Oral Microbiome and Host Health: Review on Current Advances in Genome-Wide Analysis
Abstract
1. Introduction
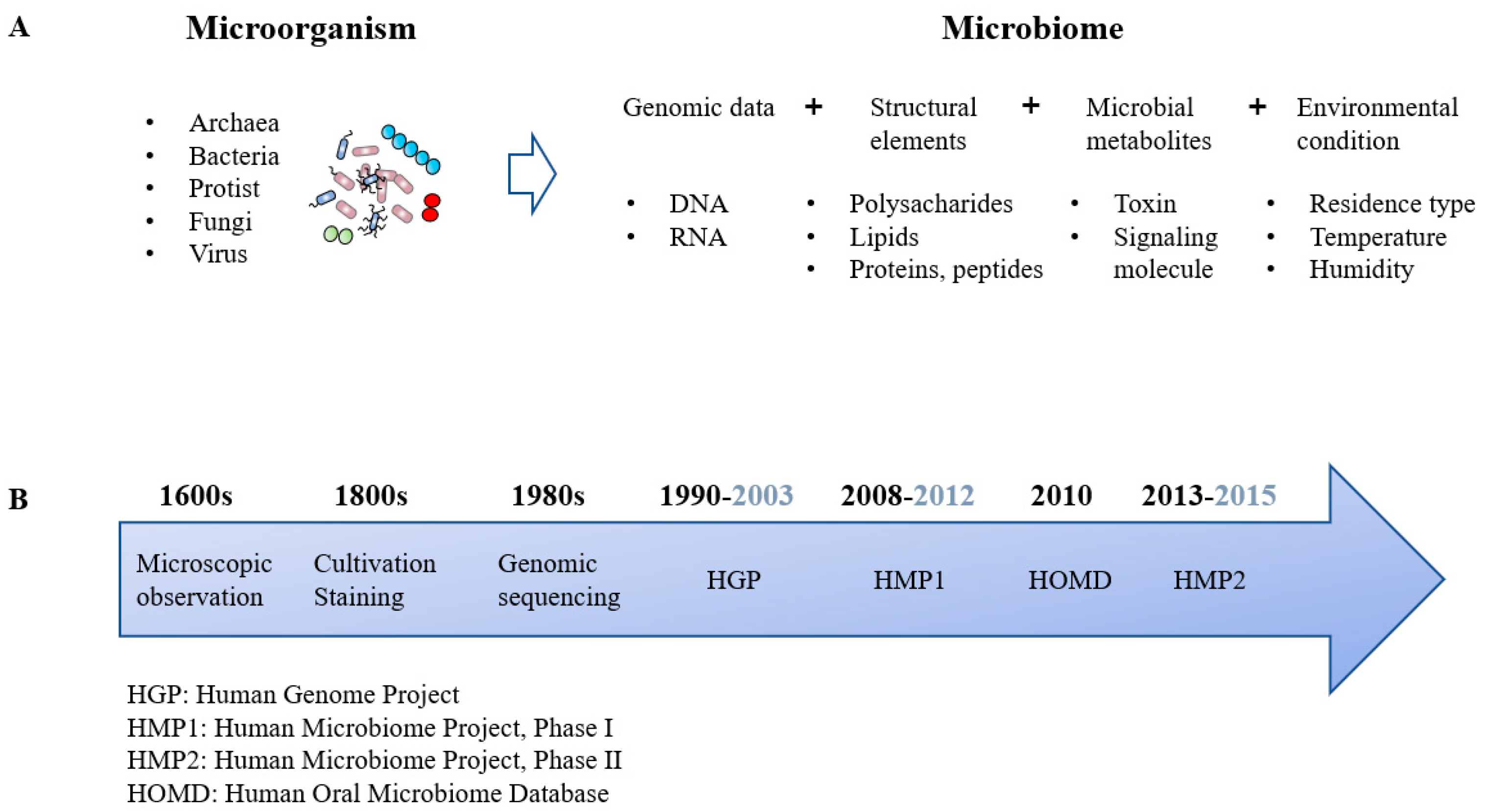
2. Paradigm Shift: Microorganisms to Microbiomes
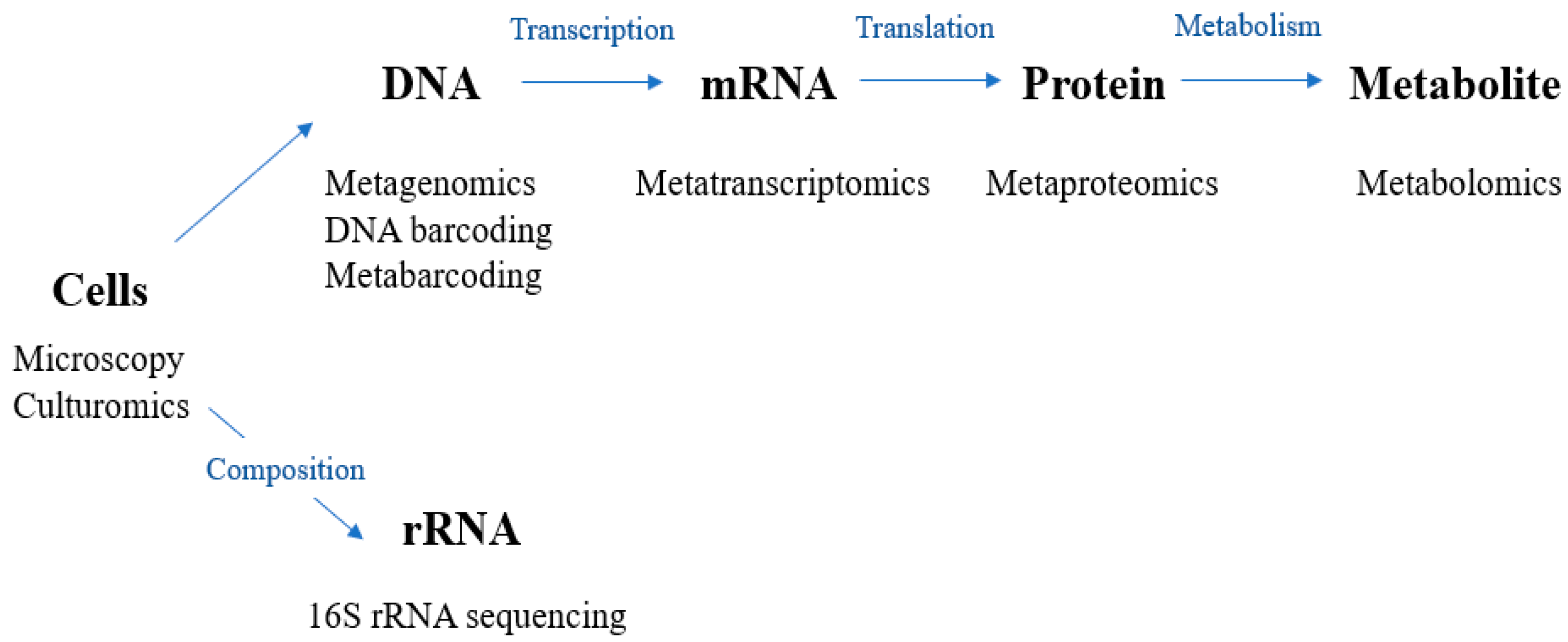
3. Methodology of the Microbiome Research
4. Core, Pathologic, and Healthy Microbiomes
5. Human Microbiome Project
6. Oral Microbiome
Human Oral Microbiome Database (HOMD)
7. Host–Oral Microbiome Interactions in Health
8. Potential Clinical Application of Oral Microbiomes
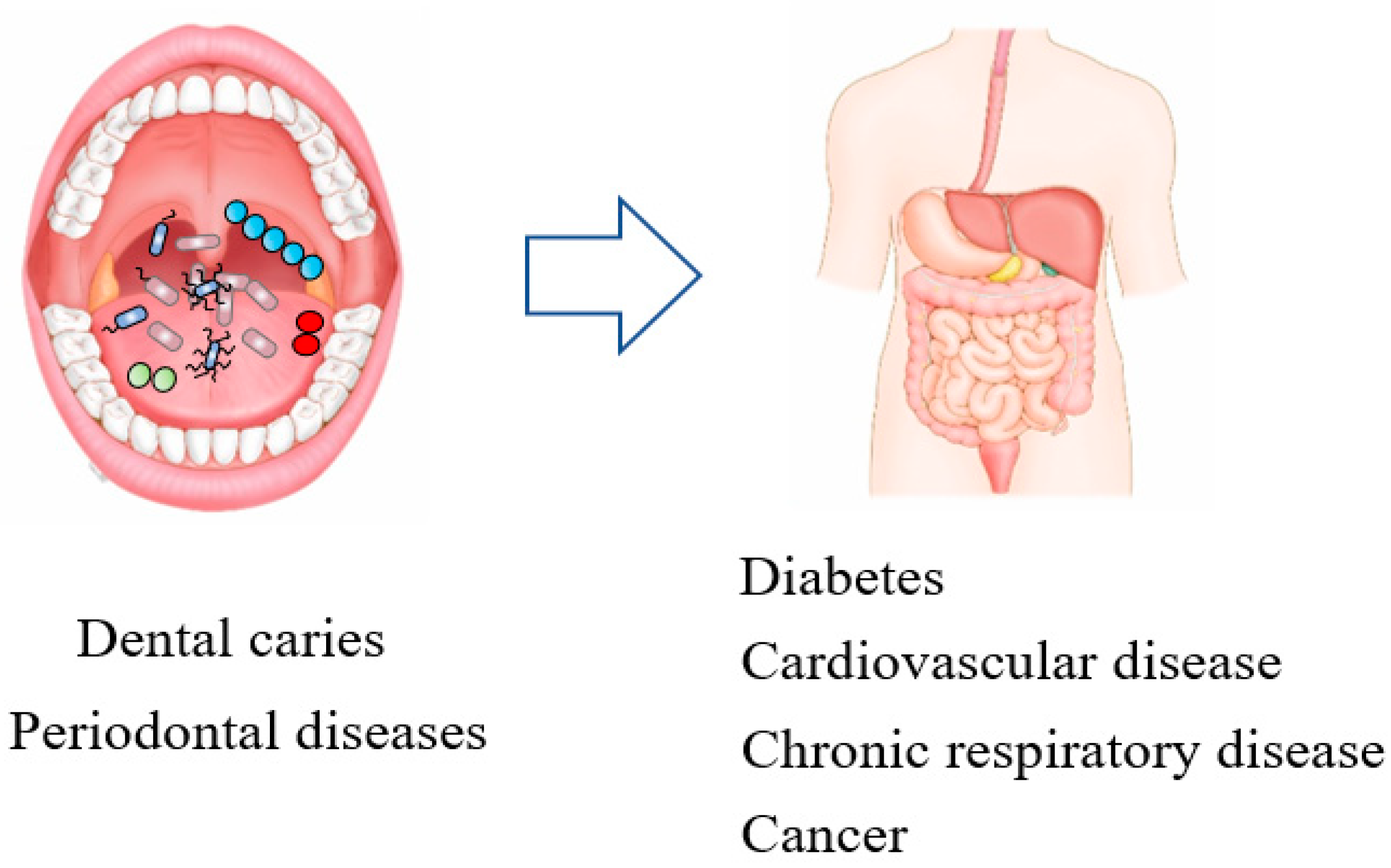
9. Oral Disease and Systemic Disease
9.1. Non-Communicable Diseases
9.1.1. Cardiovascular Disease
9.1.2. Diabetes Mellitus
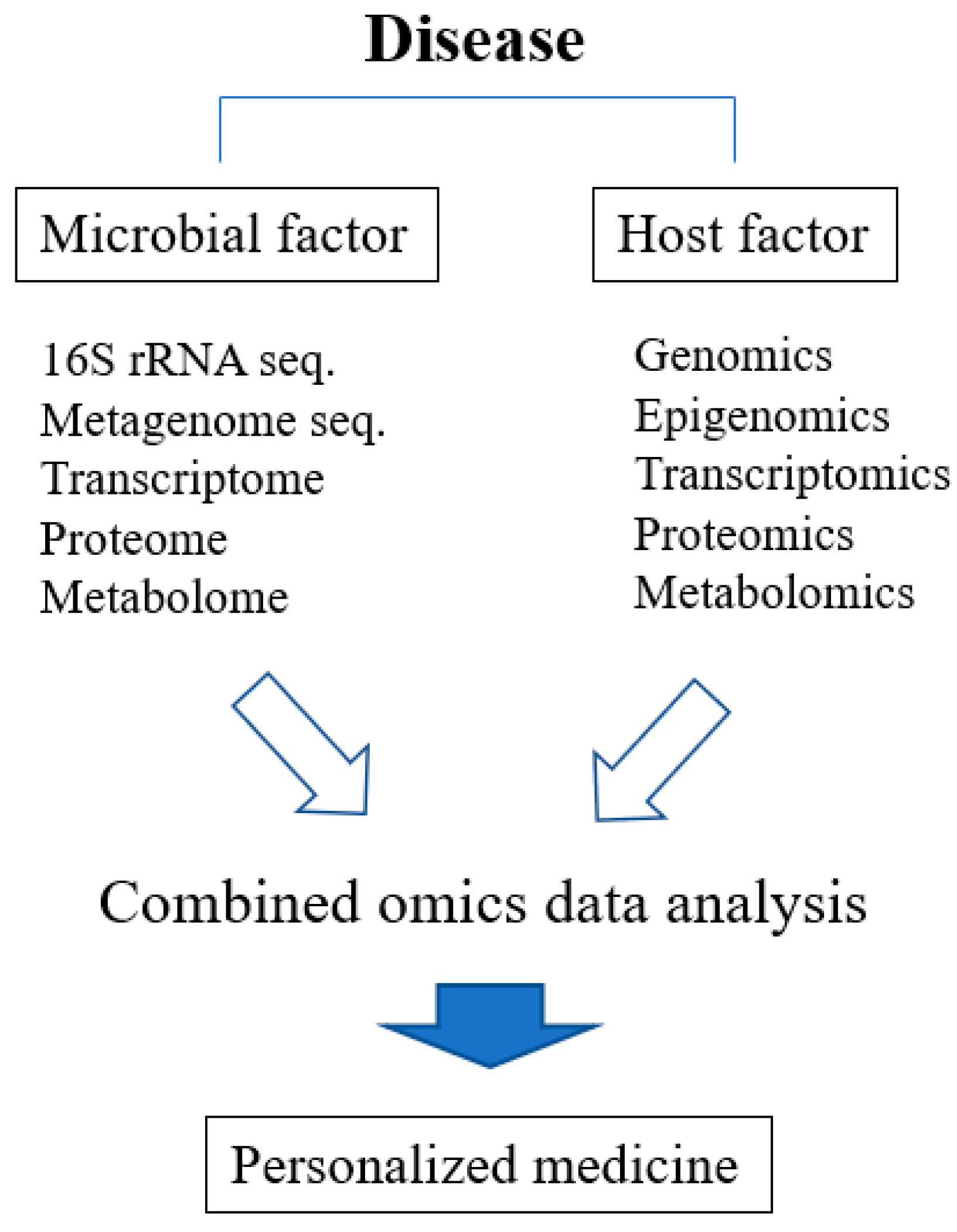
10. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priya Nimish Deo, R.D. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Xuan, S.Y.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Well. 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.S.; Chu, M.; Huang, Z.W.; Yang, X.; Ran, S.J.; Hu, B.; Zhang, C.P.; Liang, J.P. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Bauer, M.E.; Marsh, P. Advancements toward a systems level understanding of the human oral microbiome. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Pulido, V.; Huang, W.; Suarez-Ulloa, V.; Cickovski, T.; Mathee, K.; Narasimhan, G. Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis. Evol. Bioinform. Online 2016, 12, 5–16. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd Allah, E.F. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front. Immunol. 2018, 9, 2868. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. The unseen world: Reflections on Leeuwenhoek (1677) ‘Concerning little animals’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Rajfer, J. Antonie van Leeuwenhoek (1632–1723). Invest. Urol. 1976, 14, 83. [Google Scholar] [PubMed]
- Hiergeist, A.; Glasner, J.; Reischl, U.; Gessner, A. Analyses of Intestinal Microbiota: Culture versus Sequencing. ILAR J. 2015, 56, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.W.Y.; Devkota, S. The new microbiology: Cultivating the future of microbiome-directed medicine. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G639–G645. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Ramiro, F.; Peiro-Pastor, R.; Aguado, B. Human genomics projects and precision medicine. Gene Ther. 2017, 24, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Zaura, E. Next-generation sequencing approaches to understanding the oral microbiome. Adv. Dent. Res. 2012, 24, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.J.; Zhou, D.; Caporaso, J.G.; Knight, R.; Angenent, L.T. Comparison of Illumina paired-end and single-direction sequencing for microbial 16S rRNA gene amplicon surveys. ISME J. 2012, 6, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, P.; Goebel, B.M.; Pace, N.R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 1998, 180, 4765–4774. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17. [Google Scholar] [CrossRef]
- Simon, C.; Daniel, R. Metagenomic analyses: Past and future trends. Appl. Environ. Microbiol. 2011, 77, 1153–1161. [Google Scholar] [CrossRef]
- Hettich, R.L.; Pan, C.; Chourey, K.; Giannone, R.J. Metaproteomics: Harnessing the power of high performance mass spectrometry to identify the suite of proteins that control metabolic activities in microbial communities. Anal. Chem. 2013, 85, 4203–4214. [Google Scholar] [CrossRef]
- Bostanci, N.; Bao, K. Contribution of proteomics to our understanding of periodontal inflammation. Proteomics 2017, 17. [Google Scholar] [CrossRef]
