Assessment of Characteristics of Acid Mine Drainage Treated with Fly Ash
Abstract
1. Introduction
2. Materials and Methods
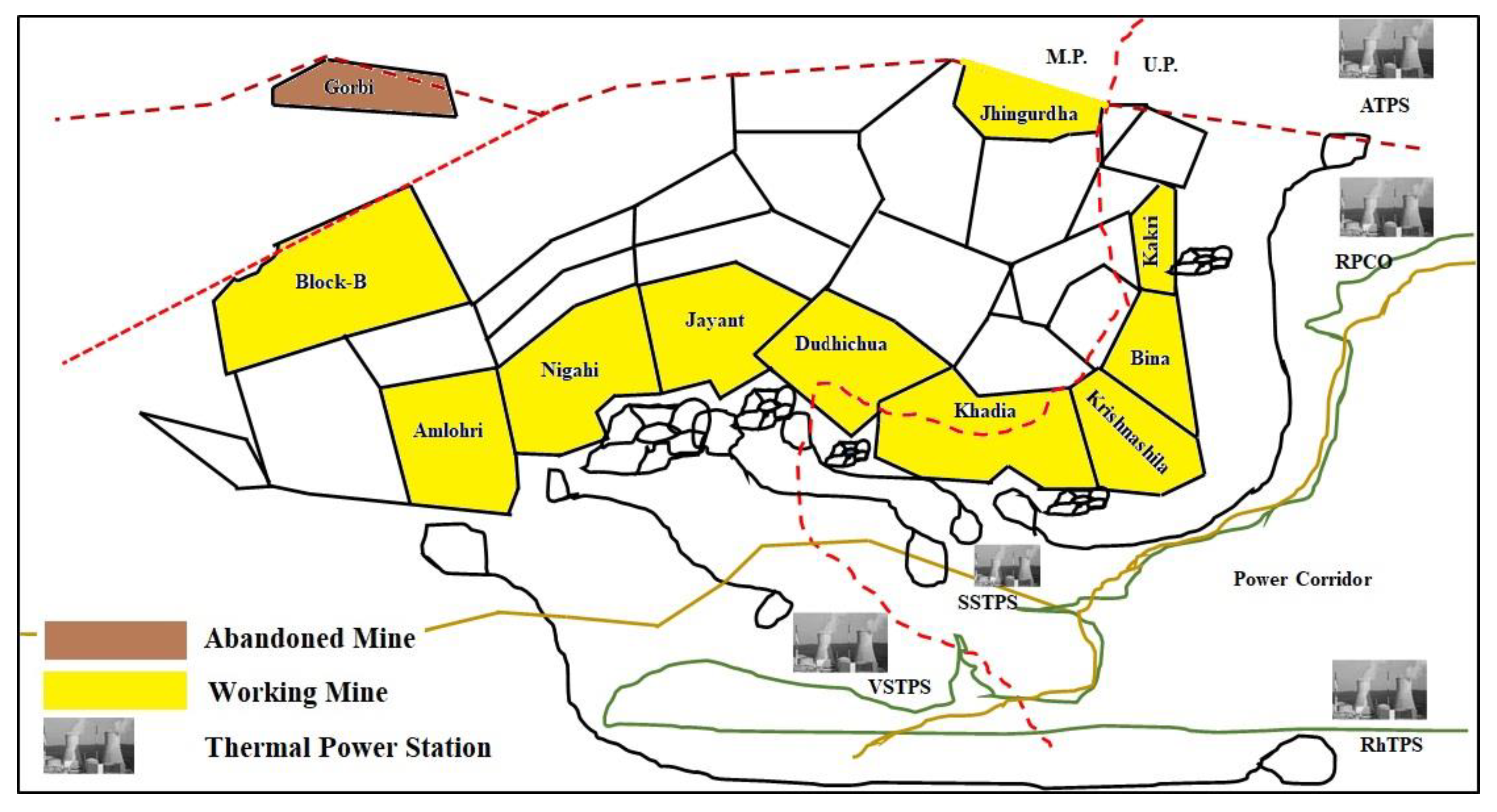
2.1. Field Investigation and Sample Collection
2.2. Fly Ash Characterization
2.3. Neutralization Experimental Setup
3. Results and Discussion
3.1. XRF Analysis of Fly Ash
- (i)
- Silica (SiO₂) is stable below 870 °C. It will not react with any base or acid except hydrofluoric acid. Hence, SiO₂ will not have an impact on AMD.
