Abstract
Damaged dental pulp undergoes oxidative stress and 4-hexylresorcinol (4HR) is a well-known antioxidant. In this study, we aimed to evaluate the therapeutic effects of a 4HR ointment on damaged dental pulp. Pulp cells from rat mandibular incisor were cultured and treated with 4HR or resveratrol (1–100 μM). These treatments (10–100 μM) exerted a protective effect during subsequent hydrogen peroxide treatments. The total antioxidant capacity and glutathione peroxidase activity were significantly increased following 4HR or resveratrol treatment (p < 0.05), while the expression levels of TNF-α and IL1β were decreased following the exposure to 4HR pre-treatment in an in vitro model. Additionally, the application of 4HR ointment in an exposed dental pulp model significantly reduced the expression of TNF-α and IL1β (p < 0.05). Conclusively, 4HR exerted protective effects against oxidative stress in dental pulp tissues through downregulating TNF-α and IL1β.
1. Introduction
Dental pulp tissue acts as a frontline marker of dental stress. The teeth undergo many different types of stress during dental treatment and there are many examples of the causal stressors, including the removal of hard tissue during operative dentistry or tooth movement during orthodontic treatment [1]. Like other dental treatments, orthodontic treatment-induced oxidative stress has been shown to increase the expression levels of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL1β) [2]. Oxidative stress may result in tissue regeneration or necrosis, depending on its duration and intensity [3]. Therefore, excessive oxidative stress should be alleviated through the administration of antioxidants, which may help in the healing of dental pulp tissue.
Reactive oxygen species (ROS) are produced as part of the inflammatory response and, in turn, cells produce antioxidants to neutralize their harmful effects [4]. Failure to regulate oxidative stress may lead to various pathophysiological conditions [5]. In case of dental pulp, ROS stress may damage a variety of organic molecules, including proteins and DNA [6]. In diabetic condition, elevated blood sugar levels lead to an increase in ROS production and promote the development of dental diseases [7]. The endoplasmic reticulum and mitochondria produce several enzymes, such as glutathione peroxidase (GPx), which reduce ROS production [8].
4-Hexylresorcinol (4HR) is a widely used ingredient in cosmetics and antiseptics [9]. 4HR inhibits ROS-induced damage in lymphocytes through activating GPx [10] and demonstrates a similar antioxidant profile to that of resveratrol in human umbilical vein endothelial cells (HUVECs) [11]. In dentistry, 4HR has been shown to increase the rate of alveolar bone turnover and helps improve orthodontic tooth movement [12]. Additionally, 4HR injection increases the speed of mandibular incisor eruption in rat models [13] and decreases root resorption resulting from excessive orthodontic force and increases the expression of osteoprotegerin, a receptor activator of nuclear factor kappa-Β ligand, alkaline phosphatase and runt-related transcription factor 2 [14]. However, the effects of 4HR treatment on dental pulp under oxidative stress have not been studied yet.
Many models have been developed to simulate the condition of oxidative stress. Use of hydrogen peroxide is a classic procedure to simulate oxidative stress in cellular experiments [15]. Dental therapeutics that influence pulp cell homeostasis can be applied as in vivo models for such applications. The application of heavy orthodontic force or the removal of hard dental tissues are often used in in vivo models to induce dental pulp stress. When cells undergo oxidative stress, they often induce the expression of TNF-α and IL1β [16]. In this study, we aimed to evaluate the antioxidant effect of 4HR on ROS induced by oxidative stress in dental pulp cells. First, primary cultured pulp cells were subjected to oxidative stress through the application of hydrogen peroxide and then, the protective effect of 4HR was evaluated. Thereafter, the removal of hard dental tissue was used as an in vivo animal model to evaluate the antioxidant effect of 4HR on pulp tissues. For this, the total antioxidant capacity (TAC) and GPx activity were measured. The expression levels of TNF-α and IL1β were also investigated.