- Bostanci, N.; Grant, M.; Bao, K.; Silbereisen, A.; Hetrodt, F.; Manoil, D.; Belibasakis, G.N. Metaproteome and metabolome of oral microbial communities. Periodontol. 2000 2021, 85, 46–81. [Google Scholar] [CrossRef] [PubMed]
- Risely, A. Applying the core microbiome to understand host-microbe systems. J. Anim. Ecol. 2020, 89, 1549–1558. [Google Scholar] [CrossRef]
- Hamady, M.; Knight, R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009, 19, 1141–1152. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Lee, S.M.; Shen, Y.; Khosravi, A.; Mazmanian, S.K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 2010, 107, 243–274. [Google Scholar] [CrossRef] [PubMed]
- Keijser, B.J.; Zaura, E.; Huse, S.M.; van der Vossen, J.M.; Schuren, F.H.; Montijn, R.C.; ten Cate, J.M.; Crielaard, W. Pyrosequencing analysis of the oral microflora of healthy adults. J. Dent. Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Li, X.; Poveda, L.; Qi, W.; Selevsek, N.; Gumus, P.; Emingil, G.; Grossmann, J.; Diaz, P.I.; Hajishengallis, G.; et al. Proteome and Microbiome Mapping of Human Gingival Tissue in Health and Disease. Front. Cell. Infect. Microbiol. 2020, 10, 588155. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. A framework for human microbiome research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Jumpstart Reference Strains Consortium; Nelson, K.E.; Weinstock, G.M.; Highlander, S.K.; Worley, K.C.; Creasy, H.H.; Wortman, J.R.; Rusch, D.B.; Mitreva, M.; Sodergren, E.; et al. A catalog of reference genomes from the human microbiome. Science 2010, 328, 994–999. [Google Scholar] [CrossRef]
- Wylie, K.M.; Truty, R.M.; Sharpton, T.J.; Mihindukulasuriya, K.A.; Zhou, Y.; Gao, H.; Sodergren, E.; Weinstock, G.M.; Pollard, K.S. Novel bacterial taxa in the human microbiome. PLoS ONE 2012, 7, e35294. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Petrosino, J.; Keitel, W.; Watson, M.; Katancik, J.; Garcia, N.; Patel, S.; Cutting, M.; Madden, T.; Hamilton, H.; et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013, 27, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.V.; Gevers, D.; Giannoukos, G.; Earl, A.M.; Methe, B.A.; Sodergren, E.; Feldgarden, M.; Ciulla, D.M.; Tabbaa, D.; Arze, C.; et al. Evaluation of 16S rDNA-Based Community Profiling for Human Microbiome Research. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Gevers, D.; Pop, M.; Schloss, P.D.; Huttenhower, C. Bioinformatics for the Human Microbiome Project. PLoS Comp. Biol. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, V.M.; Chen, I.M.A.; Chu, K.; Szeto, E.; Palaniappan, K.; Jacob, B.; Ratner, A.; Liolios, K.; Pagani, I.; Huntemann, M.; et al. IMG/M-HMP: A Metagenome Comparative Analysis System for the Human Microbiome Project. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Integrative, H.M.P. The Integrative Human Microbiome Project: Dynamic Analysis of Microbiome-Host Omics Profiles during Periods of Human Health and Disease. Cell Host Microbe 2014, 16, 276–289. [Google Scholar] [CrossRef]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.Y.; Buck, G.A.; Snyder, M.P.; Strauss, J.F.; Weinstock, G.M.; et al. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Huang, K.; Meadow, J.F.; Gevers, D.; Lemon, K.P.; Bohannan, B.J.M.; Huttenhower, C. Identifying personal microbiomes using metagenomic codes. Proc. Natl. Acad. Sci. USA 2015, 112, E2930–E2938. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, W.; Veerman, E.C.; Nieuw Amerongen, A.V.; Ligtenberg, A.J. Antimicrobial defense systems in saliva. Monogr. Oral Sci. 2014, 24, 40–51. