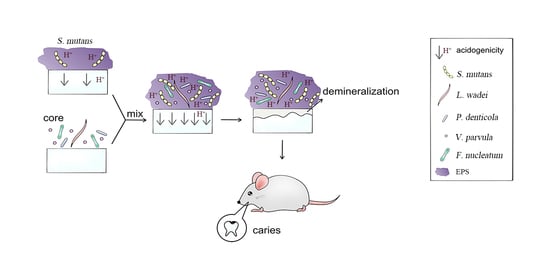
Core Microbiota Promotes the Development of Dental Caries
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Biofilm Development
2.3. Biofilm Acidogenicity and S. Mutans Counting
2.4. DNA Isolation and Quantitative Analysis of Biofilm Composition
2.5. Scanning Electron Microscopy (SEM)
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. Enamel Demineralization Assessment
2.8. Rat Caries Model and Caries Scoring
2.9. Statistical Analysis
3. Results
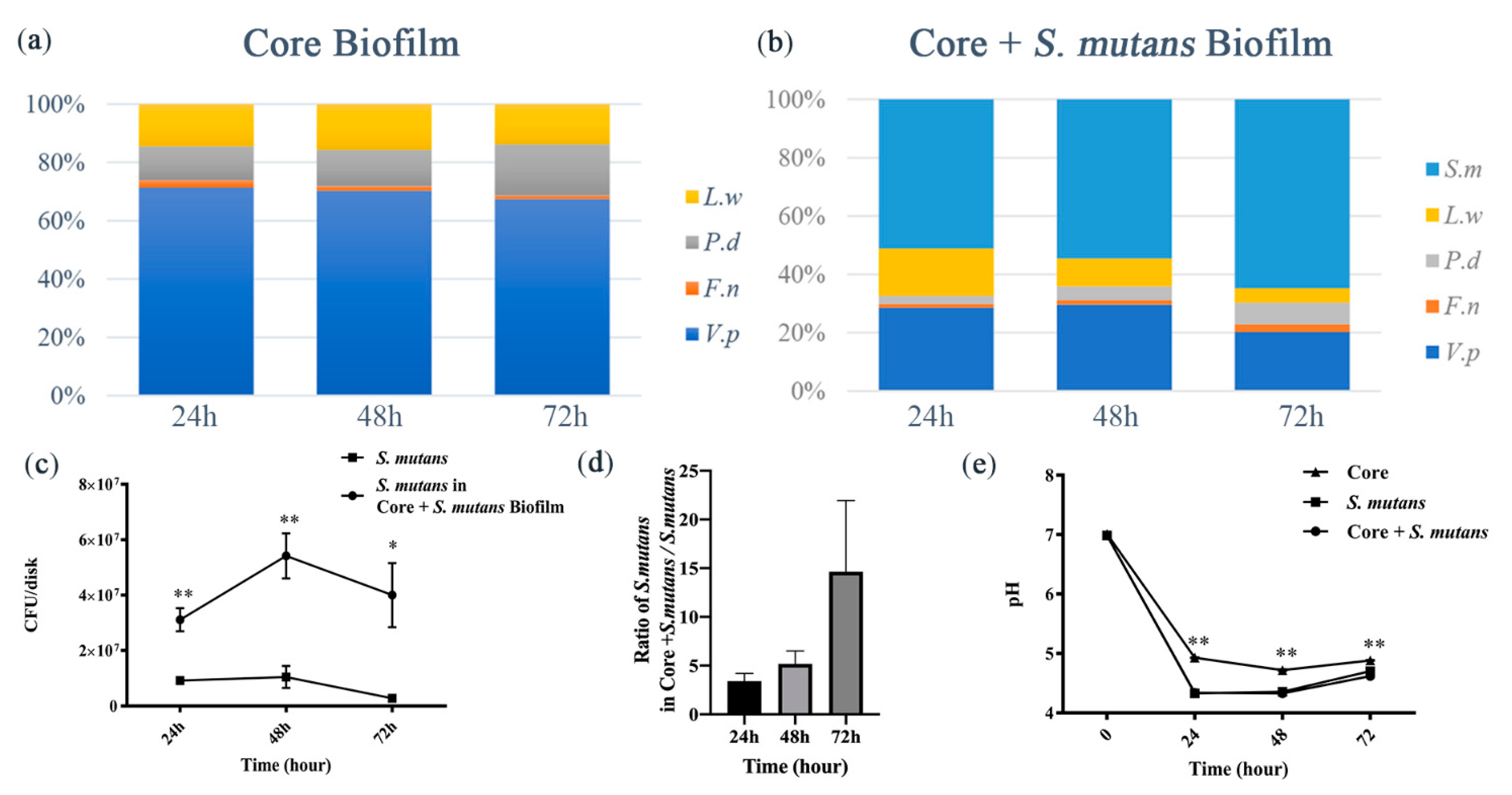
3.1. S. mutans Was Promoted by Core Microbiota and the Core + S. Mutans Biofilm Showed Increased Acidogenic Ability
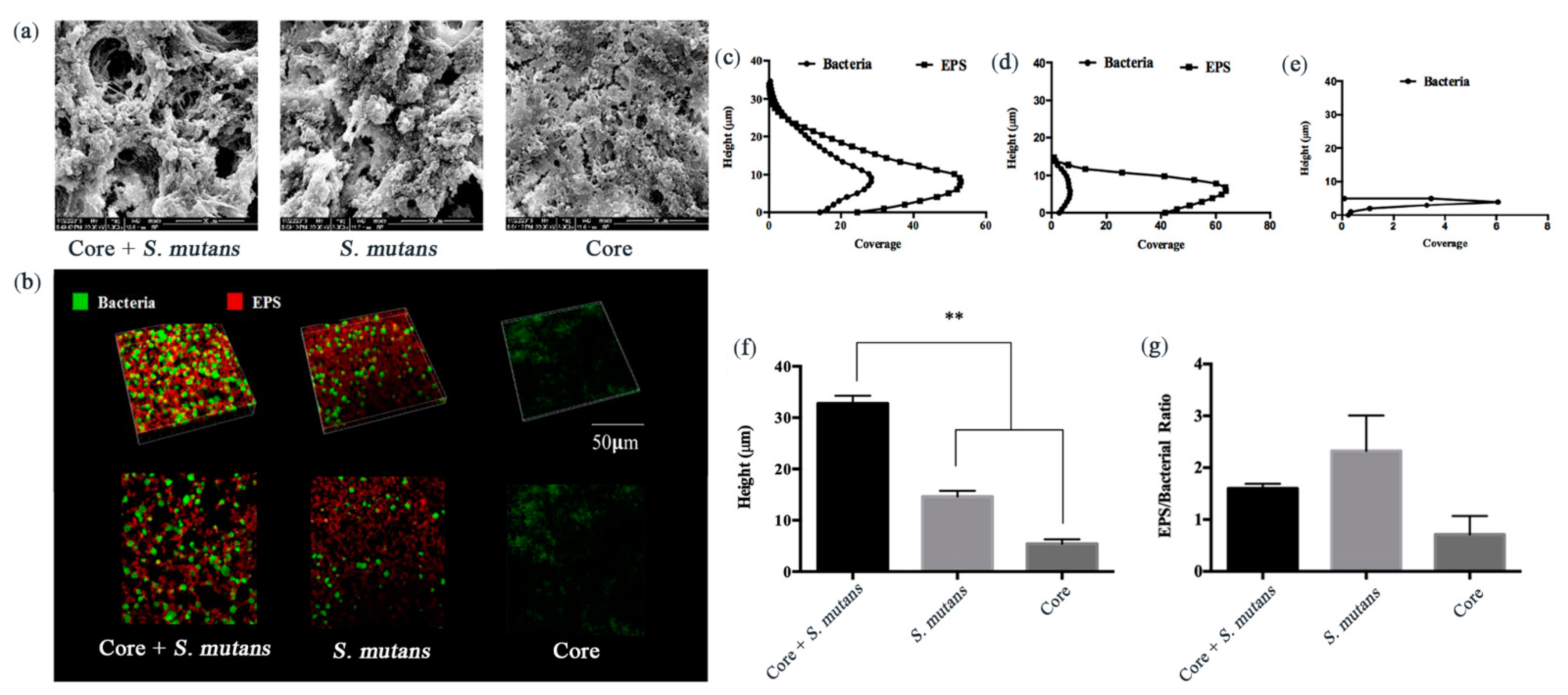
3.2. Core + S. Mutans Biofilm Showed a Net-Like Structure and Increased the Depth of the Biofilms
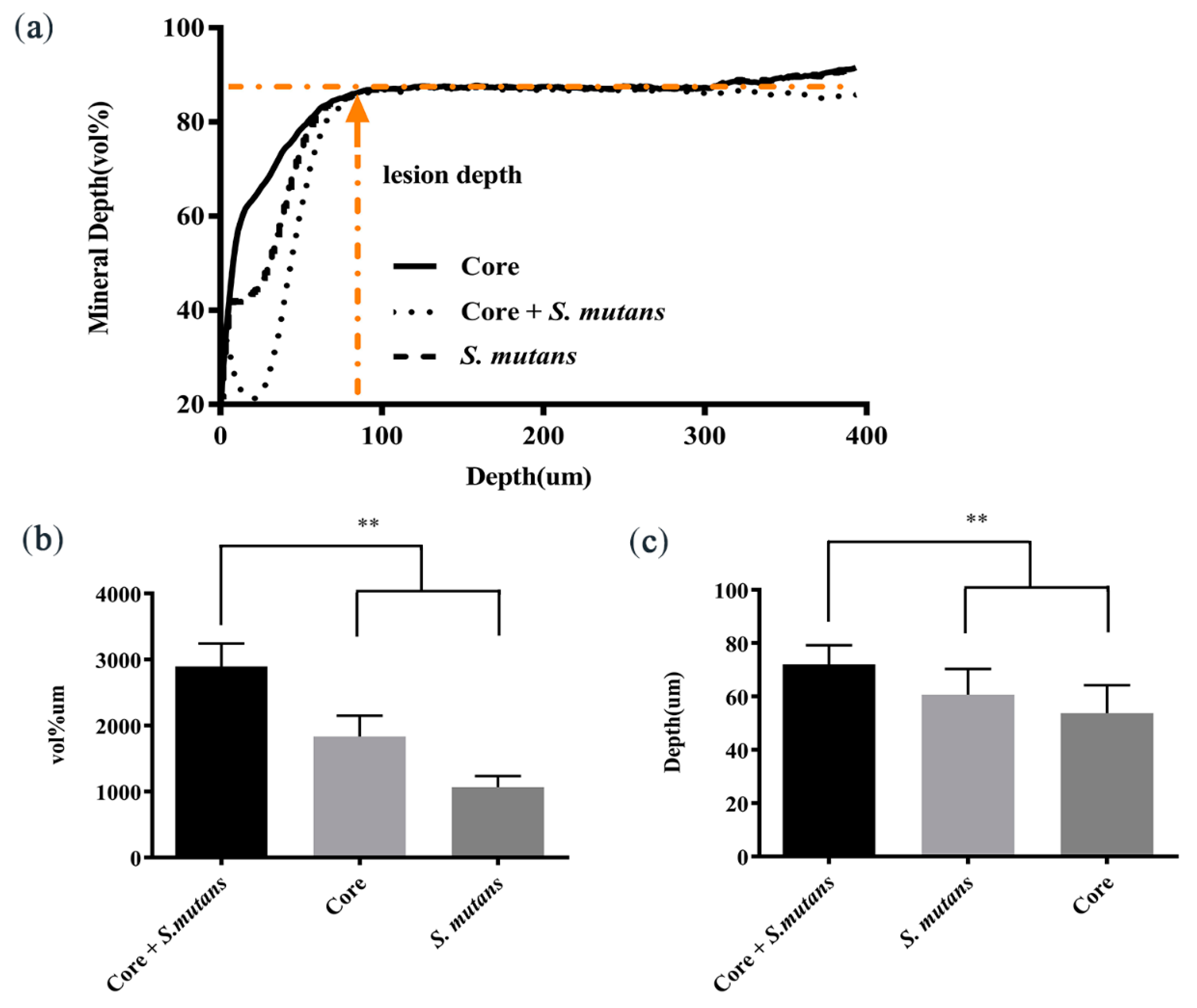
3.3. Core Microbiota Enhanced the Enamel Demineralization In Vitro
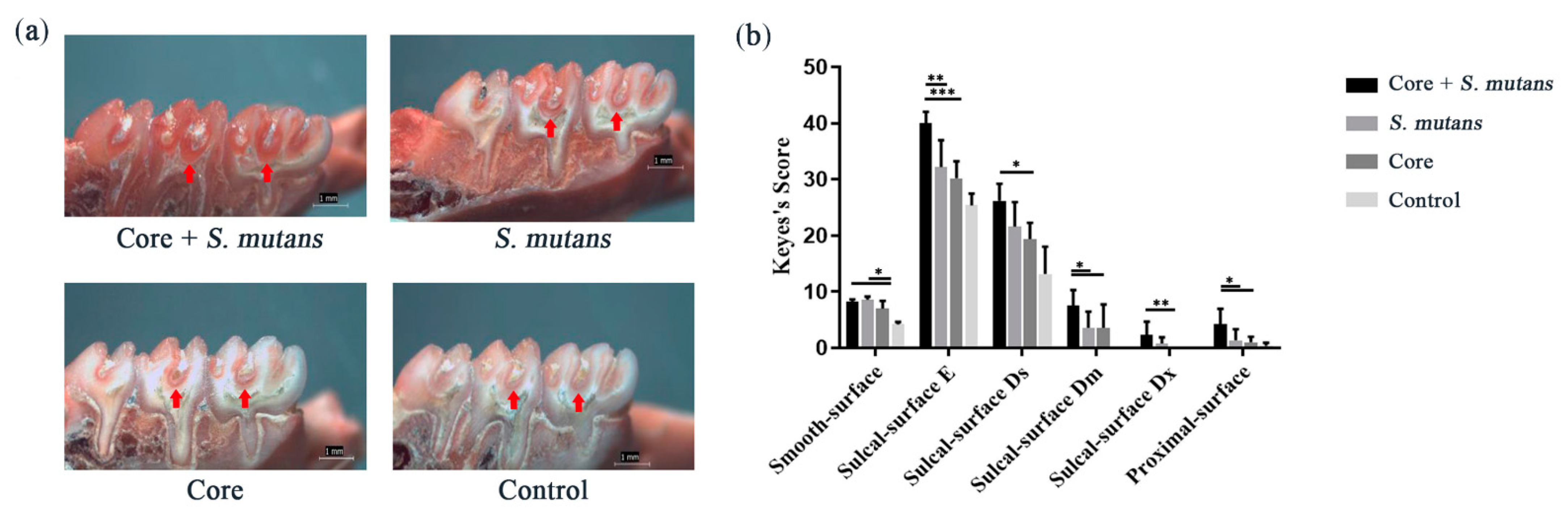
3.4. The Core Microbiota Increased Cariogenic Potential in a Rat Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hemadi, A.S.; Huang, R.; Zhou, Y.; Zou, J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral Sci. 2017, 9, e1. [Google Scholar] [CrossRef]
- Duangthip, D.; Chen, K.J.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Managing Early Childhood Caries with Atraumatic Restorative Treatment and Topical Silver and Fluoride Agents. Int. J. Environ. Res. Public Health 2017, 14, 1204. [Google Scholar] [CrossRef]
- Anil, S.; Anand, P.S. Early Childhood Caries: Prevalence, Risk Factors, and Prevention. Front. Pediatrics 2017, 5, 157. [Google Scholar] [CrossRef]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, E.; Parsaei, Y.; Klein, M.I.; Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017, 32, 24–34. [Google Scholar] [CrossRef]
