MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Proton Beam Therapy Protocol
2.3. MR Protocol
2.4. Histopathology
2.5. Image Analysis
3. Results
3.1. Patients
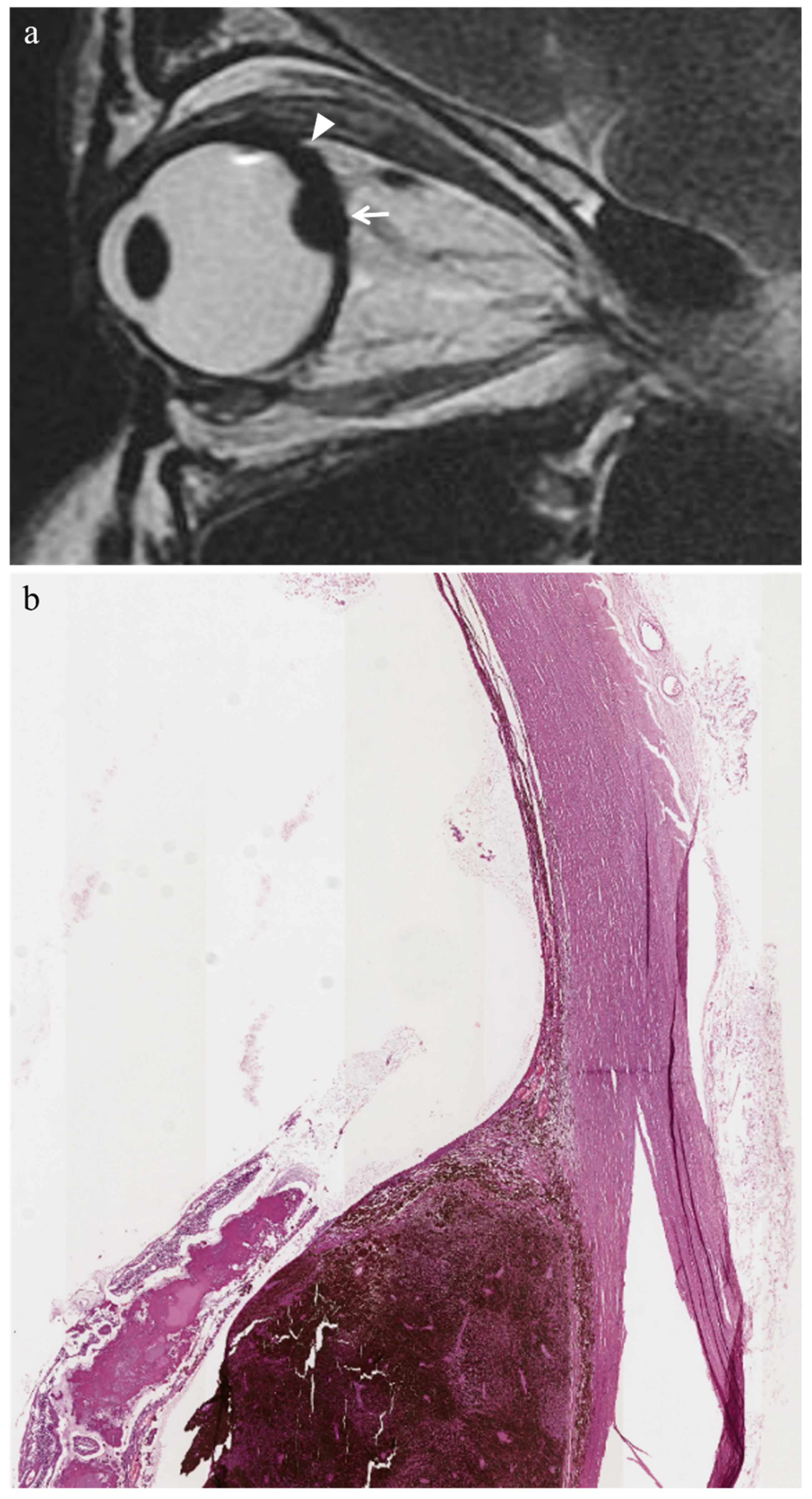
3.2. Histopathologic Findings
3.2.1. Histopathologic Findings with Respect to Irradiated Tumors
3.2.2. Extratumoral Histopathologic Findings
- Radiation-related intraocular inflammation (uveitis, endophthalmitis, and chronic conjunctivitis) (3/9 patients): presence of a conspicuous inflammatory infiltrate—mainly composed of lymphocytes, plasma cells, and granulocytes—populating the extratumoral ocular tissues.
- Vitreous hemorrhage (2/9 patients): extravasation of red blood cells within and around the vitreous body.
- Optic nerve degeneration (3/9 patients): gliosis and loss of axonal tissue, which was replaced by fibrosis.
- Iris neovascularization (1/9 patients): enrichment of blood vessels on the iris’ surface.
- Periocular fibrotic adhesions (1/9 patients): bands of scar-like tissue.