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Oilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Holgerson, P.L.; Harnevik, L.; Hernell, O.; Tanner, A.C.R.; Johansson, I. Mode of Birth Delivery Affects Oral Microbiota in Infants. J. Dent. Res. 2011, 90, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Holgerson, P.L.; Vestman, N.R.; Claesson, R.; Ohman, C.; Domellof, M.; Tanner, A.C.R.; Hernell, O.; Johansson, I. Oral Microbial Profile Discriminates Breast-fed From Formula-fed Infants. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 127–136. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent. Res. J. (Isfahan) 2014, 11, 291–301. [Google Scholar]
- Xu, X.; He, J.Z.; Xue, J.; Wang, Y.; Li, K.; Zhang, K.K.; Guo, Q.; Liu, X.H.; Zhou, Y.; Cheng, L.; et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ. Microbiol. 2015, 17, 699–710. [Google Scholar] [CrossRef]
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, W.H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010, 2010, baq013. [Google Scholar] [CrossRef] [PubMed]
- Cross, K.L.; Chirania, P.; Xiong, W.L.; Beall, C.J.; Elkins, J.G.; Giannone, R.J.; Griffen, A.L.; Guss, A.M.; Hettich, R.L.; Joshi, S.S.; et al. Insights into the Evolution of Host Association through the Isolation and Characterization of a Novel Human Periodontal Pathobiont, Desulfobulbus oralis. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, P.C.; van der Veen, M.H.; Krom, B.P. Review: Modulation of the oral microbiome by the host to promote ecological balance. Odontology 2019, 107, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.P.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Perez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Devine, D.A. How is the development of dental biofilms influenced by the host? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 28–35. [Google Scholar] [CrossRef]
- Chimenos-Kustner, E.; Giovannoni, M.L.; Schemel-Suarez, M. Dysbiosis as a determinant factor of systemic and oral pathology: Importance of microbiome. Med. Clin. (Barc.) 2017, 149, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Lips, A.; Antunes, L.S.; Antunes, L.A.; Abreu, J.G.B.; Barreiros, D.; Oliveira, D.S.B.; Batista, A.C.; Nelson-Filho, P.; Silva, L.; Silva, R.; et al. Genetic Polymorphisms in DEFB1 and miRNA202 Are Involved in Salivary Human beta-Defensin 1 Levels and Caries Experience in Children. Caries Res. 2017, 51, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, F.; Koruyucu, M.; Kuchler, E.C.; Bayram, M.; Tuna, E.B.; Deeley, K.; Pierri, R.A.; Souza, J.F.; Fragelli, C.M.; Paschoal, M.A.; et al. Genes expressed in dental enamel development are associated with molar-incisor hypomineralization. Arch. Oral Biol. 2013, 58, 1434–1442. [Google Scholar] [CrossRef]
- Kulkarni, G.V.; Chng, T.; Eny, K.M.; Nielsen, D.; Wessman, C.; El-Sohemy, A. Association of GLUT2 and TAS1R2 genotypes with risk for dental caries. Caries Res. 2013, 47, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Espinoza, J.L.; Harkins, D.M.; Leong, P.; Saffery, R.; Bockmann, M.; Torralba, M.; Kuelbs, C.; Kodukula, R.; Inman, J.; et al. Host Genetic Control of the Oral Microbiome in Health and Disease. Cell Host Microbe 2017, 22, 269–278. [Google Scholar] [CrossRef]
- Demmitt, B.A.; Corley, R.P.; Huibregtse, B.M.; Keller, M.C.; Hewitt, J.K.; McQueen, M.B.; Knight, R.; McDermott, I.; Krauter, K.S. Genetic influences on the human oral microbiome. BMC Genomics 2017, 18, 659. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Konkel, J.E. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol. 2018, 39, 276–287. [Google Scholar] [CrossRef]
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar] [CrossRef]
