Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target
Abstract
:1. Introduction
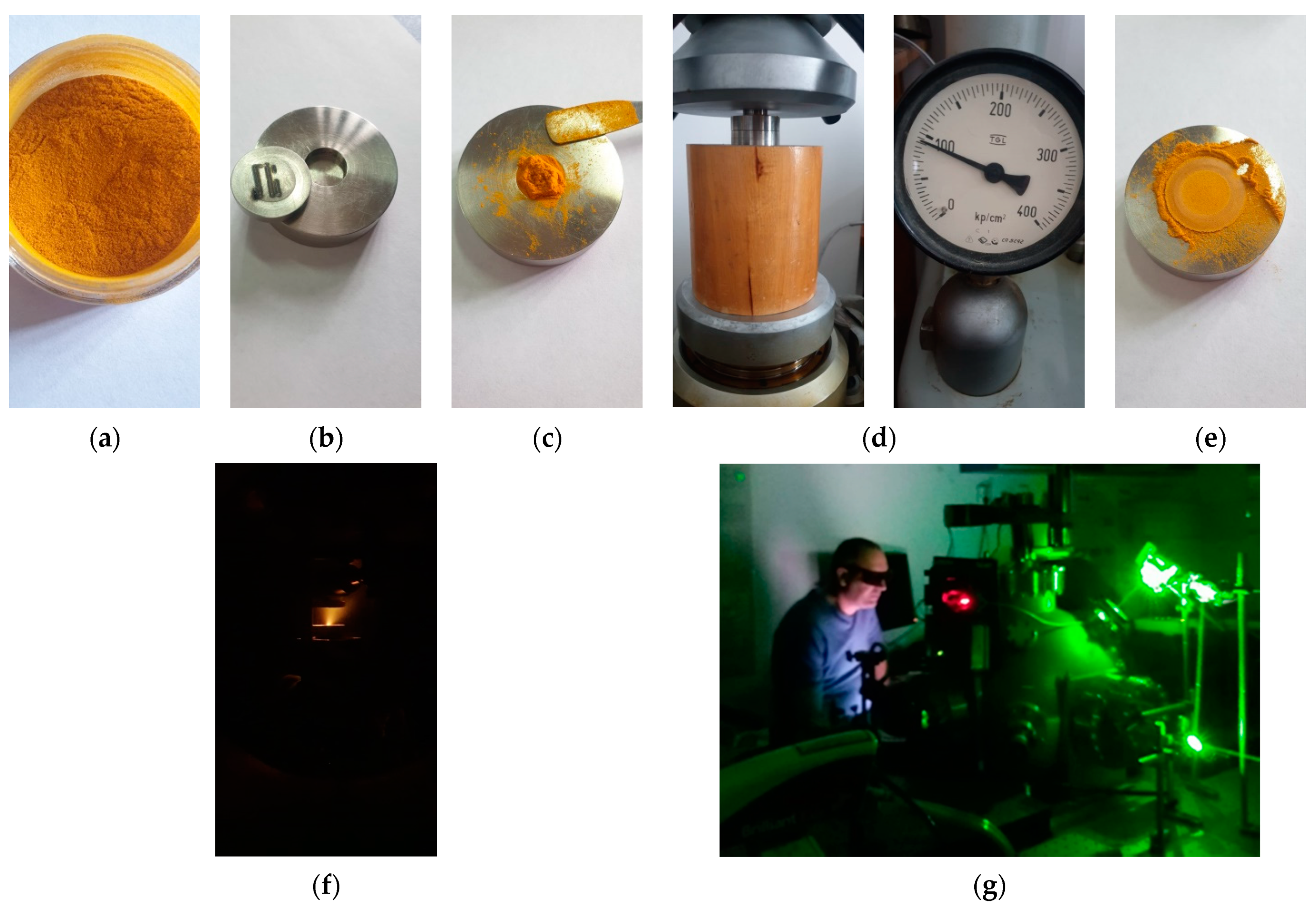
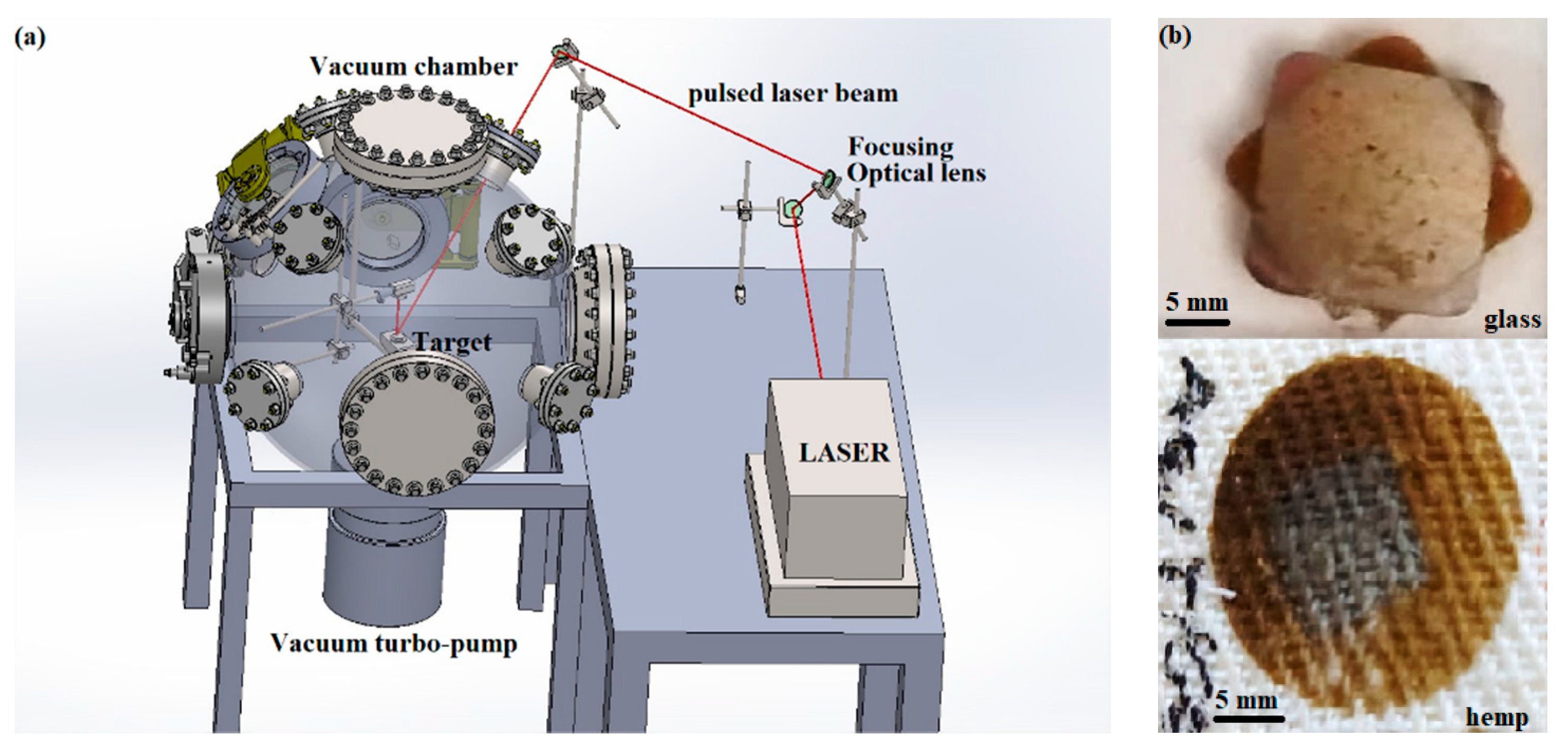
2. Materials and Methods
3. Results
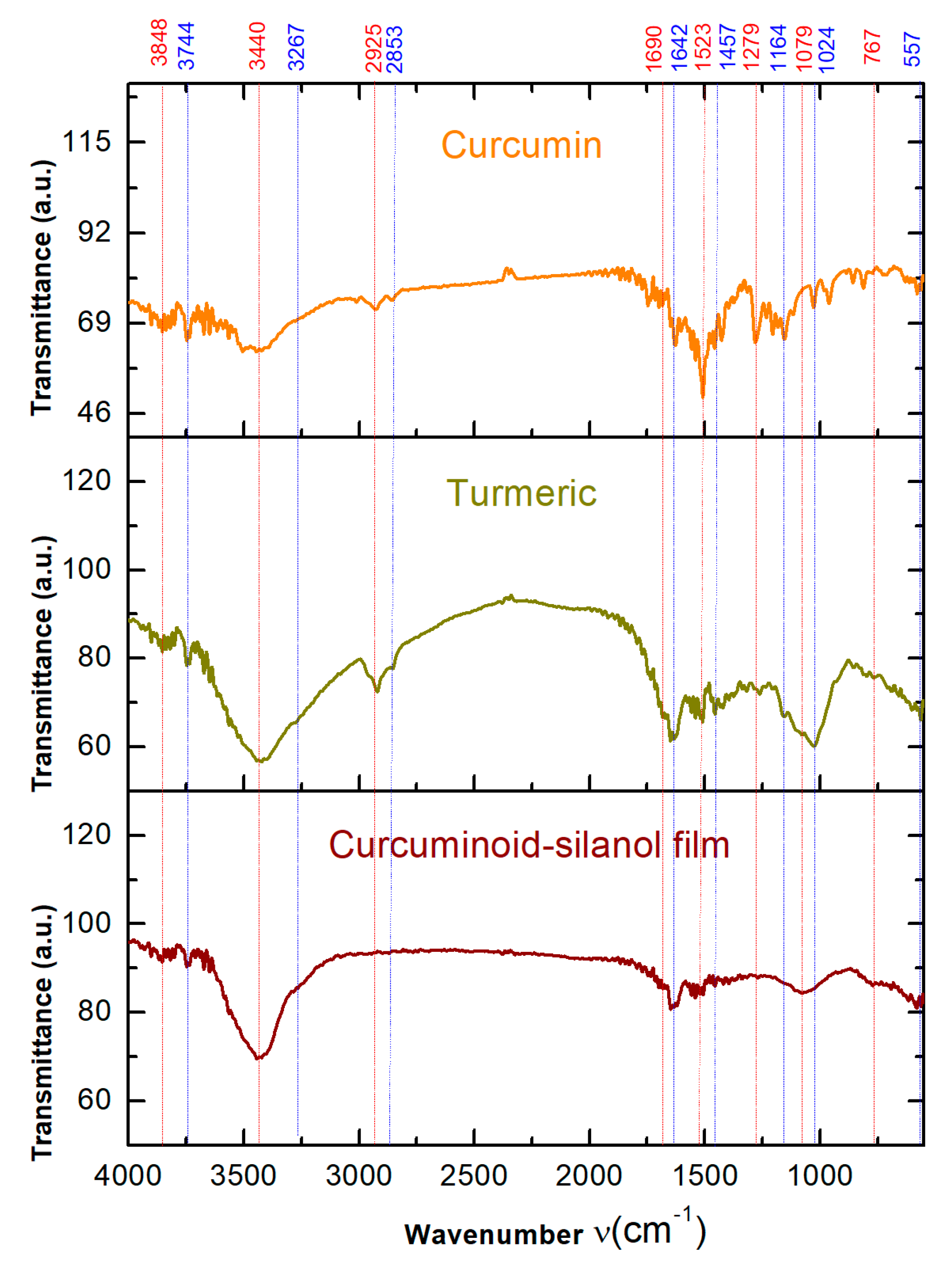
3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
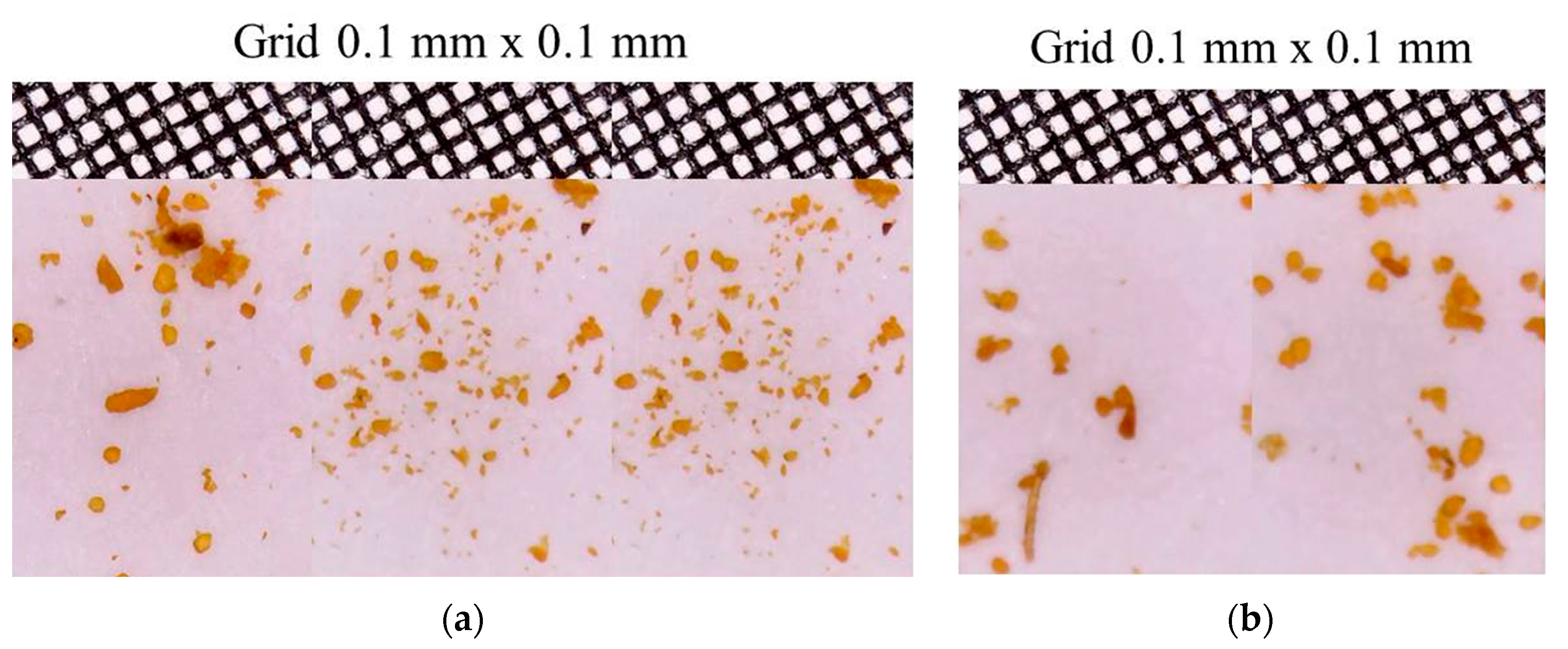
3.2. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS)
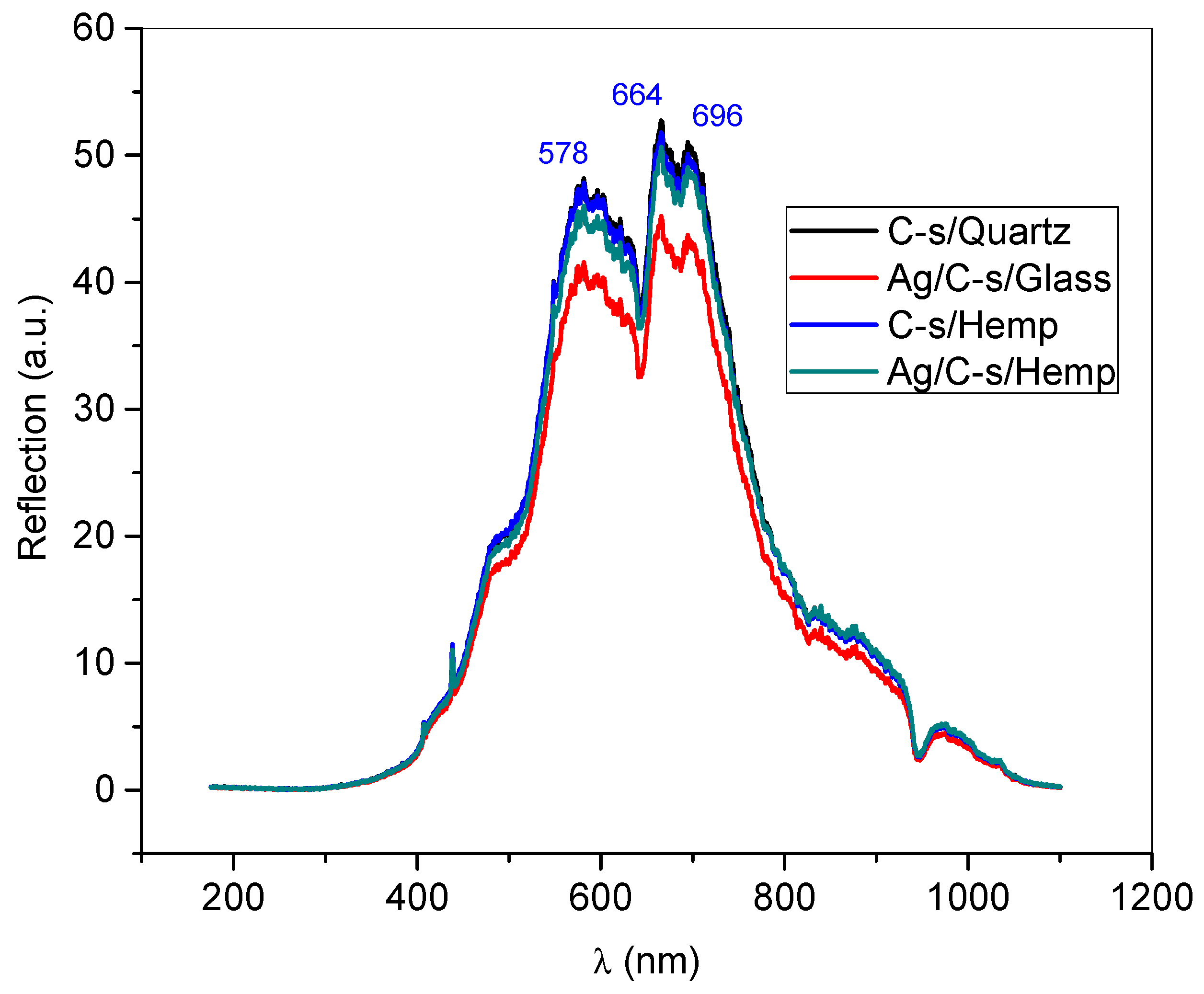
3.3. UV–VIS Spectra of Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kumar, A.; Singh, A.K.; Kaushik, M.S.; Mishra, S.K.; Raj, P.; Singh, P.K.; Pandey, K.D. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: A review. 3 Biotech 2017, 7, 357. [Google Scholar] [CrossRef]
- Niranjan, A.; Prakash, D. Chemical constituents and biological activities of turmeric (Curcuma longa L.)—A review. J. Food Sci. Technol. 2008, 45, 109–116. [Google Scholar]
