Convolutional Neural Networks for Differential Diagnosis of Raynaud’s Phenomenon Based on Hands Thermal Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Paticipants
2.2. Procedure
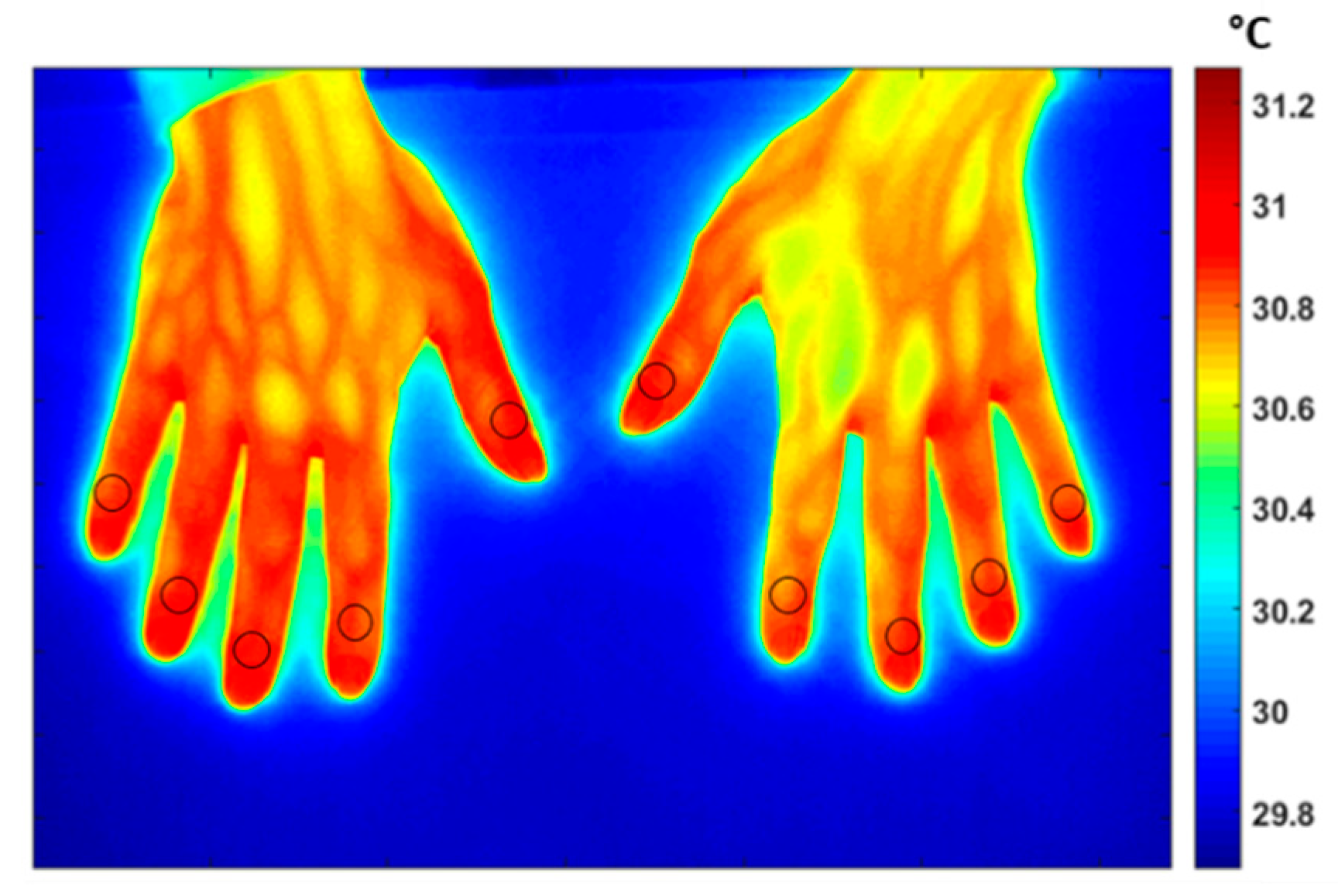
2.3. Data Acquisition
2.4. Data Analysis
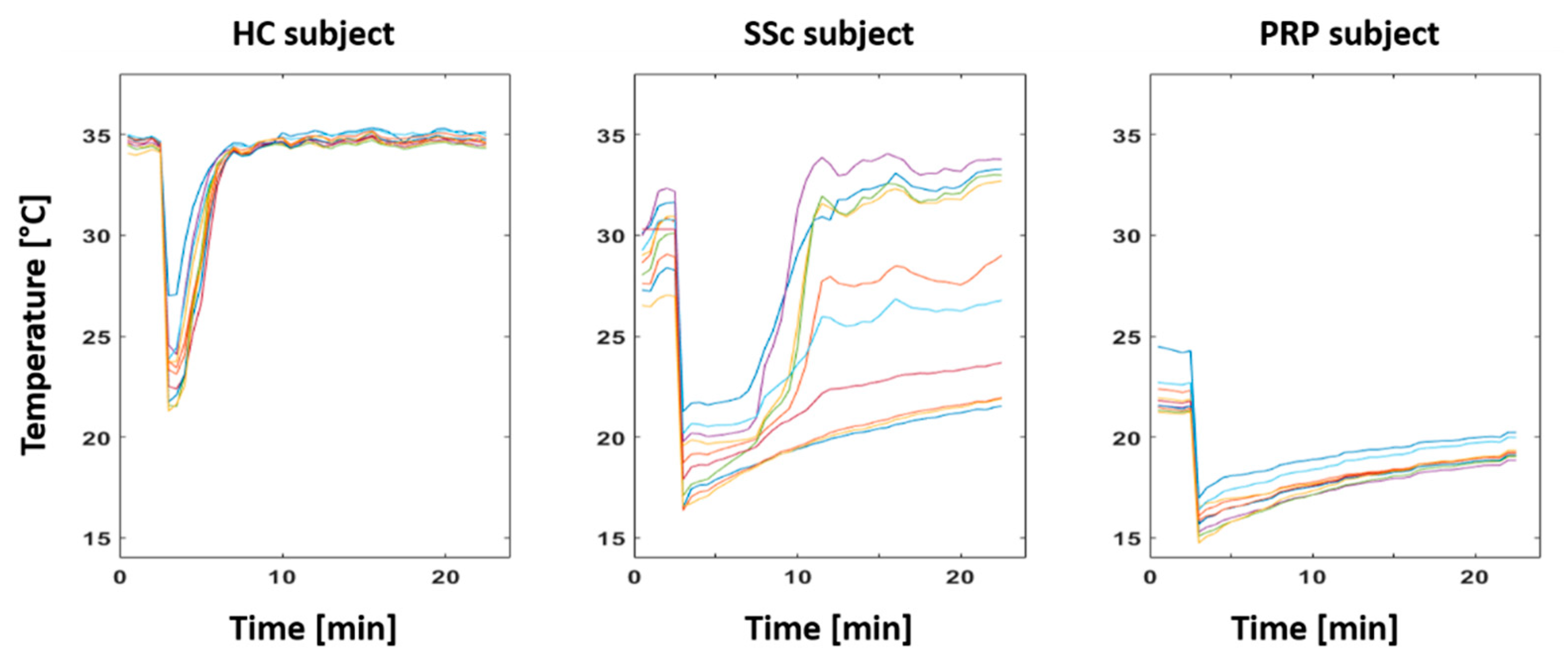
2.4.1. Baseline Analysis
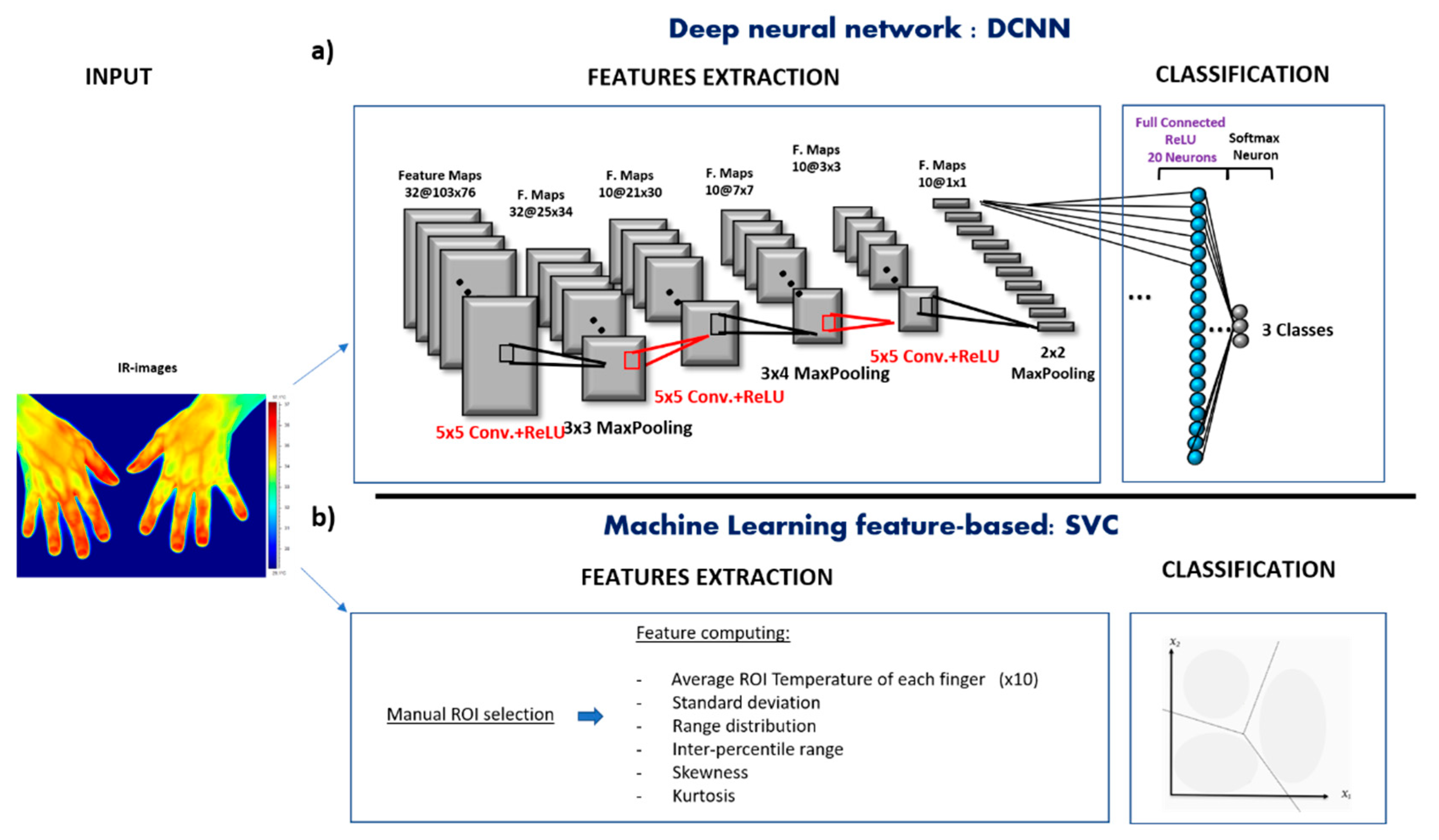
DCNN Model
- Input images preprocess. Firstly, the IR images were down sampled from 320 × 240 to 80 × 107 pixels and normalized by subtracting the average value of the entire set of images. A Principal Components Analysis was then adopted to reduce the number of spatial features while keeping the information characterizing the object to be analyzed. The number of components kept was 20 with a cumulative explained variance ratio of 0.95.
- Model architecture design. The DCNN structure employed in this work was heuristically chosen in similarity with previously reported DCNN structures on biological images or signals classification [41]. The DCNN was composed of 3 convolutional layers, 3 pooling layers, and 1 fully connected prior to the output layer. The first convolutional layer consisted in 32 filters (size 5 × 5) applied to the input images to obtain 32 feature maps of the images. The other 2 convolutional layer were both composed of 10 filters (size 5 × 5). The activation function employed in all the 3 convolutional layers to add nonlinearity to the network, were the Rectified Linear Unit (ReLU) function. Then, as pooling layer (or down-sampling layer) a MaxPooling were chosen, where the largest element from the rectified feature map was retained. A filter size of 3 × 3, 3 × 4 and 2 × 2 for the 3 MaxPooling layers, respectively, was implemented to reduce the dimensionality of each feature to 1 after the 3 convolutional layers and before the fully connected layer. The fully connected layer consisted in 20 neurons employed to summarize information and compute the class score. Lastly, a softamx function was used in the output layer, which outputs a probability value from 0 to 1 for each of the 3 classification classes (PRP, SSc, and healthy). All the biases of the DCNN were initialized to a small constant, i.e., 0.1, whereas the weights were initialized in a pseudo-random manner employing a truncated normal distribution (standard deviation = 0.1). The DCNN architecture is shown in Figure 1a.