3.3. MR Findings
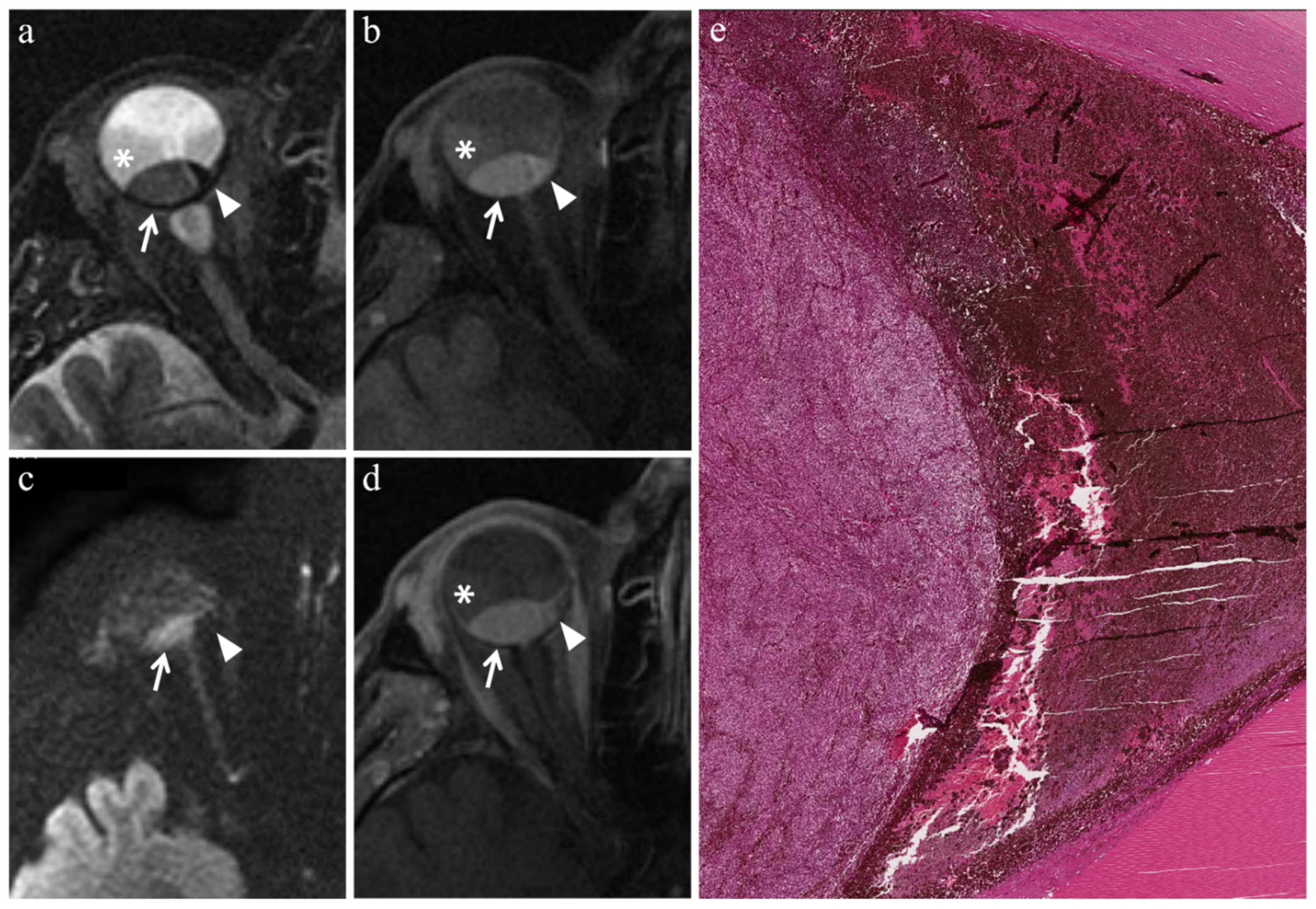
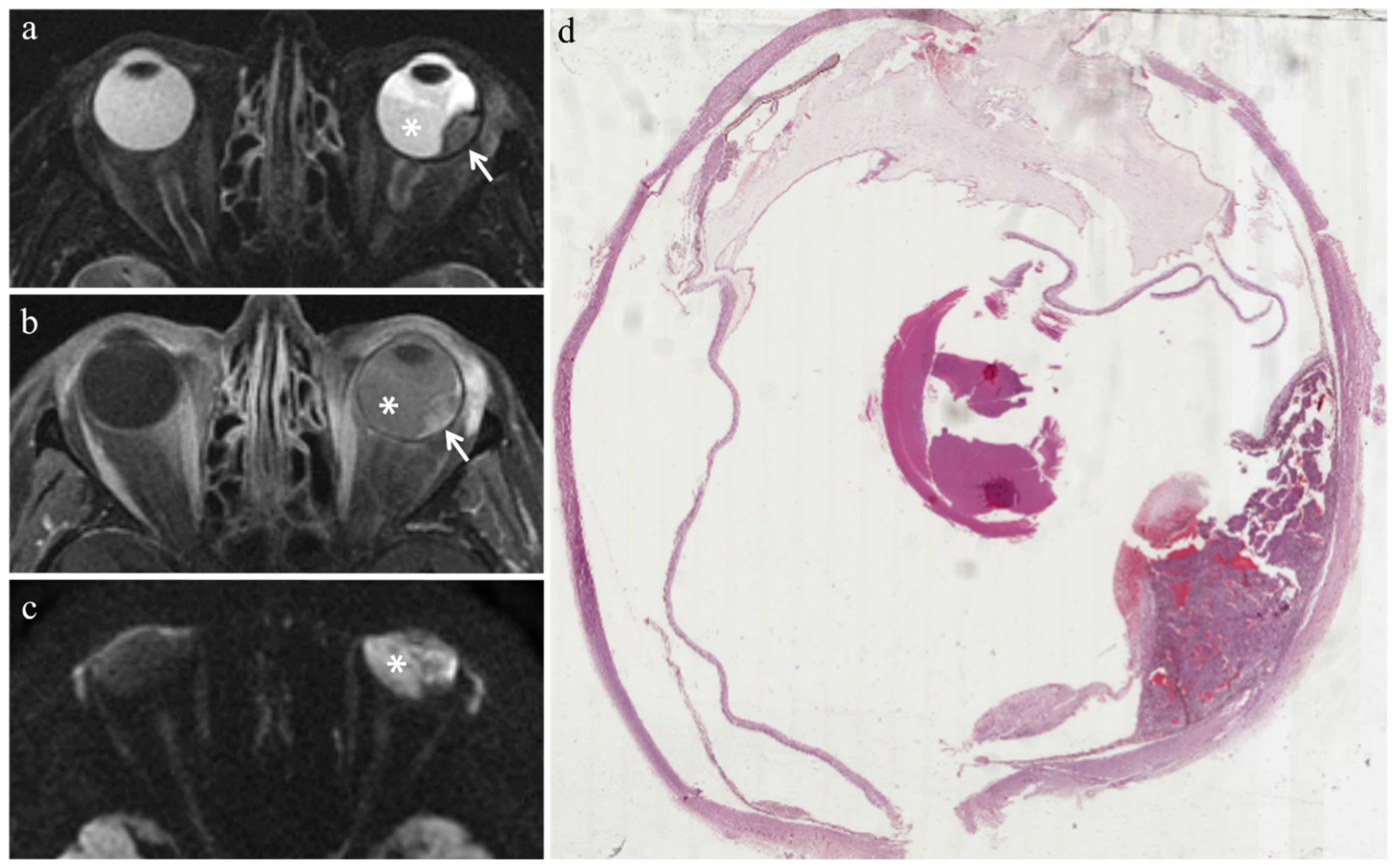
3.3.1. MR Findings with Respect to Irradiated Tumors
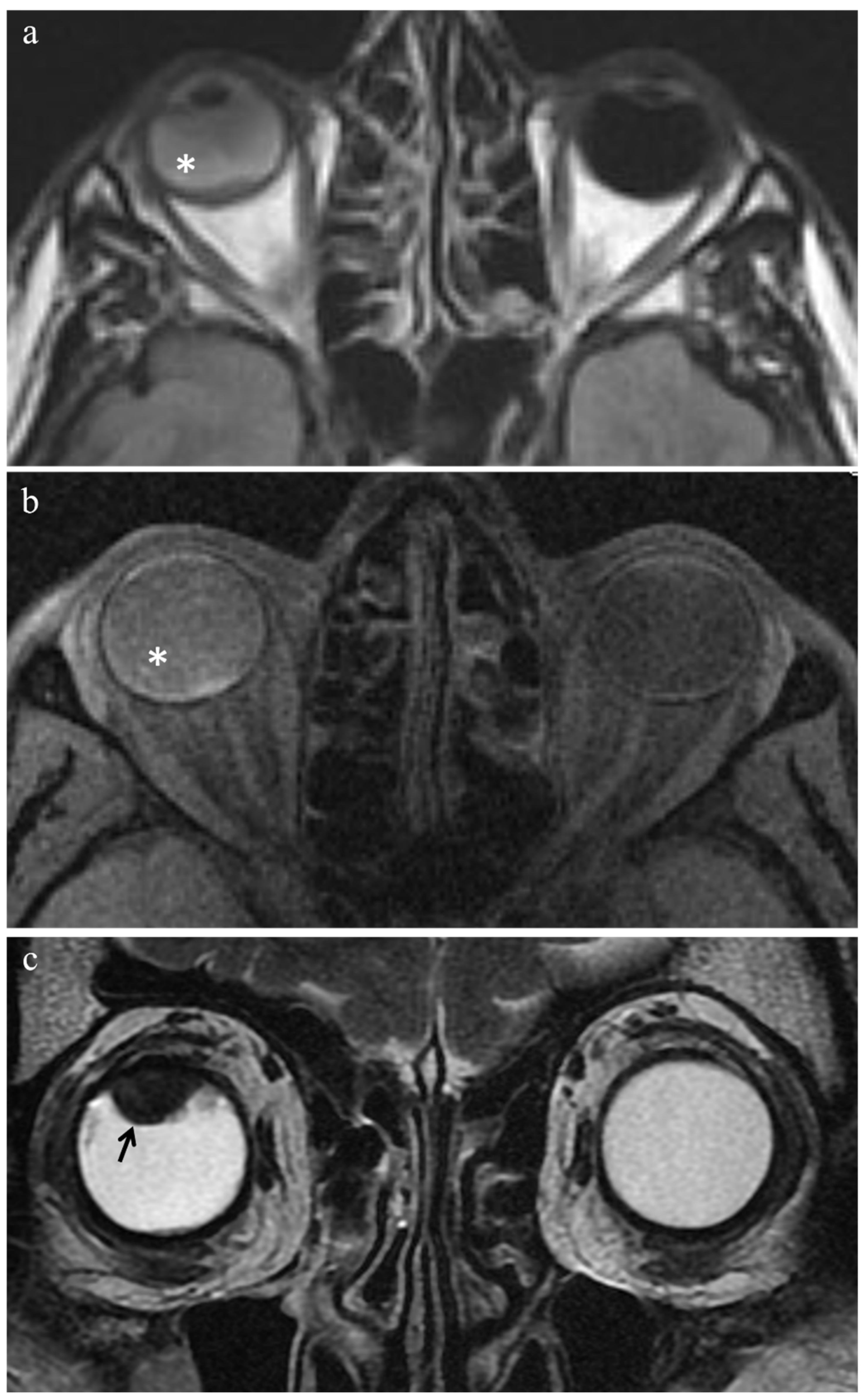
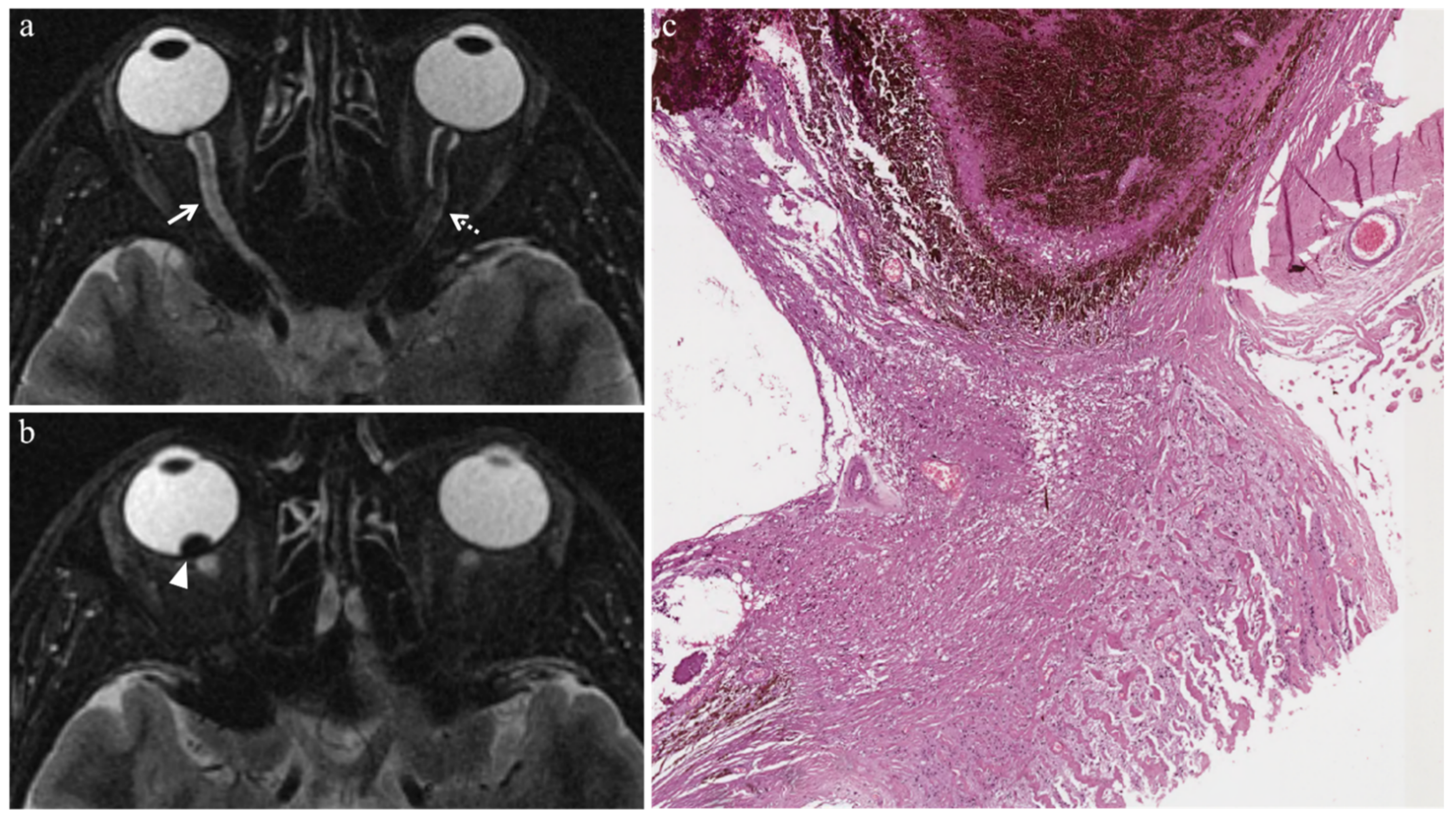
3.3.2. Extratumoral MR Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yonekawa, Y.; Kim, I.K. Epidemiology and Management of Uveal Melanoma. Hematol. Clin. N. Am. 2012, 26, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Kivela, T.; Simpson, E.R.; Grossniklaus, H.E.; Jager, M.J.; Singh, A.D.; Caminal, J.M.; Pavlick, A.C.; Kujala, E.; Coupland, S.E.; Finger, P. Uveal melanoma. In AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2017; pp. 805–817. [Google Scholar]
- Russo, A.; Avitabile, T.; Reibaldi, M.; Bonfiglio, V.; Pignatelli, F.; Fallico, M.; Caltabiano, R.; Broggi, G.; Russo, D.; Varricchio, S.; et al. Iris Melanoma: Management and Prognosis. Appl. Sci. 2020, 10, 8766. [Google Scholar] [CrossRef]
- Broggi, G.; Russo, A.; Reibaldi, M.; Russo, D.; Varricchio, S.; Bonfiglio, V.; Spatola, C.; Barbagallo, C.; Foti, P.V.; Avitabile, T.; et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Appl. Sci. 2020, 10, 8081. [Google Scholar] [CrossRef]
- Broggi, G.; Ieni, A.; Russo, D.; Varricchio, S.; Puzzo, L.; Russo, A.; Reibaldi, M.; Longo, A.; Tuccari, G.; Staibano, S.; et al. The Macro-Autophagy-Related Protein Beclin-1 Immunohistochemical Expression Correlates with Tumor Cell Type and Clinical Behavior of Uveal Melanoma. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074. [Google Scholar] [CrossRef]
- Foti, P.V.; Farina, R.; Coronella, M.; Palmucci, S.; Montana, A.; Sigona, A.; Reibaldi, M.; Longo, A.; Russo, A.; Avitabile, T.; et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting the response of ocular melanoma to proton beam therapy: Initial results. Radiol. Med. 2015, 120, 526–535. [Google Scholar] [CrossRef]
- Russo, A.; Mariotti, C.; Longo, A.; Foti, P.V.; Avitabile, T.; Uva, M.G.; Franco, L.M.; Bonfiglio, V.; Milone, P.; Ettorre, G.C.; et al. Diffusion-weighted magnetic resonance imaging and ultrasound evaluation of choroidal melanomas after proton-beam therapy. Radiol. Medica 2015, 120, 634–640. [Google Scholar] [CrossRef]