- Meyle, J.; Dommisch, H.; Groeger, S.; Giacaman, R.A.; Costalonga, M.; Herzberg, M. The innate host response in caries and periodontitis. J. Clin. Periodontol. 2017, 44, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Secretory immunity with special reference to the oral cavity. J. Oral Microbiol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- De Castro, D.T.; do Nascimento, C.; Alves, O.L.; Santos, E.D.; Agnelli, J.A.M.; dos Reis, A.C. Analysis of the oral microbiome on the surface of modified dental polymers. Arch. Oral Biol. 2018, 93, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.; Pedersen, O. Impact of a vegan diet on the human salivary microbiota. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Matthews, C.; Aspiras, M.; de Jager, M.; Ward, M.; Kumar, P. Smoking decreases structural and functional resilience in the subgingival ecosystem. J. Clin. Periodontol. 2014, 41, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Al-Zyoud, W.; Hajjo, R.; Abu-Siniyeh, A.; Hajjaj, S. Salivary Microbiome and Cigarette Smoking: A First of Its Kind Investigation in Jordan. Int. J. Environ. Res. Public Health 2020, 17, 256. [Google Scholar] [CrossRef]
- Grine, G.; Royer, A.; Terrer, E.; Diallo, O.O.; Drancourt, M.; Aboudharam, G. Tobacco Smoking Affects the Salivary Gram-Positive Bacterial Population. Front. Public Health 2019, 7, 196. [Google Scholar] [CrossRef]
- O’Grady, I.; Anderson, A.; O’Sullivan, J. The interplay of the oral microbiome and alcohol consumption in oral squamous cell carcinomas. Oral Oncol. 2020, 110. [Google Scholar] [CrossRef] [PubMed]
- Snider, J. Alcohol Consumption, Particularly among Heavy Drinkers, May Affect Oral Microbiome Composition, Researchers Find. J. Am. Dent. Assoc. 2018, 149, 574–575. [Google Scholar]
- Fan, X.Z.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.Y.; Pei, Z.H.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tadakamadla, J.; Johnson, N.W. Effect of Toothbrushing Frequency on Incidence and Increment of Dental Caries: A Systematic Review and Meta-Analysis. J. Dent. Res. 2016, 95, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Crawford, N.D. Socioeconomic position indicators and periodontitis: Examining the evidence. Periodontol. 2000 2012, 58, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Boyce, W.T.; Den Besten, P.K.; Stamperdahl, J.; Zhan, L.; Jiang, Y.; Adler, N.E.; Featherstone, J.D. Social inequalities in childhood dental caries: The convergent roles of stress, bacteria and disadvantage. Soc. Sci. Med. 2010, 71, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Belstrom, D.; Holmstrup, P.; Nielsen, C.H.; Kirkby, N.; Twetman, S.; Heitmann, B.L.; Klepac-Ceraj, V.; Paster, B.J.; Fiehn, N.E. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Inchingolo, A.D.; Xhajanka, E.; Altini, V.; Bordea, I.R.; Dipalma, G.; Inchingolo, F. Management of patients suffering from xerostomia with a combined mouthrinse containing sea salt, xylitol and lysozyme. J. Biol. Regul. Homeost. Agents 2020, 34, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Chiniforush, N.; Pourhajibagher, M.; Parker, S.; Benedicenti, S.; Bahador, A.; Salagean, T.; Bordea, I.R. The Effect of Antimicrobial Photodynamic Therapy Using Chlorophyllin-Phycocyanin Mixture onEnterococcus faecalis: The Influence of Different Light Sources. Appl. Sci. 2020, 10, 4290. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the oral microbiome in personalized dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef]