2. Materials and Methods
2.1. Cell Culture and 4HR Treatment
The dental pulp tissue was collected as described previously [13]. Pulp tissue was extracted from the mandibular incisors of rats. The collected pulp tissue was washed with phosphate-buffered saline (PBS; CAT# 17–602E; Lonza, Walkersville, MD, USA), then sliced with sterilized scissors and placed in a culture dish. Type I collagenase (CAT# 17100017, Gibco™, Carlsbad, CA, USA) was then added to the dish and the sample was incubated for 2 h. The cells were cultured in 10 mL of α-MEM (CAT# SH30265, Hyclone Laboratories, Logan, UT, USA) supplemented with 10% FBS (CAT# 12483020, Gibco™), 50 U/mL of penicillin G, 50 μg/mL of streptomycin sulfate, 2 g/L of sodium carbonate and 0.11 g/L of sodium pyruvate. This cell mixture was centrifuged at 15,000 rpm for 10 min and the resuspended cells were placed on a 60-mm Petri dish. Resveratrol and 4HR were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO) was used as the solvent to resuspend 4HR and then, it was diluted in the culture medium prior to its application. Final concentration of DMSO used was 0.1%.
2.2. Assessing GPx Activity and TAC
Changes in TAC in response to 4HR treatment were evaluated using commercial kits. The culture conditions for the primary cultured dental pulp cells and H2O2 treatment were the same as those described in Section 2.1. Resveratrol was used as a positive control. Resveratrol or 4HR was added to the dental pulp cells at a concentration of 1, 10, or 100 μM for 45 min. The pulp cells were then treated with 0.1 mM H2O2 for 30 min. TAC was evaluated after 24 h of resveratrol or 4HR treatment using the total antioxidant capacity assay kit (CAT#: ab65329, Abcam, Cambridge, UK) according to the manufacturer’s instructions. After 90 min of incubation at room temperature, the optical density was measured at 570 nm. The GPx assay was performed using a commercial kit (CAT#: ab102530, Abcam) as per manufacturer’s instruction. After adding cumen hydroperoxide, the optical density was measured at 340 nm in the kinetic mode.
2.3. Evaluating TNF-α and IL1β Expression in Dental Pulp Cells after 4HR Treatment
Primary antibodies against TNF-α and IL1β (Santa Cruz Biotech, Santa Cruz, CA, USA) were used in all evaluation assays. The cell culture and H2O2 treatment conditions were the same as those described in Section 2.2. Primary cultured pulp cells were treated with 1, 10, or 100 μM 4HR. The positive control included the H2O2 group without 4HR pretreatment and the negative control included the no treatment group. Cells were harvested at 8 or 24 h post 4HR treatment and lysed using radio-immunoprecipitation assay (RIPA) buffer. The lysates were then sonicated for 10 s, following which, their concentration was determined. Total protein concentration was measured using the RC DC™ protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein were then loaded onto the gel and subjected to electrophoresis. After separating the proteins, they were transferred onto a nitrocellulose membrane and subsequent protein blocking was performed, followed by antibody incubation steps as described previously [13]. The dilution ratio of the primary antibody was 1:500. The blot images were obtained using a ChemiDoc XRS system (Bio-Rad Laboratories). β-Actin was used as an internal control.
2.4. Animals and Experimental Design
The animals used in this study were purchased from Orientbio Inc. (Sungnam, Korea). This animal study was approved by the Gangneung-Wonju National University committee for animal research (GWNU-2021-01). Twenty male rats (8-week-old Crl:CD specific pathogen-free/viral antibody free) were used in this study. The conditions for caging and breeding were determined as described previously [13].
Eighteen rats were subjected to the incisor-cutting experiment. Two rats were used for pulp tissue extraction to determine the baseline activity of TAC and GPx. The right incisor was cut at the free gingival margin and pulp exposure was identified as the pin-point bleeding. The experimental group was administered 2% 4HR ointment and the control group was administered the ointment base (lanolin) only. Three animals from each group were sacrificed 3 days post application and the tooth specimen was sent for protein extraction and Western blot analysis. Then, another six animals from each group were sacrificed at 5 days after application. Among these, three from each group were subjected to histological and immunohistochemical analyses. The pulp tissue in the incisor was extracted from the other three animals in each group. They were then processed and homogenized for TAC and GPx analyses.