- Model optimization. The model optimization was primarily focused on how to reduce overfitting. Indeed, developing a DCNN model with a small sample size inevitably involves high risk of overfitting. A technique used to address overfitting is regularization [42]. In this study, a Ridge Regression (L2 regularization) was implemented by adding the sum of the squared values of the model coefficient (weights) as a penalty term to the loss function. The loss function employed in this study was the categorical cross-entropy, therefore after performing the regularization technique it resulted in the following Equation:where is the kth scalar value of the model output, yk is the corresponding target value, and the constant times the sum of the squared weight () values is the regularization term. The value was set to 0.01. This was intended to reduce model complexity and make the model less prone to overfitting. In addition, instead of using a fixed learning rate hyperparameter for the presented model, which could lead the model to converge too quickly to a suboptimal solution, a tunable learning rate over the training process was implemented. In detail, a function was implemented to reduce learning rate by a factor of 0.1 once learning stop improving during at least 10 epochs.
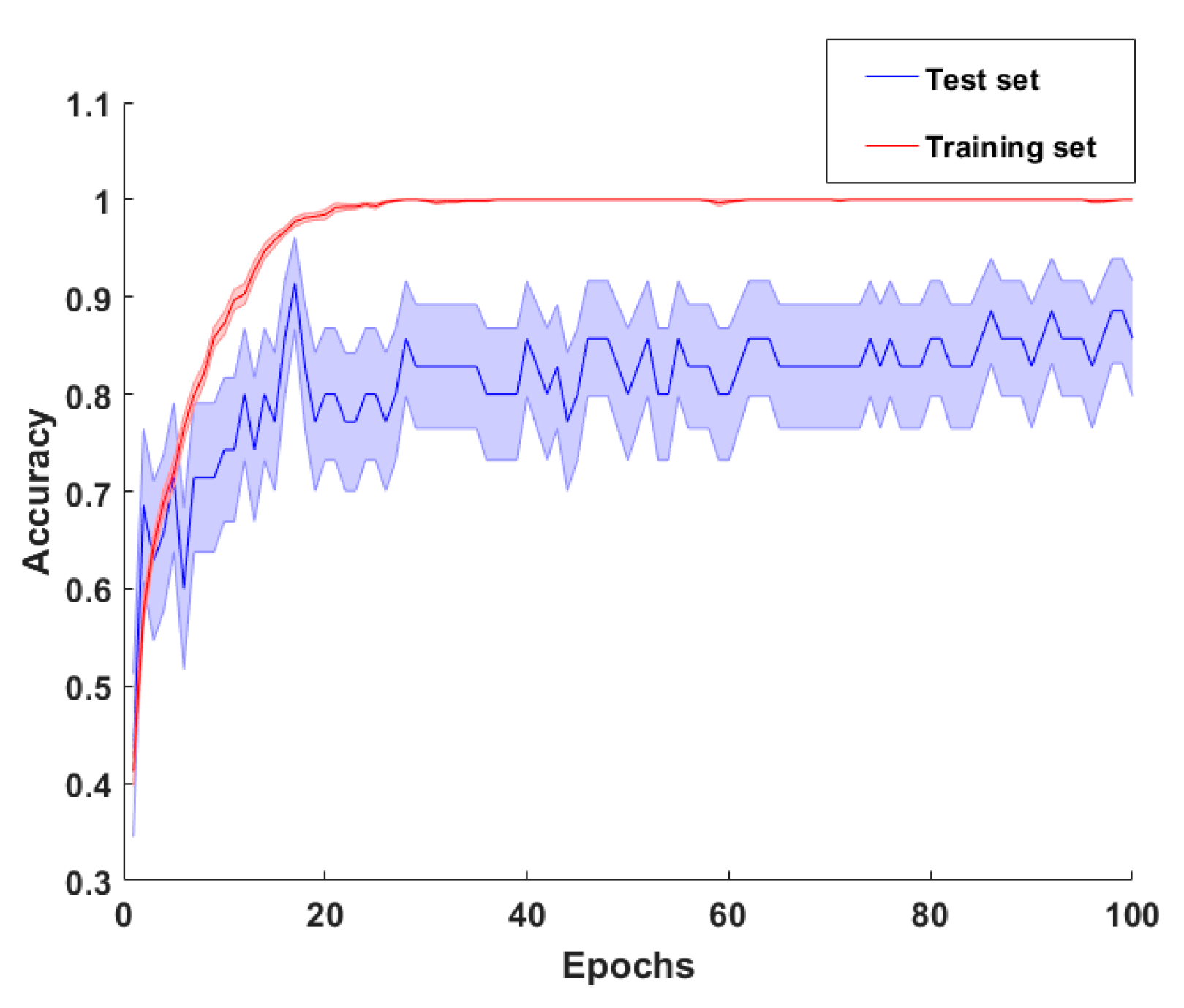
- Model evaluation. To evaluate the model performance, a categorical cross-entropy was employed, which is suitable for multiclass classification task. The optimization procedure was iterated for 100 epochs with a batch size of 6 samples. To address the model generalization performance, a leave-one-out cross-validation procedure was performed [43]. The metrics used for evaluating the model was the accuracy, sensitivity, and specificity. Accuracy represents the percentage of correct predictions out of the total number of test samples. Sensitivity is the proportion of predicted true positives out of all patients with the disease, whereas specificity represent the percentage of the predicted true negatives out of all participants who do not have the disease [44]. Those metrics were performed by counting the number of correct DCNN predictors after an argmax evaluation of the DCNN output vector, averaged among the plateau iterations. This procedure was conducted following the leave one-out cross-validation. The overall sensitivity and specificity of the classifier were obtained by averaging the sensitivity and specificity of each class, respectively.