- (ii)
- Alumina (Al₂O₃) will also not have any impact on AMD, at least at room temperature.
- (iii)
- Hematite (Fe₂O₃) will have negligible impact on AMD, up to the most extreme temperature and pressure found in the field.
- (iv)
- Lime (CaO) present in fly ash will react with H₂O to give Ca(OH)₂.
- (v)
- Magnesia (MgO) present in fly ash will react with H₂O to give Mg (OH)₂, which will also neutralize acidic solutions
- (vi)
- Na₂O will react with H₂O to give NaOH.
- (vii)
- K₂O will react with H₂O to give KOH.
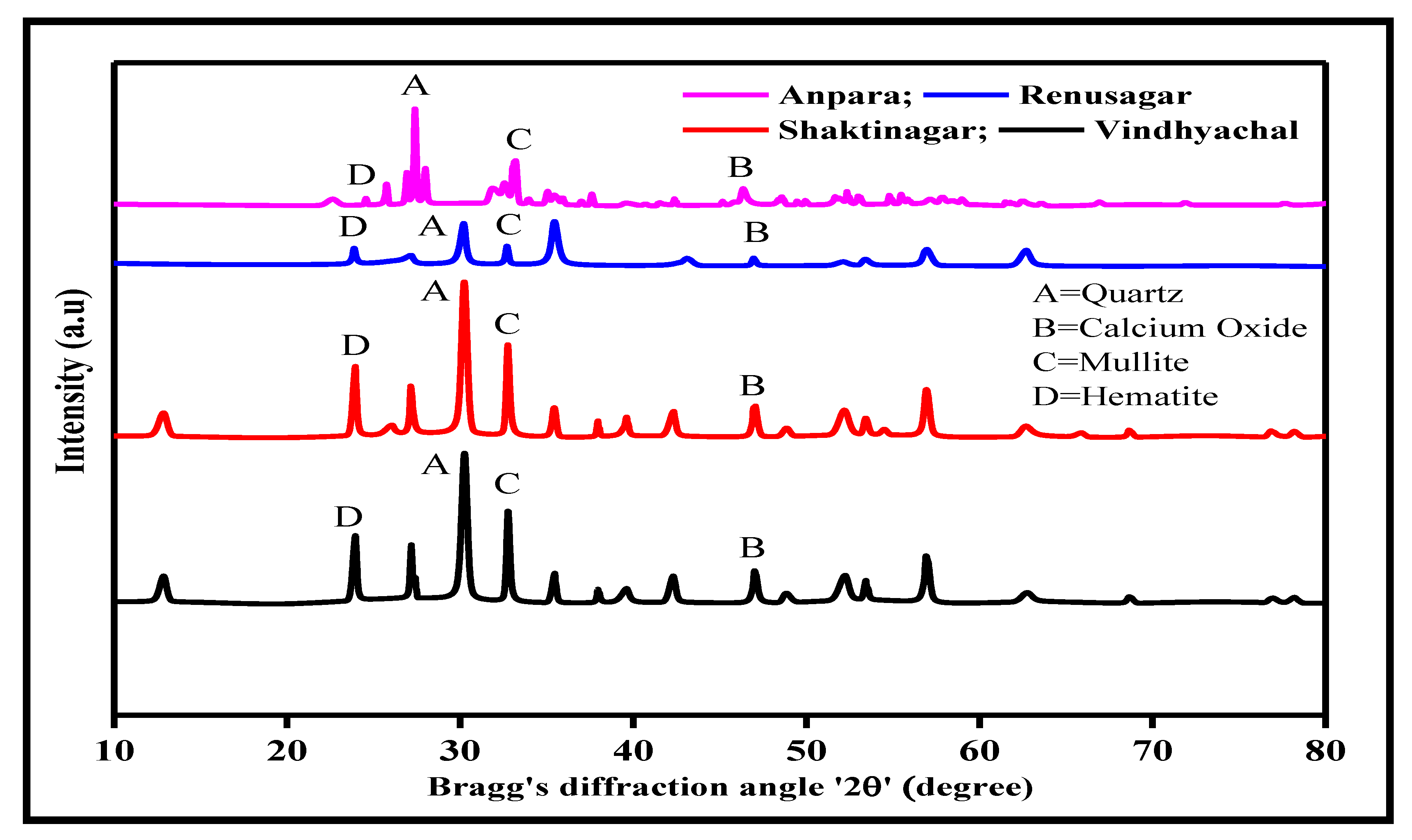
3.2. XRD Analysis of Fly Ash
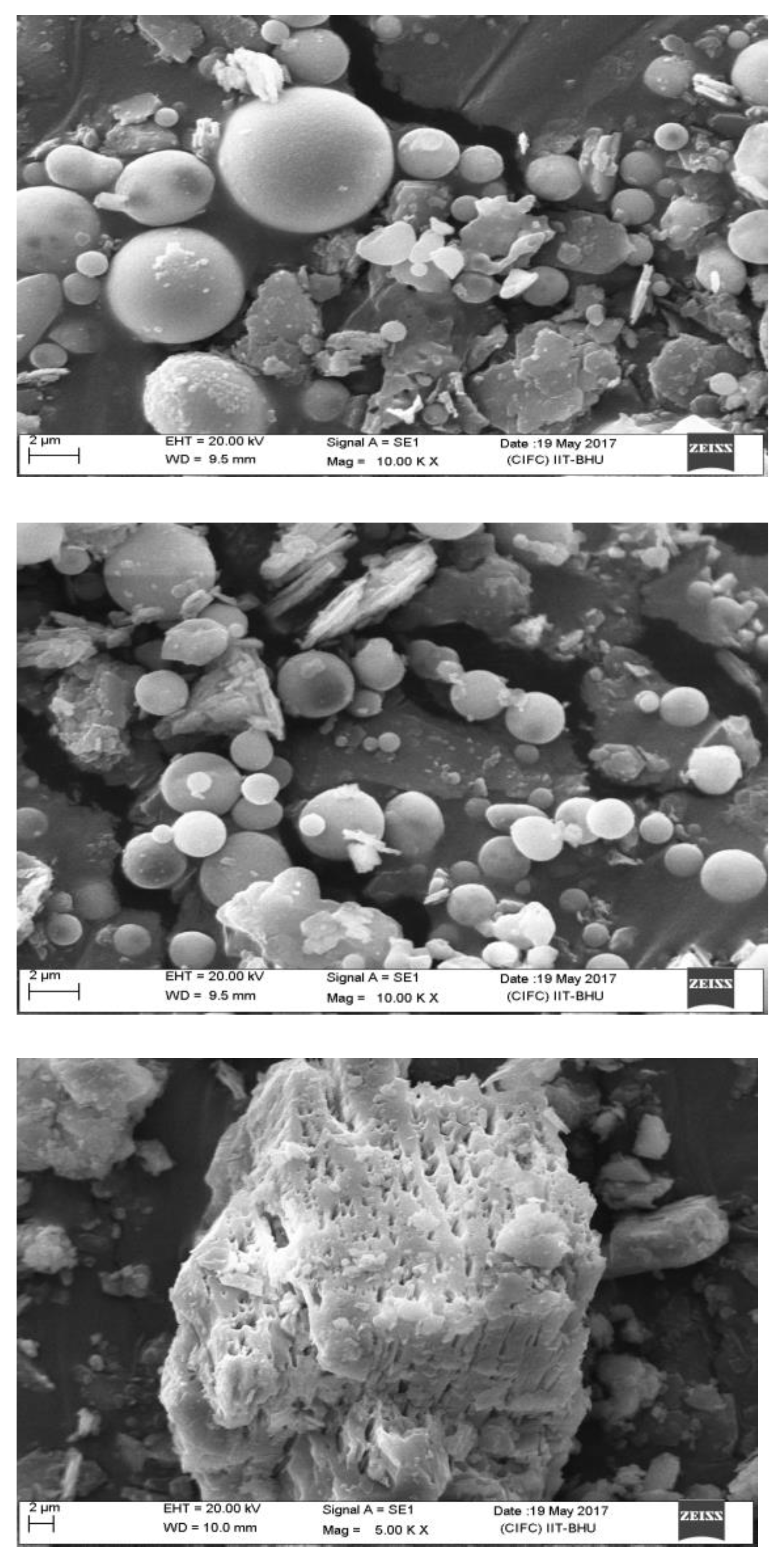
3.3. SEM Analysis of Fly Ash
3.4. AMD Quality Analysis and Neutralization Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Angelakoglou, K.; Gaidajis, G. A Conceptual Framework to Evaluate the Environmental Sustainability Performance of Mining Industrial Facilities. Sustainability 2020, 12, 2135. [Google Scholar] [CrossRef]
- Chockalingam, E.; Subramanian, S. Studies on removal of metal ions and sulphate reduction using rice husk and Desulfotomaculum nigrificans with reference to remediation of acid mine drainage. Chemosphere 2006, 62, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Brar, K.K.; Magdouli, S.; Etteieb, S.; Zolfaghari, M.; Fathollahzadeh, H.; Calugaru, L.; Komtchou, S.-P.; Tanabene, R.; Brar, S.K. Integrated bioleaching-electrometallurgy for copper recovery—A critical review. J. Clean. Prod. 2021, 291, 125257. [Google Scholar] [CrossRef]