- Subtaweesin, C.; Woraharn, W.; Taokaew, S.; Chiaoprakobkij, N.; Sereemaspun, A.; Phisalaphong, M. Characteristics of Curcumin-Loaded Bacterial Cellulose Films and Anticancer Properties against Malignant Melanoma Skin Cancer Cells. Appl. Sci. 2018, 8, 1188. [Google Scholar] [CrossRef] [Green Version]
- Abu-Rizq, H.A.; Mansour, M.H.; Safer, A.M.; Afzal, M. Cyto-protective and immunomodulating effect of Curcuma longa in Wistar rats subjected to carbon tetrachloride-induced oxidative stress. Inflammopharmacology 2008, 16, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C. Curcumin—Pharmacological Actions and its Role in Oral Submucous Fibrosis: A Review. J. Clin. Diagn. Res. 2015, 9, ZE01–ZE03. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Nagpal, M. Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 2013, 4, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrei, F.; Vlad, A.; Birjega, R.; Tozar, T.; Secuc, M.; Urzica, I.; Dinescu, M.; Zavoianu, R. Hybrid layered double hydroxides-curcumin thin films deposited via Matrix Assisted Pulsed Laser Evaporation-MAPLE with photoluminescence properties. Appl. Surf. Sci. 2019, 478, 754–761. [Google Scholar] [CrossRef]