Feature-Based Analysis
2.4.2. CCP Analysis
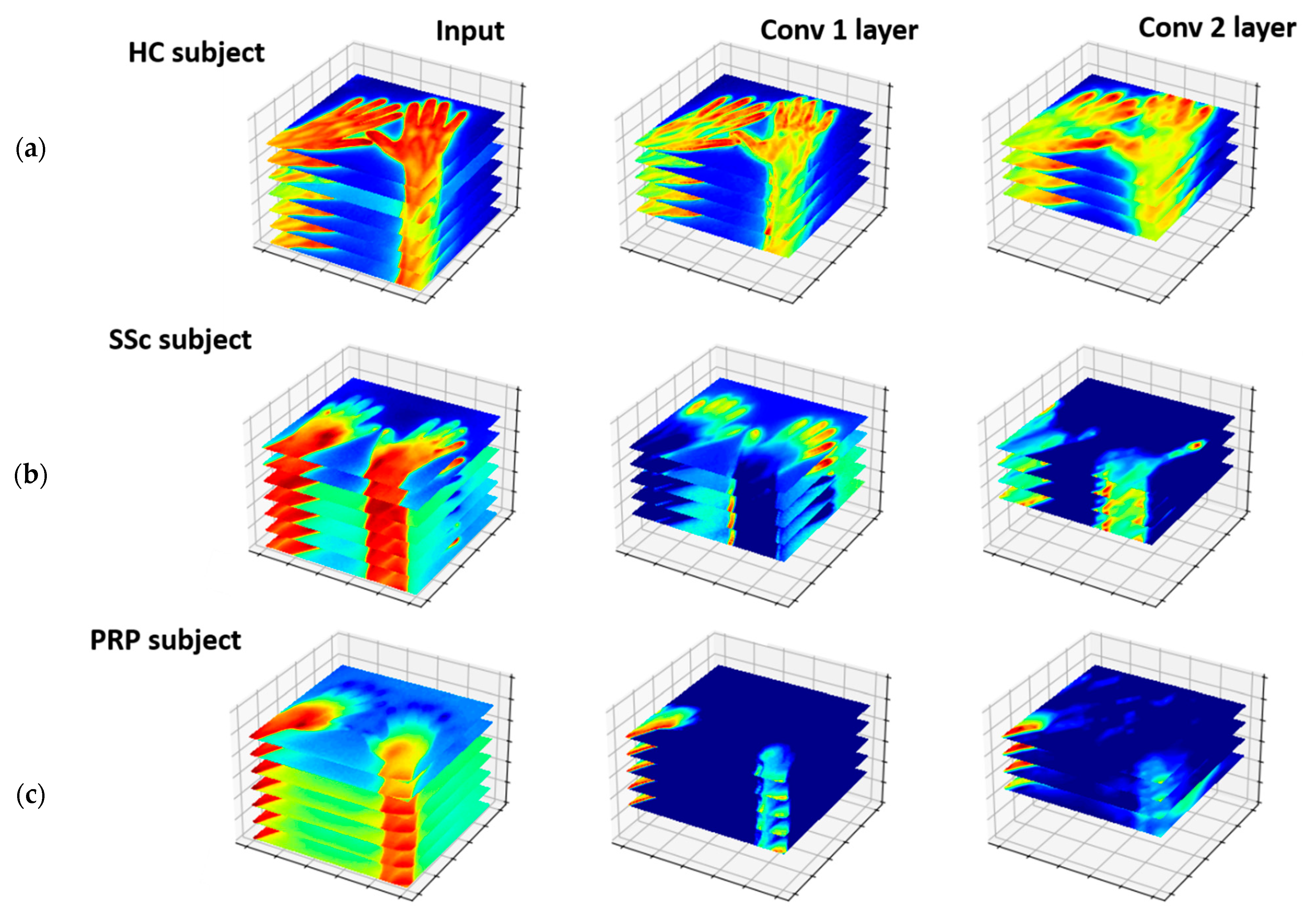
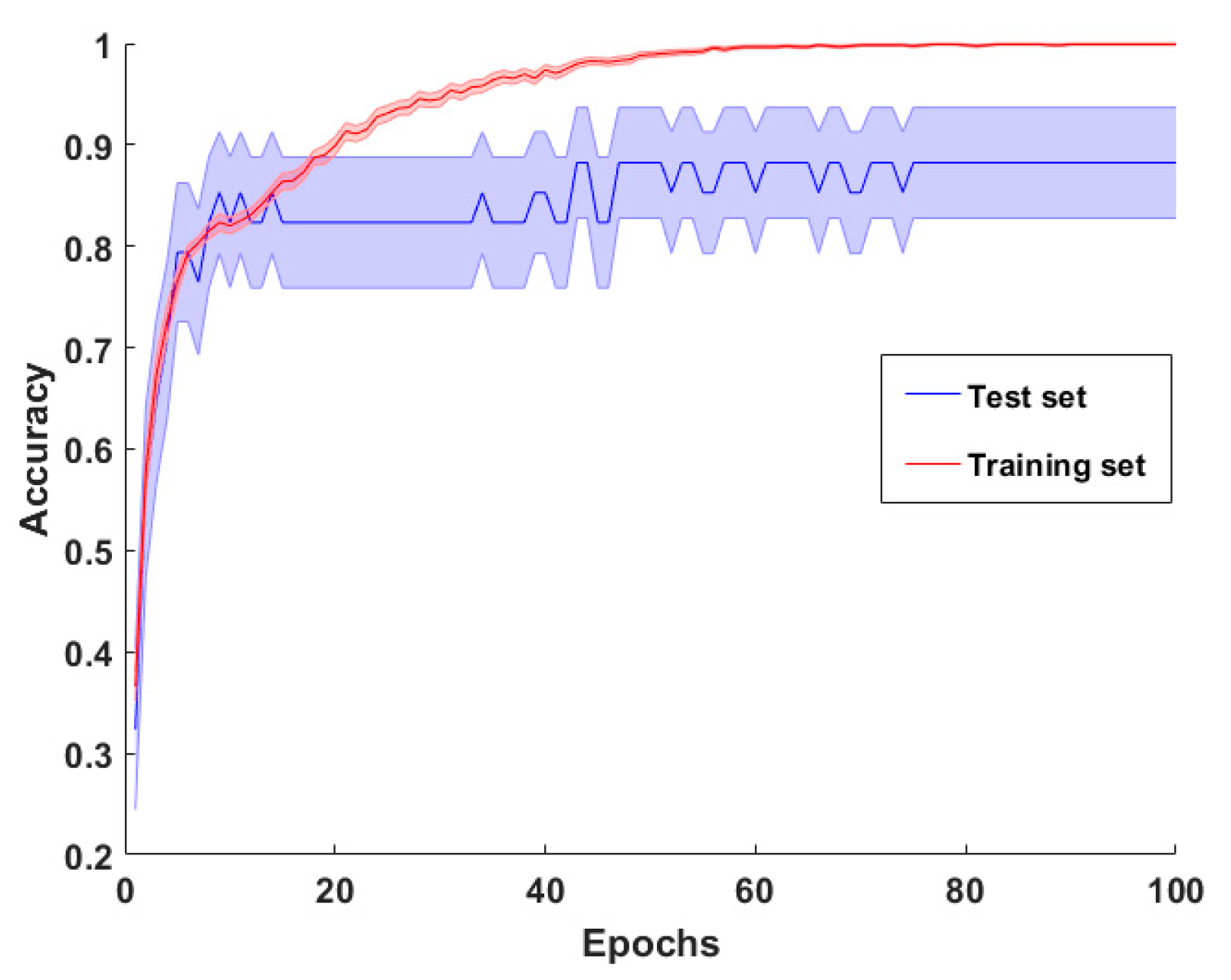
3-Dimensional DCNN
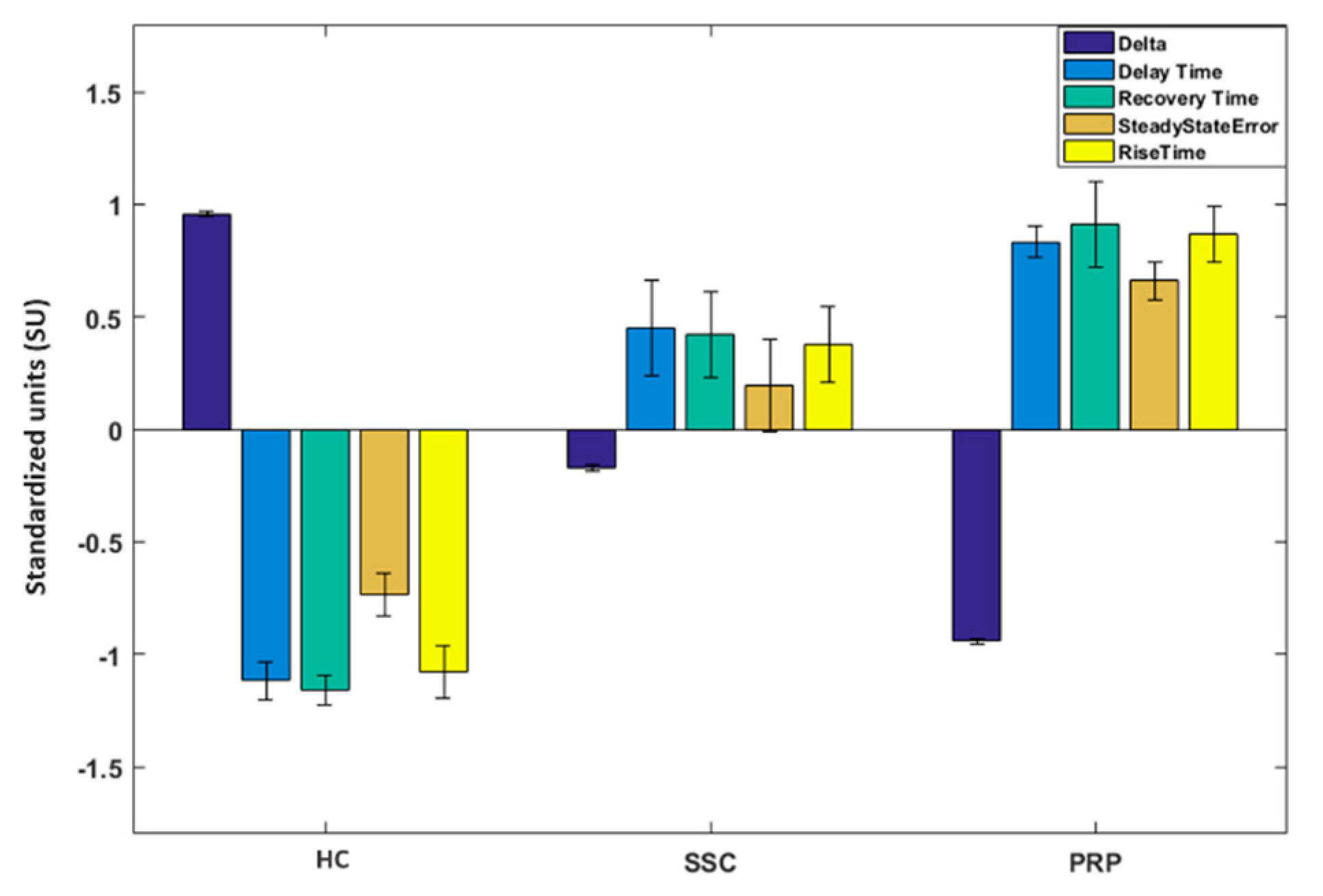
Feature-Based Analysis
- Delay time: the time required for the finger’s temperature after the cold challenge to reach 50% of its final value (i.e., the recovery temperature after 20 min from the cold stress).
- Rise time: the time required for the finger’s temperature to rise from 10% to 90% of its final value.
- Recovery time: time required for the finger’s temperature to reach the 68% of the difference value between the baseline temperature and the temperature soon after the cold challenge.
- Steady state error: the difference between the baseline temperature value and the final recovery temperature value.
- Delta: difference between the finger’s temperature on its final recovery point and the temperature soon after the stimulus.
3. Results
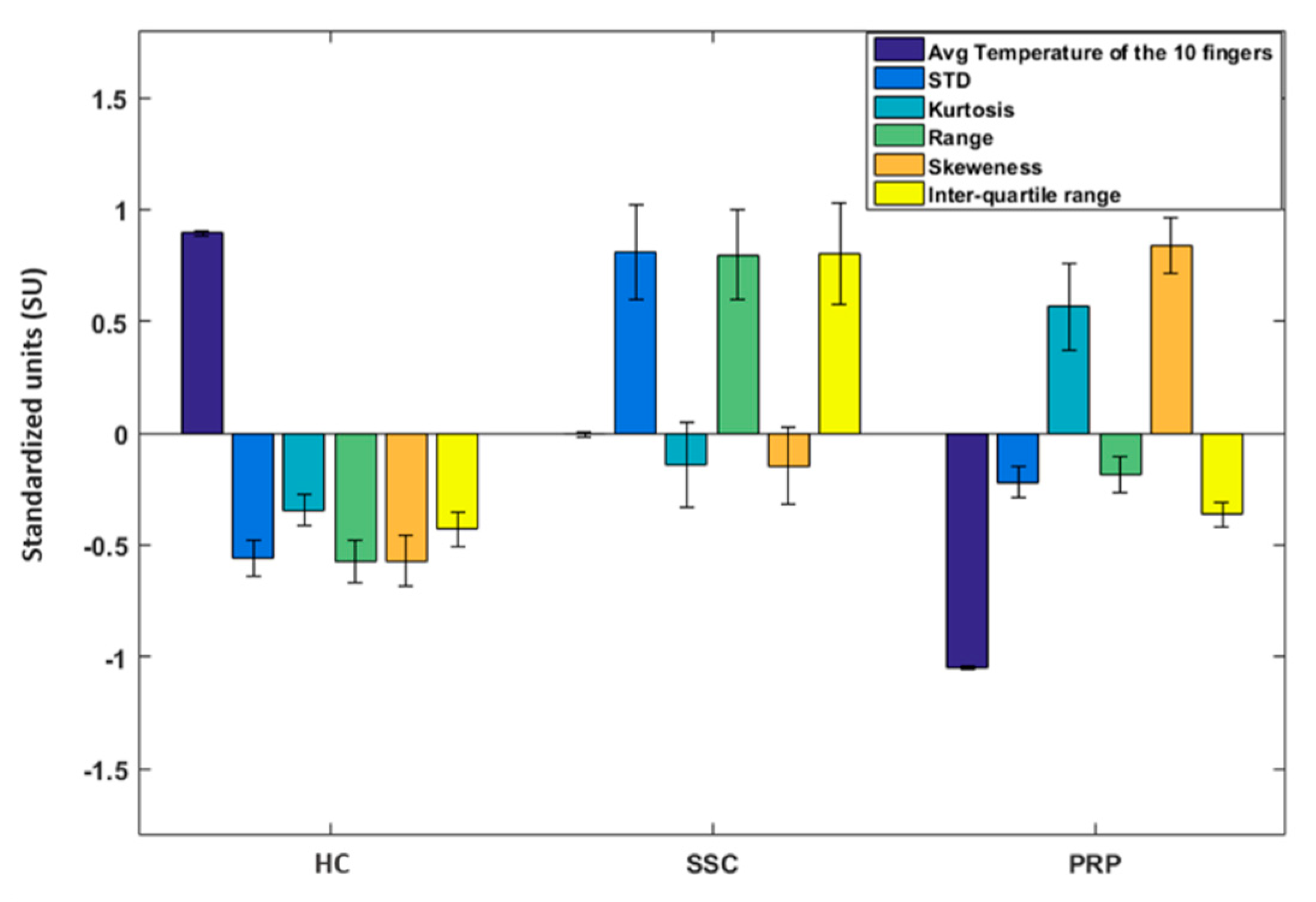
3.1. Baseline Results
3.2. CCP Results
3.3. Baseline vs. CCP Results
4. Discussion
4.1. Thermoregulatory Difference between Classes
4.2. Comparisons with Previous Studies and Discussion on Machine Learning Results
4.3. Study’s Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prete, M.; Fatone, M.C.; Favoino, E.; Perosa, F. Raynaud’s Phenomenon: From Molecular Pathogenesis to Therapy. Autoimmun. Rev. 2014, 13, 655–667. [Google Scholar] [CrossRef]
- Mariotti, A.; Grossi, G.; Amerio, P.; Orlando, G.; Mattei, P.A.; Tulli, A.; Romani, G.L.; Merla, A. Finger Thermoregulatory Model Assessing Functional Impairment in Raynaud’s Phenomenon. Ann. Biomed. Eng. 2009, 37, 2631–2639. [Google Scholar] [CrossRef]
- Ruaro, B.; Smith, V.; Sulli, A.; Pizzorni, C.; Tardito, S.; Patané, M.; Paolino, S.; Cutolo, M. Innovations in the Assessment of Primary and Secondary Raynaud’s Phenomenon. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Maricq, H.R.; Carpentier, P.H.; Weinrich, M.C.; Keil, J.E.; Palesch, Y.; Biro, C.; Vionnet-Fuasset, M.; Jiguet, M.; Valter, I. Geographic Variation in the Prevalence of Raynaud’s Phenomenon: A 5 Region Comparison. J. Rheumatol. 1997, 24, 879–889. [Google Scholar]
- Hughes, M.; Allanore, Y.; Chung, L.; Pauling, J.D.; Denton, C.P.; Matucci-Cerinic, M. Raynaud Phenomenon and Digital Ulcers in Systemic Sclerosis. Nat. Rev. Rheumatol. 2020, 16, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L. The Pathogenesis, Diagnosis and Treatment of Raynaud Phenomenon. Nat. Rev. Rheumatol. 2012, 8, 469–479. [Google Scholar] [CrossRef]
- Kahaleh, M.B. Raynaud Phenomenon and the Vascular Disease in Scleroderma. Curr. Opin. Rheumatol. 2004, 16, 718–722. [Google Scholar] [CrossRef]
- Abraham, D.J.; Varga, J. Scleroderma: From Cell and Molecular Mechanisms to Disease Models. Trends Immunol. 2005, 26, 587–595. [Google Scholar] [CrossRef]