- Foti, P.V.; Longo, A.; Reibaldi, M.; Russo, A.; Privitera, G.; Spatola, C.; Raffaele, L.; Salamone, V.; Farina, R.; Palmucci, S.; et al. Uveal melanoma: Quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol. Med. 2017, 122, 131–139. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Fonk, L.G.; Jaarsma-Coes, M.G.; Van Haren, G.G.R.; Marinkovic, M.; Beenakker, J.-W.M. MRI of Uveal Melanoma. Cancers 2019, 11, 377. [Google Scholar] [CrossRef]
- Zimmerman, L.E.; McLean, I.W.; Foster, W.D. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br. J. Ophthalmol. 1978, 62, 420–425. [Google Scholar] [CrossRef]
- Tarlan, B.; Kıratlı, H. Uveal Melanoma: Current Trends in Diagnosis and Management. Turk. J. Ophthalmol. 2016, 46, 123–137. [Google Scholar] [CrossRef]
- Puusaari, I.; Heikkonen, J.; Summanen, P.; Tarkkanen, A.; Kivelä, T. Iodine brachytherapy as an alternative to enucleation for large uveal melanomas. Ophthalmology 2003, 110, 2223–2234. [Google Scholar] [CrossRef]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R.; Collaborative Ocular Melanoma Study Group. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma, III: Initial Mortality Findings. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef]
- Collaborative Ocular Melanoma Study Group. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Jampol, L.M.; Moy, C.S.; Murray, T.G.; Reynolds, S.M.; Albert, D.M.; Schachat, A.P.; Diddie, K.R.; Engstrom, R.; Finger, P.T.; Hovland, K.R.; et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. Ophthalmology 2002, 109, 2197–2206. [Google Scholar] [CrossRef]
- Aziz, S. Proton beam radiotherapy in the management of uveal melanoma: Clinical experience in Scotland. Clin. Ophthalmol. 2008, 3, 49. [Google Scholar] [CrossRef]
- Avery, R.B.; Diener-West, M.; Reynolds, S.M.; Grossniklaus, H.E.; Green, W.R.; Albert, D.M. Histopathologic Characteristics of Choroidal Melanoma in Eyes Enucleated After Iodine 125 Brachytherapy in the Collaborative Ocular Melanoma Study. Arch. Ophthalmol. 2008, 126, 207–212. [Google Scholar] [CrossRef]
- Hager, A.; Meissner, F.; Riechardt, A.; Bonaventura, T.; Löwen, J.; Heufelder, J.; Joussen, A.M. Breakdown of the blood-eye barrier in choroidal melanoma after proton beam radiotherapy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2323–2328. [Google Scholar] [CrossRef]