- Abramov, E.; Garti, N. Incorporation of curcumin in liquid nanodomains embedded into polymeric films for dermal application. Colloids Surf. B Biointerfaces 2021, 198, 111468. [Google Scholar] [CrossRef]
- Carvalho, D.D.M.; Takeuchi, K.P.; Geraldine, R.M.; De Moura, C.J.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 20, 3277–3296. [Google Scholar] [CrossRef]
- Herreros, F.O.C.; Cintra, M.L.; Adam, R.L.; De Moraes, A.M.; Metze, K. Remodeling of the human dermis after application of salicylate silanol. Arch. Dermatol. Res. 2007, 299, 41–45. [Google Scholar] [CrossRef]
- Cocean, I.; Cocean, A.; Postolachi, C.; Pohoata, V.; Cimpoesu, N.; Bulai, G.; Iacomi, F.; Gurlui, S. Alpha keratin amino acids BEHVIOR under high FLUENCE laser interaction. Medical applications. Appl. Surf. Sci. 2019, 488, 418–426. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Gurlui, S.; Iacomi, F. Study of the pulsed laser deposition phenomena by means of Comsol Multiphysics. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys. 2017, 79, 263–274. [Google Scholar]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of spectral data. Struct. Determ. Org. Compd. 2009, 10, 978–1007. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, N.; Kaur, P.; Kaur, S.; Kaur, J.; Kukkar, P.; Kumar, V.; Kukkar, D.; Rawat, M. Piper betle leaves mediated synthesis of biogenic SnO2 nanoparticles for photocatalytic degradation of reactive yellow 186 dye under direct sunlight. Environ. Nanotechnol. Monit. Manag. 2018, 10, 331–338. [Google Scholar] [CrossRef]
- Blanchard, M.; Méheut, M.; Delon, L.; Poirier, M.; Micoud, P.; Le Roux, C.; Martin, F. Infrared spectroscopic study of the synthetic Mg–Ni talc series. Phys. Chem. Miner. 2018, 45, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, S.A.; Ahmed, R. (Eds.) Novel Developments in Pharmaceutical and Biomedical Analysis; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018. [Google Scholar]