- Pauling, J.D.; Domsic, R.T.; Saketkoo, L.A.; Almeida, C.; Withey, J.; Jay, H.; Frech, T.M.; Ingegnoli, F.; Dures, E.; Robson, J.; et al. Multinational Qualitative Research Study Exploring the Patient Experience of Raynaud’s Phenomenon in Systemic Sclerosis. Arthritis Care Res. 2018, 70, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Tyndall, A.; Czirják, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Müller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical Risk Assessment of Organ Manifestations in Systemic Sclerosis: A Report from the EULAR Scleroderma Trials and Research Group Database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Evidence That Systemic Sclerosis Is a Vascular Disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- McMahan, Z.H.; Wigley, F.M. Raynaud’s Phenomenon and Digital Ischemia: A Practical Approach to Risk Stratification, Diagnosis and Management. Int. J. Clin. Rheumtol. 2010, 5, 355–370. [Google Scholar] [CrossRef]
- Merla, A.; Donato, L.D.; Luzio, S.D.; Farina, G.; Pisarri, S.; Proietti, M.; Salsano, F.; Romani, G.L. Infrared Functional Imaging Applied to Raynaud’s Phenomenon. IEEE Eng. Med. Biol. Mag. 2002, 21, 73–79. [Google Scholar] [CrossRef]
- Sousa, E.; Vardasca, R.; Teixeira, S.; Seixas, A.; Mendes, J.; Costa-Ferreira, A. A Review on the Application of Medical Infrared Thermal Imaging in Hands. Infrared Phys. Technol. 2017, 85, 315–323. [Google Scholar] [CrossRef]
- Quesada, J.I.P. Application of Infrared Thermography in Sports Science; Springer: Berlin, Germany, 2017; ISBN 3-319-47409-X. [Google Scholar]
- Perpetuini, D.; Filippini, C.; Cardone, D.; Merla, A. An Overview of Thermal Infrared Imaging-Based Screenings during Pandemic Emergencies. Int. J. Environ. Res. Public Health 2021, 18, 3286. [Google Scholar] [CrossRef] [PubMed]
- Filippini, C.; Perpetuini, D.; Cardone, D.; Chiarelli, A.M.; Merla, A. Thermal Infrared Imaging-Based Affective Computing and Its Application to Facilitate Human Robot Interaction: A Review. Appl. Sci. 2020, 10, 2924. [Google Scholar] [CrossRef]
- Filippini, C.; Spadolini, E.; Cardone, D.; Bianchi, D.; Preziuso, M.; Sciarretta, C.; del Cimmuto, V.; Lisciani, D.; Merla, A. Facilitating the Child–Robot Interaction by Endowing the Robot with the Capability of Understanding the Child Engagement: The Case of Mio Amico Robot. Int. J. Soc. Robot. 2020, 1–13. [Google Scholar] [CrossRef]
- Ismail, E.; Orlando, G.; Corradini, M.L.; Amerio, P.; Romani, G.L.; Merla, A. Differential Diagnosis of Raynaud’s Phenomenon Based on Modeling of Finger Thermoregulation. Phys. Meas. 2014, 35, 703. [Google Scholar] [CrossRef]
- Chand, G.; Ali, M.; Barmada, B.; Liesaputra, V.; Ramirez-Prado, G. Tracking a Person’s Behaviour in a Smart House. In Proceedings of the International Conference on Service-Oriented Computing; Springer: Cham, Switzerland, 2018; pp. 241–252. [Google Scholar]
- Cardone, D.; Perpetuini, D.; Filippini, C.; Spadolini, E.; Mancini, L.; Chiarelli, A.M.; Merla, A. Driver Stress State Evaluation by Means of Thermal Imaging: A Supervised Machine Learning Approach Based on ECG Signal. Appl. Sci. 2020, 10, 5673. [Google Scholar] [CrossRef]
- Paszkiel, S. Using neural networks for classification of the changes in the EEG signal based on facial expressions. In Analysis and Classification of EEG Signals for Brain–Computer Interfaces; Springer: Cham, Switzerland, 2020; pp. 41–69. [Google Scholar]
- Paszkiel, S. Analysis and Classification of EEG Signals for Brain-Computer Interfaces; Springer: Berlin, Germany, 2020; ISBN 3-030-30580-5. [Google Scholar]