- Shirin, S.; Jamal, A. Neutralization of acidic mine water using flyash and overburden. Rasayan J. Chem. 2018, 11, 74–79. [Google Scholar]
- Seo, J.; Kang, S.-W.; Ji, W.; Jo, H.-J.; Jung, J. Potential Risks of Effluent from Acid Mine Drainage Treatment Plants at Abandoned Coal Mines. Bull. Environ. Contam. Toxicol. 2012, 88, 990–996. [Google Scholar] [CrossRef]
- Etteieb, S.; Magdouli, S.; Zolfaghari, M.; Brar, S. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020, 698, 134339. [Google Scholar] [CrossRef]
- Jamal, A.; Dhar, B.; Ratan, S. Acid mine drainage control in an opencast coal mine. Mine Water Environ. 1991, 10, 1–16. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Jamal, A.; Ranjan, A.K.; Yadav, A.K.; Shirin, S. Prediction of mine drainage quality in a proposed coal mine. Ind. Min. Eng. J. 2015, 54, 15–19. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Magdouli, S.; Tanabene, R.; Komtchou, S.P.; Martial, R.; Saffar, T. Pragmatic strategy for the removal of ammonia from gold mine effluents using a combination of electro-coagulation and zeolite cation exchange processes: A staged approach. J. Water Process. Eng. 2020, 37, 101512. [Google Scholar] [CrossRef]
- Pradhan, A.; Deshmukh, J.P. Utilization of fly ash for treatment of acid mine water. J. Environ. Res. Dev. 2008, 3, 137–142. [Google Scholar]