- Cappello, F.; Rappa, F.; Canepa, F.; Carini, F.; Mazzola, M.; Tomasello, G.; Bonaventura, G.; Giuliana, G.; Leone, A.; Saguto, D.; et al. Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis. Int. J. Mol. Sci. 2019, 20, 5026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jiang, Y.; Li, C.; Liang, J. Investigation of supragingival plaque microbiota in different caries status of Chinese preschool children by denaturing gradient gel electrophoresis. Microb. Ecol. 2011, 61, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S. Prevention of dental caries as a non-communicable disease. Eur. J. Oral Sci. 2018, 126, 19–25. [Google Scholar] [CrossRef]
- Manji, F.; Dahlen, G.; Fejerskov, O. Caries and Periodontitis: Contesting the Conventional Wisdom on Their Aetiology. Caries Res. 2018, 52, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Ran, S.; Huang, Z.; Liang, J. Bacterial Diversity and Community Structure of Supragingival Plaques in Adults with Dental Health or Caries Revealed by 16S Pyrosequencing. Front. Microbiol. 2016, 7, 1145. [Google Scholar] [CrossRef]
- Yang, F.; Ning, K.; Zeng, X.; Zhou, Q.; Su, X.; Yuan, X. Characterization of saliva microbiota’s functional feature based on metagenomic sequencing. SpringerPlus 2016, 5, 2098. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Yang, F.; Huang, S.; Bo, C.; Xu, Z.Z.; Amir, A.; Knight, R.; Ling, J.; Xu, J. Prediction of Early Childhood Caries via Spatial-Temporal Variations of Oral Microbiota. Cell Host Microbe 2015, 18, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zheng, X.; Wei, Y.; Zhou, X.; Zhang, K.; Wang, S.; Cheng, L.; Li, Y.; Ren, B.; Xu, X.; et al. d-Alanine metabolism is essential for growth and biofilm formation of Streptococcus mutans. Mol. Oral Microbiol. 2016, 31, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Song, I.S.; Baek, K.J.; Choi, Y.; Ji, S. Immunologic characteristics of human gingival fibroblasts in response to oral bacteria. J. Periodontal Res. 2017, 52, 447–457. [Google Scholar] [CrossRef]