2.5. Histological, Immunohistochemical, Western Blot, GPx Activity and TAC Analysis
Routine hematoxylin and eosin (H&E) staining was performed to conduct the histological analysis. To investigate the expression level of TNF-α and IL1β in the pulp tissues, immunohistochemical staining was performed using anti-TNF-α and anti-IL1β antibodies (Santa Cruz Biotech). Briefly, the deparaffinized slides were treated with 1 mg of porcine trypsin (Sigma-Aldrich) for 10 min to allow antigen retrieval. Endogenous peroxidase activity was blocked following the treatment with 30% H2O2 for 7 min. The slides were then washed and subjected to a protein block step for 1 h. Then, the primary antibodies (dilution ratio 1:50) were applied and the slides were covered with parafilm. The slides were then incubated in a humid chamber at 4 °C for 8 h. After washing, a universal secondary antibody (Dako REAL™ EnVision™/HRP, Rabbit/Mouse; Dako North America Inc., Carpinteria, CA, USA) was applied. The unreacted secondary antibodies were washed with PBS and the slides were stained with a chromogen substrate (Dako REAL™ DAB+Chromogen and Dako REAL™ Substrate Buffer; Dako North America Inc.).
Fresh mandibular incisors on the affected side were separated from the mandible, homogenized in a reagent buffer and protease inhibitor cocktail and then evaluated using Western blot analysis as described above (Section 2.3). The pulp tissue in the incisor was processed and homogenized for further TAC and GPx analyses. Subsequent procedures were performed as described above (Section 2.2). The relative activities of TAC and GPx in the no-injury group were assessed for comparison with 4HR and ointment base-only (control) groups.
2.6. Statistical Analysis
Data are presented as mean ± standard deviation. Between group comparisons of the mean of different groups was performed using the independent samples t-test. The software used for the statistical analysis was SPSS 12.0 (SPSS Inc., Chicago, IL, USA). The level of significance was set at p < 0.05.
3. Results
3.1. Application of 4HR Increases GPx Activity and TAC in Dental Pulp Cells
TAC in the H2O2 treated control was 0.223 ± 0.003 mM (Figure 1a). TAC in the resveratrol group was 0.231 ± 0.002 mM, 0.236 ± 0.004 mM and 0.245 ± 0.005 mM in the 1 μM, 10 μM and 100 μM treated groups, respectively. Treatment with 10 μM and 100 μM resveratrol significantly increased TAC compared to that of the controls (p = 0.002 and 0.001, respectively); while TAC in the 4HR group was shown to increase in a dose-dependent manner, with 0.228 ± 0.003 mM, 0.235 ± 0.007 mM and 0.247 ± 0.002 mM in the 1 μM, 10 μM and 100 μM groups, respectively. Treatment with 10 μM and 100 μM 4HR significantly increased TAC compared to the controls (p = 0.013 and <0.001, respectively), while there were no significant differences between the 4HR and resveratrol groups (p > 0.05). GPx activity in the H2O2 treated control was 1.089 ± 0.022 mU/mL (Figure 1b), while for the resveratrol treatment, it was 1.244 ± 0.001 mU/mL, 1.246 ± 0.002 mU/mL and 1.559 ± 0.008 mU/mL in the 1 μM, 10 μM and 100 μM groups, respectively. Treatment with 10 μM and 100 μM resveratrol significantly increased GPx activity compared to the controls (p = 0.009 and <0.001, respectively) and GPx activity in the 4HR group was 1.247 ± 0.021 mU/mL, 1.309 ± 0.015 mU/mL and 1.411 ± 0.017 mU/mL in the 1 μM, 10 μM and 100 μM groups, respectively. Treatment with 10 μM and 100 μM 4HR significantly increased GPx activity compared to the controls (p = 0.005 and 0.001, respectively). However, no significant differences were observed between the 4HR and resveratrol groups (p > 0.05).
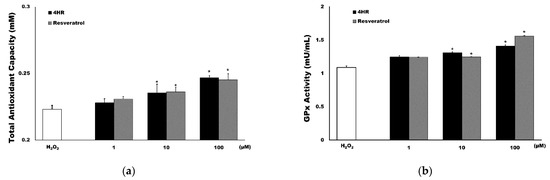
Figure 1.