- Boyd, S.R.; Gittos, A.; Richter, M.; Hungerford, J.L.; Errington, R.D.; Cree, I. Proton beam therapy and iris neovascularisation in uveal melanoma. Eye 2005, 20, 832–836. [Google Scholar] [CrossRef][Green Version]
- Ferry, A.P.; Blair, C.J.; Gragoudas, E.S.; Volk, S.C. Pathologic Examination of Ciliary Body Melanoma Treated with Proton Beam Irradiation. Arch. Ophthalmol. 1985, 103, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.M.; Stein/pokorny, K.; Jakobiec, F.A.; Friedman, A.H.; Gragoudas, E.S.; Ritch, R. Proton-Beam Irradiated Epithelioid Cell Melanoma of the Ciliary Body. Ophthalmology 1981, 88, 1315–1321. [Google Scholar] [CrossRef]
- Seddon, J.M.; Gragoudas, E.S.; Albert, D.M. Ciliary Body and Choroidal Melanomas Treated by Proton Beam Irradiation. Arch. Ophthalmol. 1983, 101, 1402–1408. [Google Scholar] [CrossRef]
- Kincaid, M.C.; Folberg, R.; Torczynski, E.; Zakov, Z.N.; Shore, J.W.; Liu, S.J.; Planchard, T.A.; Weingeist, T.A. Complications after Proton Beam Therapy for Uveal Mall nant Melanoma. Ophthalmology 1988, 95, 982–991. [Google Scholar] [CrossRef]
- Lemke, A.-J.; Hosten, N.; Bornfeld, N.; Bechrakis, N.E.; Schuler, A.; Richter, M.; Stroszczynski, C.; Felix, R. Uveal Melanoma: Correlation of Histopathologic and Radiologic Findings by Using Thin-Section MR Imaging with a Surface Coil. Radiology 1999, 210, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Saornil, M.A.; Egan, K.M.; Gragoudas, E.S.; Seddon, J.M.; Walsh, S.M.; Albert, D.M. Histopathology of Proton Beam-Irradiated vs. Enucleated Uveal Melanomas. Arch. Ophthalmol. 1992, 110, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.; Konstantinidis, L.; Damato, B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye 2013, 27, 163–171. [Google Scholar] [CrossRef]
- Gragoudas, E.S.; Egan, K.M.; Saornil, M.A.; Walsh, S.M.; Albert, D.M.; Seddon, J.M. The Time Course of Irradiation Changes in Proton Beam-treated Uveal Melanomas. Ophthalmology 1993, 100, 1555–1560. [Google Scholar] [CrossRef]
- Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Basile, A.; Foti, P.V.; Palmucci, S.; Cirrone, G.A.P.; Cuttone, G.; et al. Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy. Appl. Sci. 2020, 10, 9071. [Google Scholar] [CrossRef]