- Kaman, W.E.; Galassi, F.; de Soet, J.J.; Bizzarro, S.; Loos, B.G.; Veerman, E.C.; van Belkum, A.; Hays, J.P.; Bikker, F.J. Highly specific protease-based approach for detection of porphyromonas gingivalis in diagnosis of periodontitis. J. Clin. Microbiol. 2012, 50, 104–112. [Google Scholar] [CrossRef]
- Matsha, T.E.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Offenbacher, S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J. Periodontol. 2005, 76, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Passos, J.S.; Seixas da Cruz, S. Respiratory disease and the role of oral bacteria. J. Oral Microbiol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.F.; Alawi, F.; Castilho, R.M.; Wang, Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020, 99, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, C.; Benz, C.; Aida, J.; Campard, G. The relationship of oral health with general health and NCDs: A brief review. Int. Dent. J. 2017, 67 (Suppl. S2), 14–18. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontol. 2000 2016, 72, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Kim, W.J.; Ryoo, H.M.; Kim, H.G.; Kim, K.H.; Ku, Y.; Seol, Y.J. Current advances of epigenetics in periodontology from ENCODE project: A review and future perspectives. Clin. Epigenetics 2021, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Jan, S.; Laba, T.L.; Essue, B.M.; Gheorghe, A.; Muhunthan, J.; Engelgau, M.; Mahal, A.; Griffiths, U.; McIntyre, D.; Meng, Q.; et al. Action to address the household economic burden of non-communicable diseases. Lancet 2018, 391, 2047–2058. [Google Scholar] [CrossRef]
- Barquera, S.; Pedroza-Tobias, A.; Medina, C.; Hernandez-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S. Matrix metalloproteinases and cardiovascular disease. Eur. Heart J. 2006, 27, 121–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vita, J.A.; Loscalzo, J. Shouldering the risk factor burden: Infection, atherosclerosis, and the vascular endothelium. Circulation 2002, 106, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Spahr, A.; Klein, E.; Khuseyinova, N.; Boeckh, C.; Muche, R.; Kunze, M.; Rothenbacher, D.; Pezeshki, G.; Hoffmeister, A.; Koenig, W. Periodontal infections and coronary heart disease: Role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch. Intern. Med. 2006, 166, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Kozarov, E.V.; Dorn, B.R.; Shelburne, C.E.; Dunn, W.A., Jr.; Progulske-Fox, A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Cavrini, F.; Sambri, V.; Moter, A.; Servidio, D.; Marangoni, A.; Montebugnoli, L.; Foschi, F.; Prati, C.; Di Bartolomeo, R.; Cevenini, R. Molecular detection of Treponema denticola and Porphyromonas gingivalis in carotid and aortic atheromatous plaques by FISH: Report of two cases. J. Med. Microbiol. 2005, 54, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Elkaim, R.; Dahan, M.; Kocgozlu, L.; Werner, S.; Kanter, D.; Kretz, J.G.; Tenenbaum, H. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: A preliminary study. J. Periodontal Res. 2008, 43, 224–231. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.; Marcelino, S.L.; Feitosa, A.C.R.; Romito, G.A.; Avila-Campos, M.J. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J. Med. Microbiol. 2009, 58, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Yumoto, H.; Davey, M.; Takahashi, Y.; Miyamoto, T.; Gibson, F.C., 3rd; Genco, C.A. Porphyromonas gingivalis fimbria-dependent activation of inflammatory genes in human aortic endothelial cells. Infect. Immun. 2005, 73, 5367–5378. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Fujii, R.; Nakagawa, K.I.; Kuramitsu, H.K.; Okuda, K.; Ishihara, K. Stimulation of Fusobacterium nucleatum biofilm formation by Porphyromonas gingivalis. Oral Microbiol. Immunol. 