Results of the antioxidant activity assays. (a) Total antioxidant capacity (TAC). 4-Hexylresorcinol (4HR) (black) induced a similar level of TAC production as that of resveratrol (grey). (b) Glutathione peroxidase (GPx) activity. 4HR (black) and resveratrol (grey) significantly increased GPx activity compared to H2O2 only treated control (* p < 0.05 compared to the hydrogen peroxide group, mean ± standard deviation).
3.2. 4HR Decreases the TNF-α and IL1β Expression Induced by Hydrogen Peroxidase
Hydrogen peroxide treatment increased TNF-α and IL1β expression (Figure 2) and this effect was reversed upon the addition of 4HR in the pre-treatment step.
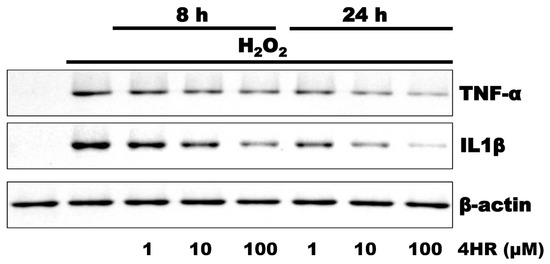
Figure 2.
Results of the Western blot analysis evaluating pulp cells after treatment with 0.1 mM hydrogen peroxide. Hydrogen peroxide treatment increased TNF-α and IL1β expression, which was then downregulated in response to pre-treatment with 4HR. The densitometric measurements are shown in Figure S1.
3.3. 4HR Decreased Inflammatory Reaction of Pulp Tissue Induced by Physical Stress
The application of 4HR ointment on exposed pulp tissue reduced the inflammation (Figure 3). The expression of both TNF-α and IL1β were lower in the 4HR group than in the ointment base-only group.
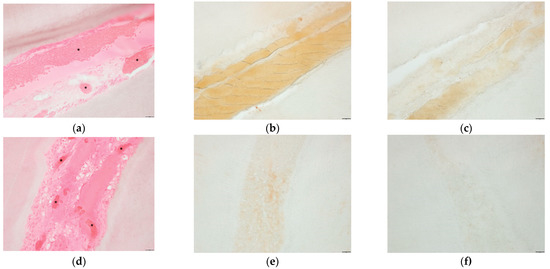
Figure 3.
Results of histological and immunohistochemical analyses of the incisor damage model. H&E staining revealed that the ointment base-only group demonstrated vascular dilatation and massive hemorrhage (*) in response to the stress (a). Degenerated pulp tissues and acellular areas were also wide. The expression level of TNF-α (b) and IL1β (c) were also higher in the ointment base-only group. However, the 4HR group displayed reduced hemorrhage areas (*) and active remodeling and regeneration (d). The expression level of TNF-α (e) and IL1β (f) were also lower in the 4HR group (bar = 20 μm, original magnification ×200). The densitometric measurements are shown in Figure S2.
The results of the Western blot analyses using the tissue samples were in accordance with the results from the immunohistochemical analysis. The administration of 4HR to the exposed incisor pulp tissues reduced the expression of TNF-α and IL1β (Figure 4a). The relative expression of TNF-α was 1.36 ± 0.32 and 0.59 ± 0.49 in the control and 4HR groups, respectively (Figure 4b) and the difference between these groups was statistically significant (p = 0.049). The relative expression level of IL1β was 0.57 ± 0.08 and 0.15 ± 0.11 in the control and 4HR groups, respectively (Figure 4b) and the difference between these groups was also statistically significant (p = 0.005).
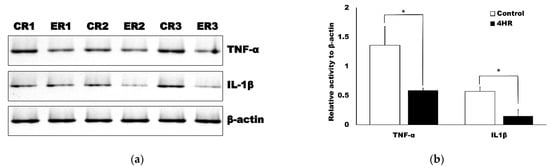
Figure 4.
Western blot results for the tissue samples. (a) The samples from the control (ointment base-only group) are represented as CR1, CR2 and CR3, while the samples from the 4HR treatment groups are represented as ER1, ER2 and ER3. Both TNF-α and IL1β expression was significantly higher in the control group compared to the 4HR group. (b) Protein expression was normalized against β-actin and presented in the form of a relative increase in TNF-α and IL1β expression versus the no treatment control and the difference between the groups was statistically significant (* p < 0.05, mean ± standard deviation).