- Goitein, M.; Miller, T. Planning proton therapy of the eye. Med. Phys. 1983, 10, 275–283. [Google Scholar] [CrossRef]
- Font, R.L.; Croxatto, J.O.; Rao, N.A. Tumors of the Eye and Ocular Adnexa (Afip Atlas of Tumor Pathology, Series 4), 1st ed.; Amer Registry of Pathology: Washington, DC, USA, 2006; ISBN 1-881041-99-9. [Google Scholar]
- Suit, H.; Skates, S.; Taghian, A.; Okunieff, P.; Efird, J.T. Clinical implications of heterogeneity of tumor response to radiation therapy. Radiother. Oncol. 1992, 25, 251–260. [Google Scholar] [CrossRef]
- Lumbroso, L.; Desjardins, L.; Levy, C.; Plancher, C.; Frau, E.; D’Hermies, F.; Schlienger, P.; Mammar, H.; Delacroix, S.; Nauraye, C.; et al. Intraocular inflammation after proton beam irradiation for uveal melanoma. Br. J. Ophthalmol. 2001, 85, 1305–1308. [Google Scholar] [CrossRef][Green Version]
- Mafee, M.F.; Karimi, A.; Shah, J.; Rapoport, M.; Ansari, S.A. Anatomy and Pathology of the Eye: Role of MR Imaging and CT. Neuroimaging Clin. N. Am. 2005, 15, 23–47. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, T.W.; Kim, S.; Choung, H.; Lee, M.J.; Kim, N.; Khwarg, S.I.; Yu, Y.S. Effects on Periocular Tissues after Proton Beam Radiation Therapy for Intraocular Tumors. J. Korean Med. Sci. 2018, 33, 120. [Google Scholar] [CrossRef]
- Desjardins, L.; Rouic, L.L.-L.; Levy-Gabriel, C.; Cassoux, N.; Dendale, R.; Mazal, A.; Delacroix, S.; Sastre, X.; Plancher, C.; Asselain, B. Treatment of Uveal Melanoma by Accelerated Proton Beam. Dev. Ophthalmol. 2011, 49, 41–57. [Google Scholar] [CrossRef]
- Polo, M.D.L.H.; Lluís, A.T.; Segura, O.P.; Bosque, A.A.; Appiani, C.E.; Mitjana, J.M.C. Ocular ultrasonography focused on the posterior eye segment: What radiologists should know. Insights Imaging 2016, 7, 351–364. [Google Scholar] [CrossRef]
- Chen, Y.J.; Nabavizadeh, S.A.; Vossough, A.; Kumar, S.; Loevner, L.A.; Mohan, S. Wallerian Degeneration Beyond the Corticospinal Tracts: Conventional and Advanced MRI Findings. J. Neuroimaging 2017, 27, 272–280. [Google Scholar] [CrossRef]
- Niwa, T.; Aida, N.; Shishikura, A.; Fujita, K.; Inoue, T. Susceptibility-Weighted Imaging Findings of Cortical Laminar Necrosis in Pediatric Patients. Am. J. Neuroradiol. 2008, 29, 1795–1798. [Google Scholar] [CrossRef]