- Filippini, C.; Perpetuini, D.; Cardone, D.; Chiarelli, A.M.; Merla, A. Thermal Infrared Imaging and Artificial Intelligence Techniques Can Support Mild Alzheimer Disease Diagnosis; CEUR Workshop Proceedings: Aachen, Germany, 2020; Volume 2804, pp. 31–39. [Google Scholar]
- Perpetuini, D.; Chiarelli, A.M.; Filippini, C.; Cardone, D.; Croce, P.; Rotunno, L.; Anzoletti, N.; Zito, M.; Zappasodi, F.; Merla, A. Working Memory Decline in Alzheimer’s Disease Is Detected by Complexity Analysis of Multimodal EEG-FNIRS. Entropy 2020, 22, 1380. [Google Scholar] [CrossRef]
- Bikmukhametov, T.; Jäschke, J. Combining Machine Learning and Process Engineering Physics towards Enhanced Accuracy and Explainability of Data-Driven Models. Comput. Chem. Eng. 2020, 138, 106834. [Google Scholar] [CrossRef]
- Shang, C.; You, F. Data Analytics and Machine Learning for Smart Process Manufacturing: Recent Advances and Perspectives in the Big Data Era. Engineering 2019, 5, 1010–1016. [Google Scholar] [CrossRef]
- Rawat, W.; Wang, Z. Deep Convolutional Neural Networks for Image Classification: A Comprehensive Review. Neural Comput. 2017, 29, 2352–2449. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sohail, A.; Zahoora, U.; Qureshi, A.S. A Survey of the Recent Architectures of Deep Convolutional Neural Networks. Artif. Intell. Rev. 2020, 53, 5455–5516. [Google Scholar] [CrossRef]
- Hecht-Nielsen, R.I. 3-Theory of the Backpropagation Neural Network. In Neural Networks for Perception; Academic Press: Cambridge, MA, USA, 1992; pp. 65–93. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Berlin, Germany, 2015; pp. 234–241. [Google Scholar]
- Li, Q.; Cai, W.; Wang, X.; Zhou, Y.; Feng, D.D.; Chen, M. Medical Image Classification with Convolutional Neural Network. In Proceedings of the 13th International Conference on Control Automation Robotics & Vision (ICARCV), Singapore, 10–12 December 2014; IEEE: Washington, DC, USA, 2014; pp. 844–848. [Google Scholar]
- World Medical Association Declaration of Helsinki. Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects. JAMA 1997, 277, 925–926. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Le Roy, E.C.; Medsger, T.A. Criteria for the Classification of Early Systemic Sclerosis. J. Rheum. 2001, 28, 1573–1576. [Google Scholar]
- Goundry, B.; Bell, L.; Langtree, M.; Moorthy, A. Diagnosis and Management of Raynaud’s Phenomenon. BMJ 2012, 344. [Google Scholar] [CrossRef]
- Cardone, D.; Merla, A. New Frontiers for Applications of Thermal Infrared Imaging Devices: Computational Psychopshysiology in the Neurosciences. Sensors 2017, 17, 1042. [Google Scholar] [CrossRef]
- Thermology Guidelines, Standards and Protocols in Clinical Thermography Imaging. Available online: https://www.researchgate.net/publication/273755657_Thermology_guidelines_standards_and_protocols_in_clinical_thermography_imaging (accessed on 22 September 2020).
- Bernard, V.; Staffa, E.; Mornstein, V.; Bourek, A. Infrared Camera Assessment of Skin Surface Temperature–Effect of Emissivity. Phys. Med. 2013, 29, 583–591. [Google Scholar] [CrossRef]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.; Costa, C.M.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I. Thermographic Imaging in Sports and Exercise Medicine: A Delphi Study and Consensus Statement on the Measurement of Human Skin Temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Bianco, F.; Perpetuini, D.; Bucciarelli, V.; Filippini, C.; Cardone, D.; Zappasodi, F.; Gallina, S.; Merla, A. Data-Driven Assessment of Cardiovascular Ageing through Multisite Photoplethysmography and Electrocardiography. Med. Eng. Phys. 2019, 73, 39–50. [Google Scholar] [CrossRef]