The application of 4HR ointment reduced the oxidative stress in the dental pulp compared to the ointment base-only control group. The relative level of TAC was 1.37 ± 0.02 and 1.20 ± 0.05 in the control and 4HR groups, respectively (Figure 5) and the difference between these groups was statistically significant (p = 0.009). The relative level of GPx activity was 1.51 ± 0.05 and 1.21 ± 0.03 in the control and 4HR groups, respectively (Figure 5) and the difference between these groups was also statistically significant (p = 0.001).
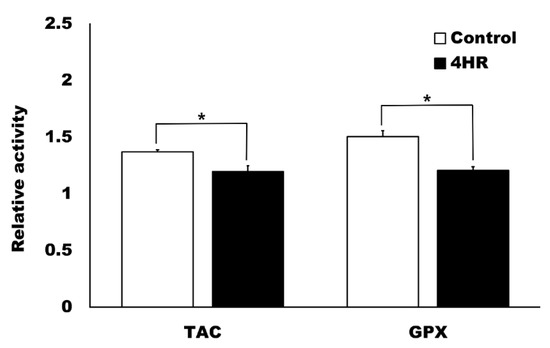
Figure 5.
Total antioxidant capacity (TAC) and glutathione peroxidase (GPx) activity in the pulp tissue samples. The control group was administered the ointment base-only. The relative activity of the uninjured incisor pulp was set as 1. The pulp injury increased TAC and GPx activity, while the application of 4HR attenuated the oxidative stress significantly compared to the ointment base-only control group (* p < 0.05, mean ± standard deviation).
4. Discussion
Dental pulp tissue repair requires transient activation of the inflammatory response, which suggests that proper repair requires that ROS inducers for this response be expressed transiently. Given this condition, the application of antioxidants may help to ensure better tissue regeneration under ROS stress [17]. In this study, hydrogen peroxide-induced oxidative stress in dental pulp cells was alleviated following 4HR treatment (Figure 1). As a result, the expression levels of TNF-α and IL1β were decreased (Figure 2). The topical application of 4HR ointment onto the exposed dental pulp also reduced the expression levels of TNF-α and IL1β (Figure 3 and Figure 4). The levels of TAC and GPX activity were also significantly reduced following 4HR treatment compared to the ointment base-only group (p < 0.05, Figure 5).
Dental pulp responds to the stress applied to the tooth [17,18]. The pathological stress in the tooth includes dental caries, periodontitis, or tooth fracture. The iatrogenic stress applied to the tooth includes orthodontic tooth movement and cavity preparation. In response to these stress, dental pulp produces ROS and induces an inflammatory response [18]. ROS in the inflammatory area alters the blood flow in the dental pulp [19]. Tooth fracture is a frequent type of dental injury where the pulp gets exposed [20]. The pulp tissue in crown-fractured teeth demonstrates increased inflammation and edema [21]. The application of antioxidants may reverse the inflammatory process in the injured dental pulp and help smoothen the transition to healing. In this study, we used an incisor cutting model, which is similar to crown fracture. The ointment base-only group showed massive hemorrhage and blood clots in the dental pulp (Figure 3a) and cellular regeneration was rare in the hemorrhagic area when the teeth were treated with the ointment base-only. However, the application of 4HR reduced the number of blood clots and markedly improved cellular regeneration (Figure 3d).
Hydrogen peroxide increases ROS production in cellular culture models. Therefore, we performed potential antioxidant screening test using a hydrogen peroxide-induced ROS production model. Catalase (CAT), GPx and superoxide dismutase (SOD) are important enzymes that act to protect cells from oxidative injury [22]. Hydrogen peroxide treatment decreases SOD, CAT and GPx activity in cardiomyocytes compared to the untreated control [15]. In this study, pretreatment with 4HR or resveratrol protected the pulp cells from oxidative damage (Figure 1). GPx activity and TAC were significantly upregulated compared to the no pre-treatment group (p < 0.05, Figure 1) and resveratrol and 4HR at 10–100 μM increased glutathione levels and GPx activity in human lymphocytes following the hydrogen peroxide stimulation [10]. Similar effects were observed in HUVECs [11,23]. Reducing oxidative stress alleviates inflammatory cytokine production, thereby reducing the availability of various signaling factors, including TNF-α and IL1β [24]. In this study, the administration of 4HR decreased the expression levels of TNF-α and IL1β, which were elevated in response to hydrogen peroxide (Figure 2).