- Donaire, A.; Carreno, M.; Gómez, B.; Fossas, P.; Bargalló, N.; Agudo, R.; Falip, M.; Setoaín, X.; Boget, T.; Raspall, T.; et al. Cortical laminar necrosis related to prolonged focal status epilepticus. J. Neurol. Neurosurg. Psychiatry 2006, 77, 104–106. [Google Scholar] [CrossRef]
- Boyko, O.B.; Burger, P.C.; Shelburne, J.D.; Ingram, P. Non-heme mechanisms for T1 shortening: Pathologic, CT, and MR elucidation. Am. J. Neuroradiol. 1992, 13, 1439–1445. [Google Scholar]
- Pham, C.M.; Custer, P.L.; Couch, S.M. Comparison of primary and secondary enucleation for uveal melanoma. Orbit 2017, 36, 422–427. [Google Scholar] [CrossRef]
| MRI Protocol | T2W FSE | T2W FSE STIR | T1W FSE | T1W FSE Fat Sat | DWI SE EPI |
|---|---|---|---|---|---|
| Acquisition plane | axial, coronal | axial, coronal | axial, coronal | axial, coronal | axial |
| Repetition time/Echo time (msec) | 3220/120 | 3700/50 | 550/14.9 | 450/15.1 | 4800/89.9 |
| Flip angle | 90° | 90° | 90° | 90° | 90° |
| Echo train length | 19 | 12 | 2 | 2 | - |
| N. of averages | 4 | 3 | 3 | 2 | 8 |
| Section thickness (mm) | 3 | 3 | 3 | 3 | 4 |
| Interslice gap (mm) | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Field of view (mm) | 160 × 160 | 160 × 160 | 160 × 160 | 160 × 160 | 200 × 200 |
| Matrix | 352 × 256 | 256 × 256 | 256 × 224 | 256 × 256 | 192 × 192 |
| Frequency direction | Superior to inferior | Anterior to posterior | Right to left | Right to left | Right to left |
| b-value (sec/mm2) | - | - | - | - | 0–1000 |
| Patient | Gender | Age | Eye | Tumor Location | Interval between Irradiation and Enucleation | Reasons for Enucleation |
|---|---|---|---|---|---|---|
| 1 | Male | 65 | Left | Choroid | 36 months | Tumor progression/recurrence and treatment-related complications |
| 2 | Female | 72 | Left | Choroid | 46 months | Treatment-related complications |
| 3 | Male | 60 | Right | Choroid | 35 months | Tumor progression/recurrence |
| 4 | Male | 29 | Right | Choroid | 34 months | Tumor progression/recurrence |
| 5 | Male | 37 | Right | Choroid | 38 months | Tumor progression/recurrence and treatment-related complications |
| 6 | Male | 58 | Right | Choroid | 12 months | Treatment-related complications |
| 7 | Male | 44 | Right | Choroid | 32 months | Tumor progression/recurrence |