- Sarkar, J.; McHardy, I.H.; Simanian, E.J.; Shi, W.; Lux, R. Transcriptional responses of Treponema denticola to other oral bacterial species. PLoS ONE 2014, 9, e88361. [Google Scholar] [CrossRef]
- Poppleton, D.I.; Duchateau, M.; Hourdel, V.; Matondo, M.; Flechsler, J.; Klingl, A.; Beloin, C.; Gribaldo, S. Outer Membrane Proteome of Veillonella parvula: A Diderm Firmicute of the Human Microbiome. Front. Microbiol. 2017, 8, 1215. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Zhou, X.; Cheng, L.; Huo, Y.; Zou, J.; Li, Y. Characterization of the clustered regularly interspaced short palindromic repeats sites in Streptococcus mutans isolated from early childhood caries patients. Arch. Oral Biol. 2017, 83, 174–180. [Google Scholar] [CrossRef]
- Tian, Y.; He, X.; Torralba, M.; Yooseph, S.; Nelson, K.E.; Lux, R.; McLean, J.S.; Yu, G.; Shi, W. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol. Oral Microbiol. 2010, 25, 357–367. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of Antibacterial Dental Adhesive on Multispecies Biofilms Formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Cheng, L.; Jiang, Y.; Melo, M.A.S.; Weir, M.D.; Oates, T.W.; Zhou, X.; Xu, H.H.K. Novel dental composite with capability to suppress cariogenic species and promote non-cariogenic species in oral biofilms. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ren, B.; Zhou, X.; Xu, H.H.K.; Wang, S.; Li, M.; Weir, M.D.; Feng, M.; Cheng, L. Novel Dental Adhesive with Biofilm-Regulating and Remineralization Capabilities. Materials 2017, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Mashima, I.; Theodorea, C.F.; Thaweboon, B.; Thaweboon, S.; Nakazawa, F. Identification of Veillonella species in the tongue biofilm by using a novel one-step polymerase chain reaction method. PLoS ONE 2016, 11, e0157516. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Weir, M.D.; Liu, H.B.; Zhou, X.D.; Xu, H.H.K. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent. Mater. 2013, 29, 462–472. [Google Scholar] [CrossRef]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.W.; Delahunty, C.M.; Yates, J.R.; Heydorn, A.; Koo, H. The Exopolysaccharide Matrix Modulates the Interaction between 3D Architecture and Virulence of a Mixed-Species Oral Biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, L.; Exterkate, R.A.; Liu, M.; Zhou, X.; Li, J.; ten Cate, J.M. Effect of pH on Galla chinensis extract’s stability and anti-caries properties in vitro. Arch. Oral Biol. 2012, 57, 1093–1099. [Google Scholar] [CrossRef]
- Han, Q.; Li, B.; Zhou, X.; Ge, Y.; Wang, S.; Li, M.; Ren, B.; Wang, H.; Zhang, K.; Xu, H.H.K.; et al. Anti-Caries Effects of Dental Adhesives Containing Quaternary Ammonium Methacrylates with Different Chain Lengths. Materials 2017, 10, 643. [Google Scholar] [CrossRef]
- Beighton, D.; Gilbert, S.C.; Clark, D.; Mantzourani, M.; Al-Haboubi, M.; Ali, F.; Ransome, E.; Hodson, N.; Fenlon, M.; Zoitopoulos, L.; et al. Isolation and identification of bifidobacteriaceae from human saliva. Appl. Environ. Microbiol. 2008, 74, 6457–6460. [Google Scholar] [CrossRef]
- Schwendicke, F.; Korte, F.; Dorfer, C.E.; Kneist, S.; Fawzy El-Sayed, K.; Paris, S. Inhibition of Streptococcus mutans Growth and Biofilm Formation by Probiotics in vitro. Caries Res. 2017, 51, 87–95. [Google Scholar] [CrossRef]
- Caglar, E.; Sandalli, N.; Twetman, S.; Kavaloglu, S.; Ergeneli, S.; Selvi, S. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol. Scand. 2005, 63, 317–320. [Google Scholar] [CrossRef]