- Sandeep, P.; Sahu, S.K.; Kothai, P.; Pandit, G.G. Leaching Behavior of Selected Trace and Toxic Metals in Coal Fly Ash Samples Collected from Two Thermal Power Plants, India. Bull. Environ. Contam. Toxicol. 2016, 97, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.G.; Webb, J.A. Application of Portland cement to control acid mine drainage generation from waste rocks. Appl. Geochem. 2017, 81, 143–154. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, H.; Fang, D.; Liang, J.; Zhou, L. A novel approach to rapidly purify acid mine drainage through chemically forming schwertmannite followed by lime neutralization. Water Res. 2019, 151, 515–522. [Google Scholar] [CrossRef]
- Choudhary, R.; Sheoran, A. Performance of single substrate in sulphate reducing bioreactor for the treatment of acid mine drainage. Miner. Eng. 2012, 39, 29–35. [Google Scholar] [CrossRef]
- Gitari, M.W.; Petrik, L.F.; Etchebers, O.; Key, D.L.; Iwuoha, E.; Okujeni, C. Treatment of Acid Mine Drainage with Fly Ash: Removal of Major Contaminants and Trace Elements. J. Environ. Sci. Health Part. A 2006, 41, 1729–1747. [Google Scholar] [CrossRef]
- Natarajan, K. Microbial aspects of acid mine drainage and its bioremediation. Trans. Nonferr. Met. Soc. China 2008, 18, 1352–1360. [Google Scholar] [CrossRef]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, E.-T.; Sarpola, A.; Hu, T.; Rämö, J.; Lassi, U. Acid mine drainage treatment using by-products from quicklime manufacturing as neutralization chemicals. Chemosphere 2014, 117, 419–424. [Google Scholar] [CrossRef]
- CEA. Report on Flyash Generation at Coal and Lignite Based Thermal Power Station and Its Utilization in the Country in Year 2017-18; Central Electricity Authority (CEA): New Delhi, India, 2018.