The activity of antioxidant enzymes was different in the animal and cellular models (Figure 5). The levels of TAC and GPx activity were significantly reduced in the 4HR group compared to the ointment base-only control group (p < 0.05). In chronic inflammatory conditions, ROS levels may be persistently elevated and the elevation of antioxidant enzyme activity follows [25,26]. Both the 4HR treated and the ointment base-only (control) groups exhibited elevated levels of GPx activity and TAC (relative activity >1, Figure 5) compared to the non-injured pulp control. CAT activity is increased in the inflamed dental pulp [25]. The application of antioxidants neutralizes ROS and decreases GPx activity and TAC in the dental pulp [26]. The beneficial effect of antioxidant application in inflamed tissues may help in the early resolution of inflammation. The injured dental pulp showed chronic inflammation (Figure 3). An increase in ROS production stimulates the production of inflammatory cytokines, such as TNF-α and IL1β. In this study, the expression levels of TNF-α and IL1β decreased after 4HR ointment application (Figure 3 and Figure 4). These results are in accordance with the in vitro data (Figure 2).
The application of antioxidants in dental practice has been shown to exert beneficial effects. Titanium-based dental implant or plates induce a redox imbalance and oxidative damage to the periosteum [27,28]. Therefore, antioxidant administration would be helpful to alleviate oxidative stress for these patients. Lactoferrin (LF) is a well-known antioxidant [29]. LF incorporated titanium disc improves antibacterial and osteoinductive ability of dental implant [30]. Similar approach has been applied for the development of bone cement [31]. According to our previous research [32], 4HR-integrated dental implant improves new bone formation in case of bacterial contamination. In this study, a single application of 4HR ointment onto the exposed dental pulp resulted in a reduction in ROS and the levels of inflammatory cytokines, TNF-α and IL1β. The application of 4HR ointment decreased TNF-α expression and increased epithelialization in a deep burn model [33] and in a diabetic animal model, 4HR topical application has been shown to increase capillary regeneration [34]. Ointment application is an easy and simple method for dental interventions. However, this study has some limitations. Rat mandibular incisors are relatively large and exposed to the external environment. The advantage of this animal model is that pulp exposure can be achieved easily using a nail cutter. However, the mandibular incisor of the rat erupts continuously and rapidly [13]. Therefore, this model is not appropriate for examining long-term changes in the dental pulp. This continuous eruption is also an important differentiator between the rat model and the human system. Despite this, limitation, the application of 4HR demonstrates a few obvious advantages, including reduced oxidative stress and downregulation of the expression of the inflammatory cytokines, which may improve the therapeutic outcomes. Altogether, this data suggests that further investigation in other models is warranted to better evaluate the effects of 4HR in stress-induced dental pulp tissue.
5. Conclusions
4HR pre-treatment alleviates hydrogen peroxide induced oxidative stress in dental pulp cells through decreasing the expression of TNF-α and IL1β. Moreover, 4HR treatment also reduced the expression of TNF-α and IL1β via antioxidant activity in vivo. In conclusion, 4HR exerts protective effects against oxidative stress in dental pulp tissues through the downregulation of TNF-α and IL1β.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11083637/s1, Figure S1: Relative expressions of TNF-α and IL1β expression after 4-hexylresorcinol administration, Figure S2: Relative expressions of TNF-α and IL1β expression after 4-hexylresorcinol administration.
Author Contributions
J.-H.C. contributed to the conception and design of the study, data acquisition, analysis and interpretation and drafted and critically revised the manuscript; S.-G.K. and T.-W.K. contributed to the study design and interpretation and critically reviewed the manuscript. D.-W.K. contributed to the data acquisition, analysis and interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the support of the ‘‘Cooperative Research Program for Agriculture Science and Technology Development (Project no. PJ01562601)’’ Rural Development Administration, Republic of Korea.
