay, M.; Grossart, H.-P. The Multifaceted Roles of Antibiotics and Antibiotic Resistance in Nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.A.S.; Sousa, E.O.; Rodrigues, F.F.G.; Campos, A.R.; Lacerda, S.R.; Costa, J.G.M. Phytochemical Screening and Synergistic Interactions between Aminoglycosides, Selected Antibiotics and Extracts from the Bryophyte Octoblepharum Albidum Hedw (Calymperaceae). Arch. Biol. Sci. 2012, 64, 465–470. [Google Scholar] [CrossRef]
- Kandpal, V.; Chaturvedi, P.; Negi, K.; Gupta, S.; Sharma, A. Evaluation of Antibiotic and Biochemical Potential of Bryophytes from Kumaun Hills and Tarai Belt of Himalayas. Int. J. Pharm. Pharm. Sci. 2016, 8, 65–69. [Google Scholar]
- Semerjyan, I.; Semerjyan, G.; Semerjyan, H.; Trchounian, A. Antibacterial Properties and Flavonoids Content of Some Mosses Common in Armenia. Iran. J. Pharm. Sci. 2021, 16, 31–42. [Google Scholar] [CrossRef]
- Tosun, A.; Küpeli Akkol, E.; Süntar, I.; Özenoğlu Kiremit, H.; Asakawa, Y. Phytochemical Investigations and Bioactivity Evaluation of Liverworts as a Function of Anti-Inflammatory and Antinociceptive Properties in Animal Models. Pharm. Biol. 2013, 51, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Süntar, İ.; Keleş, H.; Özenoğlu Kiremit, H.; Asakawa, Y.; Küpeli Akkol, E. Wound Healing Potential of Selected Liverworts Growing in Turkey. Turk. J. Pharm. Sci. 2016, 13, 285–291. [Google Scholar] [CrossRef]
- Hu, Z.; Kong, F.; Si, M.; Tian, K.; Yu, L.X.; Young, C.Y.F.; Yuan, H.; Lou, H. Riccardin D Exerts Its Antitumor Activity by Inducing DNA Damage in PC-3 Prostate Cancer Cells In Vitro and In Vivo. PLoS ONE 2013, 8, e74387. [Google Scholar] [CrossRef]
- Niu, H.; Qian, L.; Sun, B.; Liu, W.; Wang, F.; Wang, Q.; Ji, X.; Luo, Y.; Nesa, E.U.; Lou, H.; et al. Inactivation of TFEB and NF-ΚB by Marchantin M Alleviates the Chemotherapy-Driven pro-Tumorigenic Senescent Secretion. Acta Pharm. Sin. B 2019, 9, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, D.; Jiao, Y.; Guo, H.; Zheng, D.; Jia, L.; Duan, C.; Liu, Y.; Tian, X.; Shen, J.; et al. In Vitro and in Vivo Evaluation of Riccardin D Nanosuspensions with Different Particle Size. Colloids Surf. B Biointerfaces 2013, 102, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Kinghorn, A.D. Drug Discovery from Medicinal Plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Cuevas, F.; Mojoli, A.; Maldonado, M.; Rojas de Arias, A.; Pandolfi, E.; Segovia-Corrales, A. In Vivo Analysis of the Genotoxic Potential of 14-Hydroxylunularin, a Molecule with Leishmanicidal Effect. Rev. Latinoam. Química 2011, 39, 100–106. [Google Scholar]
- Ozturk, S.; Yayintas, O.T. Investigation of Hepatotoxic Effect of Bryophytes (Homalothecium Sericeum (HEDW) Schimp.) on Rat Liver. Fresenius Environ. Bull. 2021, 30, 1134–1146. [Google Scholar]
- Parasuraman, S. Toxicological Screening. J. Pharmacol. Pharm. 2011, 2, 74–79. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).