- Qureshi, A.; Jia, Y.; Maurice, C.; Öhlander, B. Potential of fly ash for neutralisation of acid mine drainage. Environ. Sci. Pollut. Res. 2016, 23, 17083–17094. [Google Scholar] [CrossRef]
- Qureshi, A.; Maurice, C.; Öhlander, B. Effects of the co-disposal of lignite fly ash and coal mine waste rocks on AMD and leachate quality. Environ. Sci. Pollut. Res. 2018, 26, 4104–4115. [Google Scholar] [CrossRef]
- Vadapalli, V.R.; Gitari, M.W.; Petrik, L.F.; Etchebers, O.; Ellendt, A. Integrated acid mine drainage management using fly ash. J. Environ. Sci. Health Part A 2012, 47, 60–69. [Google Scholar] [CrossRef]
- Yeheyis, M.B.; Shang, J.Q.; Yanful, E.K. Long-term evaluation of coal fly ash and mine tailings co-placement: A site-specific study. J. Environ. Manag. 2009, 91, 237–244. [Google Scholar] [CrossRef]
- Reash, R.J.; Van Hassel, J.H.; Wood, K.V. Ecology of a Southern Ohio stream receiving fly ash pond discharge: Changes from acid mine drainage conditions. Arch. Environ. Contam. Toxicol. 1988, 17, 543–554. [Google Scholar] [CrossRef] [PubMed]
- IS:3025. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater Part 5 Odour; Bureau of Indian Standards: New Delhi, India, 1983.
- IS:3025. Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater Part 1; Bureau of Indian Standards: New Delhi, India, 1987.
- IS:2488. Methods of Sampling and Test for Industrial Effluents Part 1; Bureau of Indian Standards: New Delhi, India, 1966.
- IS:2488. Methods of Sampling and Test for Industrial Effluents Part 5; Bureau of Indian Standards: New Delhi, India, 1976.