Institutional Review Board Statement
This animal study was approved by the Gangneung-Wonju National University committee for animal research (GWNU-2021-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
There was no publicly archived datasets in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salla, J.T.; Taddei, S.R.; Queiroz, C.M., Jr.; Andrade, I., Jr.; Teixeira, M.M.; Silva, T.A. The effect of IL-1 receptor antagonist on orthodontic tooth movement in mice. Arch. Oral. Biol. 2012, 57, 519–524. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Fuchs, D.; Sucher, R. Redox regulation of the immune response. Redox Rep. 2013, 18, 88–94. [Google Scholar] [CrossRef]
- Shah, D.; Lynd, T.; Ho, D.; Chen, J.; Vines, J.; Jung, H.-D.; Kim, J.-H.; Zhang, P.; Wu, H.; Jun, H.-W.; et al. Pulp–Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches. J. Clin. Med. 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, D.; Fedorova, J.; He, Z.; Li, J. SIRT1/SIRT3 modulates redox homeostasis during ischemia/reperfusion in the aging heart. Antioxidants 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Buczko, P.; Zalewska, A.; Szarmach, I. Saliva and oxidative stress in oral cavity and in some systemic disorders. J. Physiol. Pharmacol. 2015, 66, 3–9. [Google Scholar] [PubMed]
- Leite, M.F.; De Lima, A.; Massuyama, M.M.; Otton, R. In vivo astaxanthin treatment partially prevents antioxidant alterations in dental pulp from alloxan-induced diabetic rats. Int. Endod. J. 2010, 43, 959–967. [Google Scholar] [CrossRef]
- Dahiya, P.; Bhardwaj, R.; Chaudhary, K.; Kamal, R.; Gupta, R.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Gaté, L.; Paul, J.; Ba, G.N.; Tew, K.; Tapiero, H. Oxidative stress induced in pathologies: The role of antioxidants. Biomed. Pharmacother. 1999, 53, 169–180. [Google Scholar] [CrossRef]
- Kim, S.-G. Immunomodulation for maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 5. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Lin, C.-W. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic. Res. 2003, 37, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Kim, D.-W.; Choi, J.-Y.; Kim, S.-G. 4-Hexylresorcinol and silk sericin increase the expression of vascular endothelial growth factor via different pathways. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Kim, D.-W.; Lee, S.K.; Kim, S.-G.; Kim, T.-W. The Administration of 4-Hexylresorcinol Accelerates Orthodontic Tooth Movement and Increases the Expression Level of Bone Turnover Markers in Ovariectomized Rats. Int. J. Mol. Sci. 2020, 21, 1526. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, D.-W.; Kim, S.-G.; Kim, T.-W. 4-Hexylresorcinol Administration Increases Dental Hard Tissue Formation and Incisor Eruption Rate in Rats. Appl. Sci. 2020, 10, 5511. [Google Scholar] [CrossRef]
- Jang, J.-K.; Kim, D.-W.; Kim, S.-G.; Kim, T.-W. Inhibitory Effects of 4-Hexylresorcinol on Root Resorption Induced by Orthodontic Tooth Movement. Appl. Sci. 2020, 10, 6313. [Google Scholar] [CrossRef]
- Movahed, A.; Yu, L.; Thandapilly, S.; Louis, X.; Netticadan, T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef]
- Calabrese, V.; Scuto, M.; Salinaro, A.T.; Dionisio, G.; Modafferi, S.; Ontario, M.L.; Greco, V.; Sciuto, S.; Schmitt, C.P.; Calabrese, E.J.; et al. Hydrogen Sulfide and Carnosine: Modulation of Oxidative Stress and Inflammation in Kidney and Brain Axis. Antioxidants 2020, 9, 1303. [Google Scholar] [CrossRef]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and Regeneration in the Dentin-Pulp Complex: A Double-edged Sword. J. Endod. 2014, 40, S46–S51. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Guarnieri, S.; D’Attilio, M.; Cavalcanti, M.F.; Mariggiò, M.A.; Pizzicannella, J.; Trubiani, O. A novel role of ascorbic acid in anti-inflammatory pathway and ROS generation in HEMA treated dental pulp stem cells. Materials 2020, 13, 130. [Google Scholar] [CrossRef]