2008, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kurita-Ochiai, T.; Hashizume, T.; Du, Y.; Oguchi, S.; Yamamoto, M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol. Med. Microbiol. 2010, 59, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Khlgatian, M.; Nassar, H.; Chou, H.H.; Gibson, F.C., 3rd; Genco, C.A. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect. Immun. 2002, 70, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Davey, M.; Yumoto, H.; Gibson, F.C., 3rd; Genco, C.A. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell. Microbiol. 2006, 8, 738–757. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, T.; Karlsson, H.; Gunnarsson, P.; Skoglund, C.; Elison, C.; Leanderson, P.; Lindahl, M. The periodontal pathogen Porphyromonas gingivalis cleaves apoB-100 and increases the expression of apoM in LDL in whole blood leading to cell proliferation. J. Intern. Med. 2008, 263, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, A.M.; Jauhiainen, M.; Kovanen, P.T.; Metso, J.; Paju, S.; Pussinen, P.J. Aggregatibacter actinomycetemcomitans induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice. Microb. Pathog. 2008, 44, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, H.; Chou, H.H.; Takahashi, Y.; Davey, M.; Gibson, F.C., 3rd; Genco, C.A. Sensitization of human aortic endothelial cells to lipopolysaccharide via regulation of Toll-like receptor 4 by bacterial fimbria-dependent invasion. Infect. Immun. 2005, 73, 8050–8059. [Google Scholar] [CrossRef]
- Nakamura, N.; Yoshida, M.; Umeda, M.; Huang, Y.; Kitajima, S.; Inoue, Y.; Ishikawa, I.; Iwai, T. Extended exposure of lipopolysaccharide fraction from Porphyromonas gingivalis facilitates mononuclear cell adhesion to vascular endothelium via Toll-like receptor-2 dependent mechanism. Atherosclerosis 2008, 196, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gibson, F.C., 3rd; Hong, C.; Chou, H.H.; Yumoto, H.; Chen, J.; Lien, E.; Wong, J.; Genco, C.A. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004, 109, 2801–2806. [Google Scholar] [CrossRef]
- Brodala, N.; Merricks, E.P.; Bellinger, D.A.; Damrongsri, D.; Offenbacher, S.; Beck, J.; Madianos, P.; Sotres, D.; Chang, Y.L.; Koch, G.; et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1446–1451. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Hofmann, M.A.; Bucciarelli, L.; Jerud, A.P.; Tucker, S.; Lu, Y.; Papapanou, P.N.; Schmidt, A.M. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1405–1411. [Google Scholar] [CrossRef]
- Pizzo, G.; Guiglia, R.; Lo Russo, L.; Campisi, G. Dentistry and internal medicine: From the focal infection theory to the periodontal medicine concept. Eur. J. Intern. Med. 2010, 21, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Lalla, E.; Cheng, B.; Lal, S.; Kaplan, S.; Softness, B.; Greenberg, E.; Goland, R.S.; Lamster, I.B. Diabetes mellitus promotes periodontal destruction in children. J. Clin. Periodontol. 2007, 34, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann. Periodontol. 2001, 6, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Negrato, C.A.; Tarzia, O.; Jovanovic, L.; Chinellato, L.E.M. Periodontal disease and diabetes mellitus. J. Oral Microbiol. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S113–S134. [Google Scholar] [CrossRef]
- Mealey, B.L.; Oates, T.W.; American Academy of, P. Diabetes mellitus and periodontal diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef]