| 8 | Female | 75 | Left | Choroid | 14 months | Tumor progression/recurrence and treatment-related complications |
| 9 | Female | 78 | Right | Choroid | 18 months | Tumor progression/recurrence |
| Patient | Histologic Type | Radiation-Induced Necrosis Histology MRI | Fibrosis Histology MRI | Viable Tumor Tissue Histology MRI | |||
|---|---|---|---|---|---|---|---|
| 1 | Epithelioid cell | - | - | - | - | Yes | Yes |
| 2 | Necrosis without viable tumor tissue | Yes | Yes | - | - | - | - |
| 3 | Spindle cell | Yes | Yes | - | - | Yes | Yes |
| 4 | Epithelioid cell | Yes | Yes | - | - | Yes | Yes |
| 5 | Spindle cell | - | - | - | - | Yes | Yes |
| 6 | Fibrotic scar without viable tumor tissue | - | - | Yes | Yes | - | - |
| 7 | Spindle cell | Yes | Yes | - | - | Yes | Yes |
| 8 | Mixed cell type | - | - | - | - | Yes | Yes |
| 9 | Mixed cell type | Yes | - | - | - | Yes | Yes |
| Patient | Radiation-Related Intraocular Inflammation | Vitreous Hemorrhage | Optic Nerve Degeneration | Iris Neovascularization | Periocular Fibrotic Adhesion |
|---|---|---|---|---|---|
| 1 | - | Yes | - | - | - |
| 2 | Yes | - | - | - | - |
| 3 | - | - | Yes | - | - |
| 4 | - | - | - | - | - |
| 5 | Yes | - | Yes | - | - |
| 6 | Yes | - | - | Yes | - |
| 7 | - | - | Yes | - | Yes |
| 8 | - | Yes | - | - | - |
| 9 | - | - | - | - | - |
| MR Finding | T2 | T1 | Gd-T1 | DWI |
|---|---|---|---|---|
| Radiation-induced necrosis |  Low signal |  High signal |  No enhancement |  Low signal |
| Fibrosis |  Low signal |  Intermediate signal |  Moderate enhancement |  Low signal |
| Viable tumor tissue, pigmented melanoma |  Low signal |  High signal |  Enhancement of viable tissue |  High signal |
| Viable tumor tissue, poorly pigmented melanoma |  Intermediate signal |  Intermediate signal |  Enhancement of viable tissue |  High signal |
 low signal;
low signal;  intermediate signal;
intermediate signal;  high signal;
high signal;  no enhancement;
no enhancement;  enhancement of the viable tissue.
enhancement of the viable tissue.| MR Finding | T2 | T1 | Gd-T1 | DWI |
|---|---|---|---|---|
| Uveitis |  High signal |  Low signal |  Enhancement |  High signal |
| Endophthalmitis |  High signal of the anterior chamber and vitreous body on T2-FLAIR |  High signal of the anterior chamber and vitreous body | - |  High signal |