- Nam, H.J.; Kim, Y.M.; Kwon, Y.H.; Kim, I.R.; Park, B.S.; Son, W.S.; Lee, S.M.; Kim, Y.I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M. In situ induced demineralization in irradiated and non-irradiated human dentin. Eur. J. Oral Sci. 2000, 108, 214–221. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wu, T.M.; Zhou, X.D.; Zhang, B.; Huo, S.B.; Yang, Y.T.; Zhang, K.K.; Cheng, L.; Xu, X.; Li, M.Y. Nicotine is a risk factor for dental caries: An in vivo study. J. Dent. Sci. 2018, 13, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Keyes, P.H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J. Dent. Res. 1958, 37, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S5–S11. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, J.; Chen, H. Pyrosequencing analysis of oral microbiota in children with severe early childhood dental caries. Curr. Microbiol. 2013, 67, 537–542. [Google Scholar] [CrossRef]
- Jiang, W.; Ling, Z.X.; Lin, X.L.; Chen, Y.D.; Zhang, J.; Yu, J.J.; Xiang, C.; Chen, H. Pyrosequencing Analysis of Oral Microbiota Shifting in Various Caries States in Childhood. Microb. Ecol. 2014, 67, 962–969. [Google Scholar] [CrossRef]
- Kanasi, E.; Dewhirst, F.E.; Chalmers, N.I.; Kent, R., Jr.; Moore, A.; Hughes, C.V.; Pradhan, N.; Loo, C.Y.; Tanner, A.C. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010, 44, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Kong, J.; Jia, P.; Wei, C.; Wang, Y.; Pan, Z.; Huang, W.; Li, L.; Chen, H.; Xiang, C. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb. Ecol. 2010, 60, 677–690. [Google Scholar] [CrossRef]
- Tanner, A.C.; Mathney, J.M.; Kent, R.L.; Chalmers, N.I.; Hughes, C.V.; Loo, C.Y.; Pradhan, N.; Kanasi, E.; Hwang, J.; Dahlan, M.A.; et al. Cultivable anaerobic microbiota of severe early childhood caries. J. Clin. Microbiol. 2011, 49, 1464–1474. [Google Scholar] [CrossRef]