- Signorini, L.; Inchingolo, A.D.; Santacroce, L.; Xhajanka, E.; Altini, V.; Bordea, I.R.; Dipalma, G.; Cantore, S.; Inchingolo, F. Efficacy of combined sea salt based oral rinse with xylitol in improving healing process and oral hygiene among diabetic population after oral surgery. J. Biol. Regul. Homeost. Agents 2020, 34, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Cai, Q.; Steinwandel, M.; Hargreaves, M.K.; Bordenstein, S.R.; Blot, W.J.; Zheng, W.; Shu, X.O. Association of oral microbiome with type 2 diabetes risk. J. Periodontal Res. 2017, 52, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Honda-Ogawa, M.; Ikebe, K.; Notomi, Y.; Iwamoto, Y.; Shirobayashi, I.; Hata, S.; Kibi, M.; Masayasu, S.; Sasaki, S.; et al. Characterizations of oral microbiota in elderly nursing home residents with diabetes. J. Oral Sci. 2017, 59, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Saeb, A.T.M.; Al-Rubeaan, K.A.; Aldosary, K.; Raja, G.K.U.; Mani, B.; Abouelhoda, M.; Tayeb, H.T. Relative reduction of biological and phylogenetic diversity of the oral microbiota of diabetes and pre-diabetes patients. Microb. Pathog. 2019, 128, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Taylor, J.J.; Jaedicke, K.M.; De Jager, M.; Bikker, J.W.; Selten, W.; Bissett, S.M.; Whall, K.M.; van de Merwe, R.; Areibi, A.; et al. Treatment of periodontitis reduces systemic inflammation in type 2 diabetes. J. Clin. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. Revista FOB 2020, 28, e20190248. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.M. Does Treatment of Periodontal Disease Improve Glycemic Control in Diabetes? Am. J. Nurs. 2012, 112, 22. [Google Scholar] [CrossRef]
- Janket, S.J.; Wightman, A.; Baird, A.E.; Van Dyke, T.E.; Jones, J.A. Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J. Dent. Res. 2005, 84, 1154–1159. [Google Scholar] [CrossRef]
- Sastrowijoto, S.H.; Hillemans, P.; van Steenbergen, T.J.; Abraham-Inpijn, L.; de Graaff, J. Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J. Clin. Periodontol. 1989, 16, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tervonen, T.; Oliver, R.C.; Wolff, L.F.; Bereuter, J.; Anderson, L.; Aeppli, D.M. Prevalence of periodontal pathogens with varying metabolic control of diabetes mellitus. J. Clin. Periodontol. 1994, 21, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.C.; Polson, A.M.; Kang, T. Associations between periodontal diseases and systemic diseases: A review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health 2008, 122, 417–433. [Google Scholar] [CrossRef]
- Makiura, N.; Ojima, M.; Kou, Y.; Furuta, N.; Okahashi, N.; Shizukuishi, S.; Amano, A. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol. Immunol. 2008, 23, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Ojima, M.; Takeda, M.; Yoshioka, H.; Nomura, M.; Tanaka, N.; Kato, T.; Shizukuishi, S.; Amano, A. Relationship of periodontal bacterium genotypic variations with periodontitis in type 2 diabetic patients. Diabetes Care 2005, 28, 433–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, J.; Liu, C.; Zheng, X.; Jia, X.; Peng, X.; Yang, R.; Zhou, X.; Xu, X. Porphyromonas gingivalis Induces Insulin Resistance by Increasing BCAA Levels in Mice. J. Dent. Res. 2020, 99, 839–846. [Google Scholar] [CrossRef]
- Aemaimanan, P.; Amimanan, P.; Taweechaisupapong, S. Quantification of key periodontal pathogens in insulin-dependent type 2 diabetic and non-diabetic patients with generalized chronic periodontitis. Anaerobe 2013, 22, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef]
- Castrillon, C.A.; Hincapie, J.P.; Yepes, F.L.; Roldan, N.; Moreno, S.M.; Contreras, A.; Botero, J.E. Occurrence of red complex microorganisms and Aggregatibacter actinomycetemcomitans in patients with diabetes. J. Investig. Clin. Dent. 2015, 6, 25–31. [Google Scholar] [CrossRef] [PubMed]