- Okabe, E. Endogenous vasoactive substances and oxygen-derived free radicals in pulpal haemodynamics. Arch. Oral Biol. 1994, 39, S39–S45. [Google Scholar] [CrossRef]
- Viduskalne, I.; Care, R. Analysis of the crown fractures and factors affecting pulp survival due to dental trauma. Stomatologija 2010, 12, 109–115. [Google Scholar]
- Özçelïk, B.; Kuraner, T.; Kendïr, B.; Aşan, E. Histopathological Evaluation of the Dental Pulps in Crown-Fractured Teeth. J. Endod. 2000, 26, 271–273. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, S.-G.; Lee, S.-K. 4-Hexylresorcinol induced angiogenesis potential in human endothelial cells. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 23. [Google Scholar] [CrossRef]
- Xiong, W.; Ma, H.; Zhang, Z.; Jin, M.; Wang, J.; Xu, Y.; Wang, Z. The protective effect of icariin and phosphorylated icariin against LPS-induced intestinal goblet cell dysfunction. Innate Immun. 2019, 26, 97–106. [Google Scholar] [CrossRef]
- Topçu, K.Ç.; Kırıcı, D.; Evcil, M. Catalase activity in healthy and inflamed pulp tissues of permanent teeth in young people. Niger. J. Clin. Pract. 2016, 19, 600–602. [Google Scholar]
- Bagheri, A.; Ebrahimpour, S.; Nourbakhsh, N.; Talebi, S.; Esmaeili, A. Protective effect of quercetin on alteration of antioxidant genes expression and histological changes in the dental pulp of the streptozotocin-diabetic rats. Arch. Oral Biol. 2021, 125, 105088. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V Titanium Alloy Leads to Redox Abnormalities, Oxidative Stress, and Oxidative Damage in Patients Treated for Mandible Fractures. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.R.; Ładny, J.R.; Morawska, K.; Zalewska, A. Glutathione Metabolism, Mitochondria Activity, and Nitrosative Stress in Patients Treated for Mandible Fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, M.; Ma, H.; Jin, Q.; Ma, Y.; Wang, C.; Ren, J.; Liu, G.; Dai, Y. Ameliorative effect of recombinant human lactoferrin on the premature ovarian failure in rats after cyclophosphamide treatments. J. Ovarian Res. 2021, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Y.; Zhong, L.; Xie, W.; Xue, Y.; Wang, J. Lactoferrin/Calcium Phosphate-Modified Porous Ti by Biomimetic Mineralization: Effective Infection Prevention and Excellent Osteoinduction. Materials 2021, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Saruta, J.; Ozawa, R.; Hamajima, K.; Saita, M.; Sato, N.; Ishijima, M.; Kitajima, H.; Ogawa, T. Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement. Materials 2021, 14, 1289. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G.; Hahn, B.-D.; Park, D.-S.; Lee, Y.-C.; Choi, E.-J.; Chae, W.-S.; Baek, D.-H.; Choi, J.-Y. Aerosol Deposition of Hydroxyapatite and 4-Hexylresorcinol Coatings on Titanium Alloys for Dental Implants. J. Oral Maxillofac. Surg. 2011, 69, e354–e363. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, S.-G.; Kim, M.-K.; Kim, D.-W.; Lee, J.-H.; Seok, H.; Choi, J.-Y. Topical delivery of 4-hexylresorcinol promotes wound healing via tumor necrosis factor-α suppression. Burns 2016, 42, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Jo, Y.-Y.; Garagiola, U.; Choi, J.-Y.; Kang, Y.-J.; Oh, J.-H.; Kim, S.-G. Increased Level of Vascular Endothelial Growth Factors by 4-hexylresorcinol is Mediated by Transforming Growth Factor-β1 and Accelerates Capillary Regeneration in the Burns in Diabetic Animals. Int. J. Mol. Sci. 2020, 21, 3473. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).