- Agnello, M.; Marques, J.; Cen, L.; Mittermuller, B.; Huang, A.; Chaichanasakul Tran, N.; Shi, W.; He, X.; Schroth, R.J. Microbiome Associated with Severe Caries in Canadian First Nations Children. J. Dent. Res. 2017, 96, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Corby, P.M.; Lyons-Weiler, J.; Bretz, W.A.; Hart, T.C.; Aas, J.A.; Boumenna, T.; Goss, J.; Corby, A.L.; Junior, H.M.; Weyant, R.J.; et al. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 2005, 43, 5753–5759. [Google Scholar] [CrossRef]
- Unsal, G.; Topcuoglu, N.; Ulukapi, I.; Kulekci, G.; Aktoren, O. Scardovia Wiggsiae and the Other Microorganisms in Severe Early Childhood Caries. J. Dent. Oral Care Med. 2017, 3, 2454–3276. [Google Scholar]
- Kamalendran, N. Oral Microbiota of Children Undergoing Comprehensive Treatment for Severe Early Childhood Caries. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2015. [Google Scholar]
- Hurley, E.; Barrett, M.P.J.; Kinirons, M.; Whelton, H.; Ryan, C.A.; Stanton, C.; Harris, H.M.B.; O’Toole, P.W. Comparison of the salivary and dentinal microbiome of children with severe-early childhood caries to the salivary microbiome of caries-free children. BMC Oral Health 2019, 19, 13. [Google Scholar] [CrossRef]
- Peterson, S.N.; Snesrud, E.; Liu, J.; Ong, A.C.; Kilian, M.; Schork, N.J.; Bretz, W. The dental plaque microbiome in health and disease. PLoS ONE 2013, 8, e58487. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.H.; Yang, D.Q.; Xin, B.C.; Paster, B.J.; Qin, J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis. 2012, 18, 595–601. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, C.; Huang, I.H.; Merritt, J.; Qi, F. Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology 2011, 157, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E. Development of oral bacterial flora in young children. Ann. Med. 2000, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Haake, S.K.; Yoder, S.C.; Attarian, G.; Podkaminer, K. Native plasmids of Fusobacterium nucleatum: Characterization and use in development of genetic systems. J. Bacteriol. 