- Van Steen, E.; Callanan, L.H. Part 3, Studies in Surface Science and Catalyzes. In Recent Advances in the Science and Technology of Zeolites and Related Materials, Proceedings of the 14th International Zeolite Conference, Cape Town, South Africa, 25–30 April 2004; Elsevier: Amsterdam, The Netherlands, 2004; Volume 154. [Google Scholar]
- Hamdan, H.; Endud, S.; He, H.; Muhid, M.N.M.; Klinowski, J. Alumination of the purely siliceous mesoporous molecular sieve MCM-41 and its hydrothermal conversion into zeolite Na-A. J. Chem. Soc. Faraday Trans. 1998, 24, 3527–3780. [Google Scholar]
- Zakaria, M.A.; Menazea, A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 2020, 19, 100438. [Google Scholar] [CrossRef]
- El Sayed, S.; De La Torre, C.; Santos-Figueroa, L.E.; Pérez-Payá, E.; Martínez-Máñez, R.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. A new fluorescent “turn-on” chemodosimeter for the detection of hydrogen sulfide in water and living cells. RSC Adv. 2013, 3, 25690. [Google Scholar] [CrossRef]
- Boom, A.F.J.V.D.; Pujari, S.P.; Bannani, F.; Driss, H.; Zuilhof, H. Fast room-temperature functionalization of silicon nanoparticles using alkyl silanols. Faraday Discuss. 2020, 222, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, Y.; Kimoto, N.; Kabeshita, M.; Itaya, A. Pulsed laser deposition of collagen and keratin. J. Photochem. Photobiol. A Chem. 2001, 145, 209–214. [Google Scholar] [CrossRef]
- Donaldson, L.; Radotic, K.; Kalauzi, A.; Djikanovic, D.; Jeremic, M. Quantification of compression wood severity in tracheids of Pinus radiate D. Don using confocal fluorescence imaging and spectral deconvolution. J. Struct. Biol. 2010, 169, 106–115. [Google Scholar] [CrossRef]
- Radotić, K.; Kalauzi, A.; Djikanović, D.; Jeremić, M.; Leblanc, R.M.; Cerovic, Z.G. Component analysis of the fluorescence spectra of a lignin model compound. J. Photochem. Photobiol. B Biol. 2006, 83, 1–10. [Google Scholar] [CrossRef]


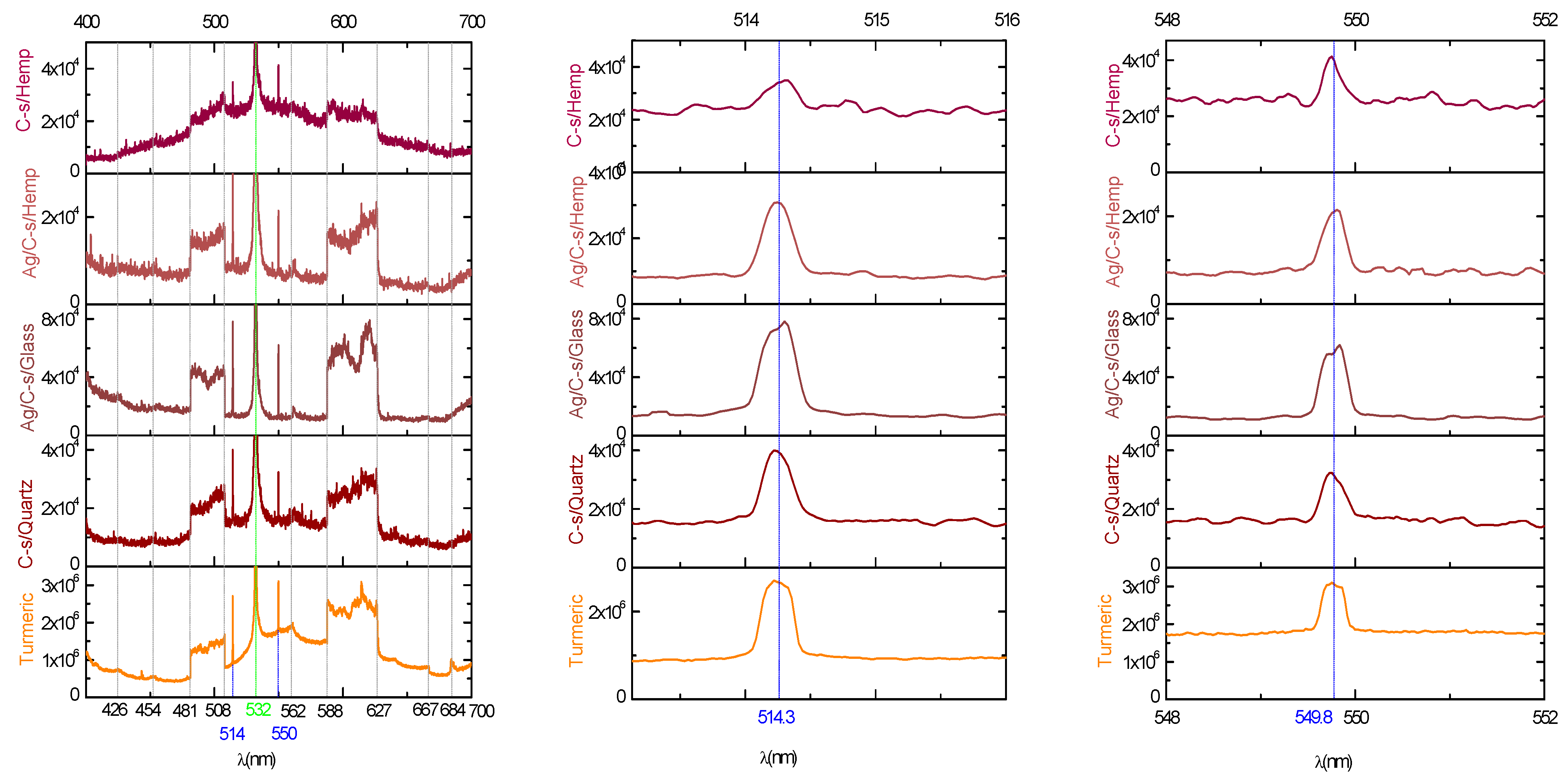
| Element | Atom (%) | Weight (%) | EDS Error % | ||||
|---|---|---|---|---|---|---|---|