- Karfakis, M.G.; Bowman, C.H.; Topuz, E. Characterization of coal-mine refuse as backfilling material. Geotech. Geol. Eng. 1996, 14, 129–150. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Kretz, R. Symbols for rock-forming minerals. Am. Mineral. 1983, 68, 277–279. [Google Scholar]
- Ríos, C.; Williams, C.; Roberts, C. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef]
- Mahedi, M.; Cetin, B.; Dayioglu, A.Y. Leaching behavior of aluminum, copper, iron and zinc from cement activated fly ash and slag stabilized soils. Waste Manag. 2019, 95, 334–355. [Google Scholar] [CrossRef] [PubMed]



| Sl. No. | Pit No. | Pit Area in Ha | Depth | Water Volume |
|---|---|---|---|---|
| 1 | I | 40.00 | 50 m * | 20.00 million m3 |
| 2 | II | 8.00 | 4.00 million m3 | |
| 3 | III | 26.00 | 13.00 million m3 | |
| 74.00 | 37.00 million m3 |
| Sampling Location | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO₂ | Al₂O₃ | CaO | FeO | MgO | MnO | Na₂O | K₂O | |
| Concentration in % | ||||||||
| Anpara TPP | 45.9 | 27.7 | 17.4 | 3.2 | 2.9 | 1.6 | 0.6 | 0.7 |
| Renusagar TPP | 40.5 | 23.0 | 16.1 | 8.1 | 8.0 | 2.2 | 1.4 | 0.7 |
| Shaktinagar TPP | 44.7 | 26.3 | 15.6 | 7.9 | 1.7 | 2.1 | 1.3 | 0.4 |
| Vindhyachal TPP | 44.0 | 20.7 | 14.9 | 8.4 | 5.8 | 4.8 | 1.2 | 0.2 |
| Parameter | Unit | Concentration |
|---|---|---|
| pH (AMD water) | -- | 2.5 |
| pH (Fly ash) | -- | 12.0 |
| EC | µS/m | 2990 |
| TDS | ppm | 2270 |
| Acidity (CaCO3) | mg/L | 3097 |
| Na | 200 | |
| Mg | 45 | |
| Al | 33.7 | |
| K | 43.5 | |
| Ca | 158 | |
| Fe | 166 | |
| Fe2+ | 145 | |
| Fe3+ | 156 | |
| SO42- | 190 | |
| NO3- | 45 | |
| Al | 37.5 | |
| B | 0.07 | |
| Ba | 0.02 | |
| Be | <0.005 | |
| Cd | 0.05 | |
| Co | 0.58 | |
| Cr | <0.005 | |
| Cu | 0.06 | |
| Mn | 45 | |
| Ni | 0.70 | |
| Pb | 0.05 | |
| Sr | 0.74 | |
| Zn | 3.0 |
| Parameter * | Unit | Thermal Power Plant | |||
|---|---|---|---|---|---|
| Anpara | Renusagar | Shaktinagar | Vindhyachal | ||
| Concentration | |||||
| pH | -- | 6.0 | 6.0 | 6.0 | 6.0 |
| EC | µS/m | 765.0 | 780.0 | 735.0 | 752.0 |
| TDS | ppm | 682.0 | 675.0 | 695.0 | 672.0 |
| Na | mg/L | 20.0 | 15.0 | 21.0 | 19.0 |
| Mg | 53.0 | 54.0 | 51.0 | 49.0 | |
| Al | 29.0 | 21.0 | 27.0 | 25.0 | |
| K | 12.0 | 10.0 | 9.0 | 13.0 | |
| Ca | 55.0 | 49.0 | 53.0 | 52.0 | |
| Fe | 3.1 | 4.5 | 2.1 | 4.7 | |
| Fe2+ | 32.0 | 32.0 | 30.0 | 31.0 | |
| Fe3+ | 21.0 | 20.0 | 19.0 | 20.0 | |
| SO42− | 32.0 | 29.0 | 24.0 | 50.0 | |
| NO3− | 15.0 | 12.0 | 24.0 | 33.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirin, S.; Jamal, A.; Emmanouil, C.; Yadav, A.K. Assessment of Characteristics of Acid Mine Drainage Treated with Fly Ash. Appl. Sci. 2021, 11, 3910. https://doi.org/10.3390/app11093910
Shirin S, Jamal A, Emmanouil C, Yadav AK. Assessment of Characteristics of Acid Mine Drainage Treated with Fly Ash. Applied Sciences. 2021; 11(9):3910. https://doi.org/10.3390/app11093910
Chicago/Turabian StyleShirin, Saba, Aarif Jamal, Christina Emmanouil, and Akhilesh Kumar Yadav. 2021. "Assessment of Characteristics of Acid Mine Drainage Treated with Fly Ash" Applied Sciences 11, no. 9: 3910. https://doi.org/10.3390/app11093910
APA StyleShirin, S., Jamal, A., Emmanouil, C., & Yadav, A. K. (2021). Assessment of Characteristics of Acid Mine Drainage Treated with Fly Ash. Applied Sciences, 11(9), 3910. https://doi.org/10.3390/app11093910