2000, 182, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O.; Scheie, A.A.; Manji, F. The Effect of Sucrose on Plaque Ph in the Primary and Permanent Dentition of Caries-Inactive and Caries-Active Kenyan Children. J. Dent. Res. 1992, 71, 25–31. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—an update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontol. 2000 2015, 68, 66–82. [Google Scholar] [CrossRef]
| Organism | PCR Primers | Source |
|---|---|---|
| S. mutans | Forward:5′-TTGACGGTGTTCGTGTTGAT-3′ Reverse:5′-AAAGCGATAGGCGCAGTTTA-3′ | This study |
| V. parvula | Forward: 5′-GTAACAAAGGTGTCGTTTCTCG-3′ Reverse: 5′-CGTAACATCTTCCGAAACTTTC-3′ | [23] |
| F. nucleatum | Forward:5′-CAACCATTACTTTAACTCTACCATGTTCA-3′ Reverse: 5′-GTTGACTTTACAGAAGGAGATTATGTAAAAATC-3′ | [24] |
| P. enticola | Forward:5′-GGGGATAAAGTGAGGGACGT-3′ Reverse:5′-GGCGCCTACATTTCACAACA-3′ | This study |
| L. wadei | Forward:5′-AAGCCTGCCCTGGAAACTAT-3′ Reverse:5′-CACTTCAGCGTCAGTTACCG-3′ | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Kong, L.; Peng, X.; Chen, Y.; Ren, B.; Li, M.; Li, J.; Zhou, X.; Cheng, L. Core Microbiota Promotes the Development of Dental Caries. Appl. Sci. 2021, 11, 3638. https://doi.org/10.3390/app11083638
Chen J, Kong L, Peng X, Chen Y, Ren B, Li M, Li J, Zhou X, Cheng L. Core Microbiota Promotes the Development of Dental Caries. Applied Sciences. 2021; 11(8):3638. https://doi.org/10.3390/app11083638
Chicago/Turabian StyleChen, Jing, Lixin Kong, Xian Peng, Yanyan Chen, Biao Ren, Mingyun Li, Jiyao Li, Xuedong Zhou, and Lei Cheng. 2021. "Core Microbiota Promotes the Development of Dental Caries" Applied Sciences 11, no. 8: 3638. https://doi.org/10.3390/app11083638
APA StyleChen, J., Kong, L., Peng, X., Chen, Y., Ren, B., Li, M., Li, J., Zhou, X., & Cheng, L. (2021). Core Microbiota Promotes the Development of Dental Caries. Applied Sciences, 11(8), 3638. https://doi.org/10.3390/app11083638