- Murugan, P.; Durairaj, S. Regularization and Optimization Strategies in Deep Convolutional Neural Network. arXiv 2017, arXiv:1712.04711. [Google Scholar]
- Kohavi, R. A Study of Cross-Validation and Bootstrap for Accuracy Estimation and Model Selection. In Proceedings of the International Joint Conference on AI Palais de Congres, Montreal, QC, Canada, 20–25 August 1995; Volume 14, pp. 1137–1145. [Google Scholar]
- Shreffler, J.; Huecker, M.R. Diagnostic Testing Accuracy: Sensitivity, Specificity, Predictive Values and Likelihood Ratios. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Ji, S.; Xu, W.; Yang, M.; Yu, K. 3D Convolutional Neural Networks for Human Action Recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 35, 221–231. [Google Scholar] [CrossRef]
- Jin, T.; Cui, H.; Zeng, S.; Wang, X. Learning Deep Spatial Lung Features by 3D Convolutional Neural Network for Early Cancer Detection. In Proceedings of the 2017 International Conference on Digital Image Computing: Techniques and Applications (DICTA); IEEE: Washington, DC, USA, 2017; pp. 1–6. [Google Scholar]
- Maturana, D.; Scherer, S. Voxnet: A 3d Convolutional Neural Network for Real-Time Object Recognition. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS); IEEE: Washington, DC, USA, 2015; pp. 922–928. [Google Scholar]
- Horikoshi, M.; Inokuma, S.; Kijima, Y.; Kobuna, M.; Miura, Y.; Okada, R.; Kobayashi, S. Thermal Disparity between Fingers after Cold-Water Immersion of Hands: A Useful Indicator of Disturbed Peripheral Circulation in Raynaud Phenomenon Patients. Intern Med. 2016, 55, 461–466. [Google Scholar] [CrossRef]
- Lachenbruch, P.A.; Lynch, C.J. Assessing Screening Tests: Extensions of McNemar’s Test. Stat. Med. 1998, 17, 2207–2217. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr. In Vivo Mechanisms of Cutaneous Vasodilation and Vasoconstriction in Humans during Thermoregulatory Challenges. J. Appl. Phys. 2006, 100, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Wigley, F.M. Vascular Disease in Scleroderma. Clin. Rev. Allergy Immun. 2009, 36, 150–175. [Google Scholar] [CrossRef]
- Suter, L.G.; Murabito, J.M.; Felson, D.T.; Fraenkel, L. The Incidence and Natural History of Raynaud’s Phenomenon in the Community. Arthritis Rheum. 2005, 52, 1259–1263. [Google Scholar] [CrossRef]
- Carpentier, P.H.; Satger, B.; Poensin, D.; Maricq, H.R. Incidence and Natural History of Raynaud Phenomenon: A Long-Term Follow-up (14 Years) of a Random Sample from the General Population. J. Vasc. Surg. 2006, 44, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Tiso, F.; Favaro, M.; Ciprian, L.; Cardarelli, S.; Rizzo, M.; Tonello, M.; Ruffatti, A.; Cozzi, F. Digital Ulcers in a Cohort of 333 Scleroderma Patients. Reumatismo 2007, 59, 215–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nihtyanova, S.I.; Brough, G.M.; Black, C.M.; Denton, C.P. Clinical Burden of Digital Vasculopathy in Limited and Diffuse Cutaneous Systemic Sclerosis. Ann. Rheum. Dis. 2008, 67, 120–123. [Google Scholar] [CrossRef]