| Turmeric Powder | Curcuminoid-Silanol Film/Glass | Curcuminoid-Silanol Film/Hemp | Turmeric Powder | Curcuminoid-Silanol Film/Glass | Curcuminoid-Silanol Film/Hemp | ||
| Carbon | 63.89 | 65.39 | 65.96 | 56.49 | 51.84 | 53.37 | 2.2 |
| Oxygen | 35.49 | 29.83 | 33.43 | 41.80 | 31.50 | 39.41 | 1.2 |
| Iron | 0.01 | 1.62 | 0.29 | 0.02 | 5.96 | 0.29 | 0.01 |
| Copper | - | 0.99 | 0.01 | - | 4.18 | 0.04 | 0.09 |
| Potassium | 0.53 | 1.17 | 0.15 | 1.52 | 3.01 | 0.43 | 0.11 |
| Chromium | 0.01 | 0.46 | 0.09 | 0.01 | 1.60 | 0.33 | 0.1 |
| Nickel | 0.31 | 0.03 | - | 1.19 | 0.11 | 0.13 | |
| Zinc | 0.12 | 0.01 | - | 0.51 | 0.01 | 0.05 | |
| Silicon | 0.08 | 0.11 | 0.04 | 0.17 | 0.21 | 0.09 | 0.05 |
| Reflection Intensity (a.u.) | |||
|---|---|---|---|
| Λ = 578 nm | Λ = 664 nm | Λ = 696 nm | |
| Curcuminoid-silanol film/Quartz | 48.26 | 52.6 | 51.2 |
| Curcuminoid-silanol film/Hemp | 47.8 | 52.3 | 50.3 |
| Ag/Curcuminoid-silanol film/Glass | 45.7 | 51.2 | 48.9 |
| Ag/Curcuminoid-silanol film/Hemp | 41.1 | 45 | 43.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocean, A.; Cocean, I.; Cimpoesu, N.; Cocean, G.; Cimpoesu, R.; Postolachi, C.; Popescu, V.; Gurlui, S. Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target. Appl. Sci. 2021, 11, 4030. https://doi.org/10.3390/app11094030
Cocean A, Cocean I, Cimpoesu N, Cocean G, Cimpoesu R, Postolachi C, Popescu V, Gurlui S. Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target. Applied Sciences. 2021; 11(9):4030. https://doi.org/10.3390/app11094030
Chicago/Turabian StyleCocean, Alexandru, Iuliana Cocean, Nicanor Cimpoesu, Georgiana Cocean, Ramona Cimpoesu, Cristina Postolachi, Vasilica Popescu, and Silviu Gurlui. 2021. "Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target" Applied Sciences 11, no. 9: 4030. https://doi.org/10.3390/app11094030
APA StyleCocean, A., Cocean, I., Cimpoesu, N., Cocean, G., Cimpoesu, R., Postolachi, C., Popescu, V., & Gurlui, S. (2021). Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target. Applied Sciences, 11(9), 4030. https://doi.org/10.3390/app11094030