| Factor | Reference |
|---|---|
| Genetics |
|
| Immunity | |
| Attachment surface | |
| Diet | |
| Cigarette smoking | |
| Alcohol |
|
| Oral hygiene |
|
| Socioeconomic status |
|
| Mechanism | Organism | Result | Reference |
|---|---|---|---|
| Endothelial cell invasion | P. gingivalis (P.g.) strain 381 | Infection of human aortic endothelial cells with invasive P. g. strain 381 resulted in the upregulation of 68 genes that code for the pro-inflammatory cytokines, adhesion molecules, and chemokines. In addition, P. g. induces procoagulant effects including enhanced tissue factor expression and activity, and suppression of tissue factor pathway inhibitors | [109] |
| F.nucleatum (F.n.) | Co-infection with F. n. resulted in a 2–20-fold increase in the invasion of endothelial cells by P. g. strains | [110] | |
| Endothelial cell activation | A. actinomycenemcomitans (A.a.) | A.a. infection in apoliopoprotein E-deficient mice increased expressions of ICAM-1, E-selectin, P-selectin, MCP-1, chemokine (C-C motif) ligand 19 (CCL19), CCL21, and CCR7 in the aorta | [111] |
| P.g. | Coculture of endothelial cells with P.g. increased ICAM-1, VCAM-1 and P-and E-selectins | [112,113] | |
| Oxidative stress-mediated mechanism | P.g. | P.g. cleaves apoB-100 and increases the expression of apoM in LDL in whole blood | [114] |
| Metalloproteinase-mediated mechanism | A.a. | A.a. induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice | [115] |
| Toll-like receptors-mediated mechanism | A.a. | A.a. infection of apolipoprotein E-deficient mice resulted in increased expression in the aorta of TLR2 and TLR4 | [111] |
| P.g. | P.g. stimulates the expression of TLR2 and TLR 4 on the surface of endothelial cells. Endothelial cells incubated with P. g. LPS expressed ICAM-1 and VCAM-1 and antibodies against TLR2 | [116,117] | |
| Acceleration of the progress of atherosclerosis | P.g. | P.g. accelerates the atherosclerosis in apolipoprotein E-null mice | [118,119,120] |
| Organism | Result | Reference |
|---|---|---|
| P.g. | P.g. triggers periodontal tissue destruction and increases insulin resistance | [140,141] |
| P.g. with type II fimbriae is a critical infectious factor in the deterioration of periodontitis with DM | [142] | |
| P.g. is involved with insulin resistance in DM | [143,144,145] | |
| Actinobacteria | Higher abundances of taxa in the phylum Actinobacteria were associated with lower diabetes risk | [131] |
| A.a. | A.a. was associated with periodontitis in DM patients | [146] |
| P.g., T. forsythia, T. denticola | Poor glycemic control is associated with increased proportions of red-complex microbes | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Oral Microbiome and Host Health: Review on Current Advances in Genome-Wide Analysis. Appl. Sci. 2021, 11, 4050. https://doi.org/10.3390/app11094050
Cho Y-D, Kim K-H, Lee Y-M, Ku Y, Seol Y-J. Oral Microbiome and Host Health: Review on Current Advances in Genome-Wide Analysis. Applied Sciences. 2021; 11(9):4050. https://doi.org/10.3390/app11094050
Chicago/Turabian StyleCho, Young-Dan, Kyoung-Hwa Kim, Yong-Moo Lee, Young Ku, and Yang-Jo Seol. 2021. "Oral Microbiome and Host Health: Review on Current Advances in Genome-Wide Analysis" Applied Sciences 11, no. 9: 4050. https://doi.org/10.3390/app11094050
APA StyleCho, Y.-D., Kim, K.-H., Lee, Y.-M., Ku, Y., & Seol, Y.-J. (2021). Oral Microbiome and Host Health: Review on Current Advances in Genome-Wide Analysis. Applied Sciences, 11(9), 4050. https://doi.org/10.3390/app11094050