| Chronic conjunctivitis | - | - |  Enhancement | - |
| Vitreous hemorrhage |  Variable signal intensity with possible fluid-fluid level |  High signal of the anterior chamber and vitreous body |  Moderately hyperintense |  High signal |
| Optic nerve degeneration |  Moderately hyperintense | - | - | - |
| Iris neovascularization |  Low signal |  Intermediate signal |  Conspicuous enhancement of the ciliary body | - |
| Radiation-induced cataract | - |  Peripheral high signal |  Peripheral high signal | - |
| Periocular fibrotic adhesion |  Low signal |  Low signal | - | - |
 low signal;
low signal;  intermediate signal;
intermediate signal;  moderately hyperintense;
moderately hyperintense;  high signal;
high signal;  enhancement;
enhancement;  peripheral high signal;
peripheral high signal;  variable signal intensity with possible fluid-fluid level.
variable signal intensity with possible fluid-fluid level.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, P.V.; Inì, C.; Travali, M.; Farina, R.; Palmucci, S.; Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; et al. MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy. Appl. Sci. 2021, 11, 4310. https://doi.org/10.3390/app11094310
Foti PV, Inì C, Travali M, Farina R, Palmucci S, Spatola C, Liardo RLE, Milazzotto R, Raffaele L, Salamone V, et al. MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy. Applied Sciences. 2021; 11(9):4310. https://doi.org/10.3390/app11094310
Chicago/Turabian StyleFoti, Pietro Valerio, Corrado Inì, Mario Travali, Renato Farina, Stefano Palmucci, Corrado Spatola, Rocco Luca Emanuele Liardo, Roberto Milazzotto, Luigi Raffaele, Vincenzo Salamone, and et al. 2021. "MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy" Applied Sciences 11, no. 9: 4310. https://doi.org/10.3390/app11094310
APA StyleFoti, P. V., Inì, C., Travali, M., Farina, R., Palmucci, S., Spatola, C., Liardo, R. L. E., Milazzotto, R., Raffaele, L., Salamone, V., Caltabiano, R., Broggi, G., Puzzo, L., Russo, A., Reibaldi, M., Longo, A., Vigneri, P., Venturini, M., & Basile, A. (2021). MR Imaging–Pathologic Correlation of Uveal Melanomas Undergoing Secondary Enucleation after Proton Beam Radiotherapy. Applied Sciences, 11(9), 4310. https://doi.org/10.3390/app11094310