- Guiducci, S.; Giacomelli, R.; Cerinic, M.M. Vascular Complications of Scleroderma. Autoimmun. Rev. 2007, 6, 520–523. [Google Scholar] [CrossRef]
- Love, T.J. Thermography as an Indicator of Blood Perfusion. Ann. N. Y. Acad. Sci. 1980, 335, 429–437. [Google Scholar] [CrossRef]
- De Campos, M.F.; Ripka, W.L.; Campos, D.; Heimbecher, C.T.; Esmanhoto, E.; Ulbricht, L. Raynaud’s Phenomenon Differentiating After Cold Stress Using Thermal Parameters from Fingers. In Proceedings of the XXVI Brazilian Congress on Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 869–874. [Google Scholar]
- Viana, J.R.; Campos, D.; Ulbricht, L.; Sato, G.Y.; Ripka, W.L. Thermography for the Detection of Secondary Raynaud’s Phenomenon by Means of the Distal-Dorsal Distance. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); IEEE: Washington, DC, USA, 2020; pp. 1528–1531. [Google Scholar]
- Lim, M.J.; Kwon, S.R.; Jung, K.-H.; Joo, K.; Park, S.-G.; Park, W. Digital Thermography of the Fingers and Toes in Raynaud’s Phenomenon. J. Korean Med. Sci. 2014, 29, 502. [Google Scholar] [CrossRef]
- Martini, G.; Cappella, M.; Culpo, R.; Vittadello, F.; Sprocati, M.; Zulian, F. Infrared Thermography in Children: A Reliable Tool for Differential Diagnosis of Peripheral Microvascular Dysfunction and Raynaud’s Phenomenon? Pediatr. Rheum. 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Herrick, A.L.; Murray, A. The Role of Capillaroscopy and Thermography in the Assessment and Management of Raynaud’s Phenomenon. Autoimmun. Rev. 2018, 17, 465–472. [Google Scholar] [CrossRef]
- Herrick, A.L.; Dinsdale, G.; Murray, A. New Perspectives in the Imaging of Raynaud’s Phenomenon. Eur. J. Rheum. 2020, 7, S212–S221. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Patel, F.; Kronenberg, D.G.; Chung, L.; Fiorentino, D.; Allanore, Y.; Guiducci, S.; Hesselstrand, R.; Hummers, L.K.; Duong, C.; et al. International Consensus Criteria for the Diagnosis of Raynaud’s Phenomenon. J. Autoimmun. 2014, 48–49, 60–65. [Google Scholar] [CrossRef] [PubMed]

| Group | Sex (Female/Male) | Age (Avg ± std, Years) | Body Mass (Avg ± std, Kg) | Physically Active (%) |
|---|---|---|---|---|
| HC | 7/6 | 48.5 ± 11.1 | 61.8 ± 5.9 | 42% |
| PRP | 5/6 | 52.2 ± 10.3 | 64.5 ± 5.5 | 38% |
| SSc | 5/7 | 50.8 ± 11.6 | 62.3 ± 6.2 | 35% |
| DCNN | SVC | |||
|---|---|---|---|---|
| Classes | Sensitivity | Specificity | Sensitivity | Specificity |
| HC | 0.84 | 0.91 | 0.84 | 0.83 |
| SSc | 0.75 | 0.87 | 0.67 | 0.90 |
| PRP | 0.90 | 0.96 | 0.81 | 0.92 |
| 3D-DCNN | SVC | |||
|---|---|---|---|---|
| Classes | Sensitivity | Specificity | Sensitivity | Specificity |
| HC | 0.92 | 0.91 | 1 | 0.95 |
| SSc | 0.83 | 0.92 | 0.58 | 0.91 |
| PRP | 0.90 | 1 | 0.81 | 0.84 |
| Baseline | CCP | |||
|---|---|---|---|---|
| Metrics | DCNN | SVC | 3D-DCNN | SVC |
| Accuracy | 0.84 | 0.77 | 0.88 | 0.83 |
| Sensitivity | 0.83 + 0.08 | 0.77 + 0,09 | 0.88 + 0.04 | 0.80 + 0.20 |
| Specificity | 0.91 + 0.04 | 0.88 + 0.05 | 0.94 + 0.05 | 0.90 + 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, C.; Cardone, D.; Perpetuini, D.; Chiarelli, A.M.; Gualdi, G.; Amerio, P.; Merla, A. Convolutional Neural Networks for Differential Diagnosis of Raynaud’s Phenomenon Based on Hands Thermal Patterns. Appl. Sci. 2021, 11, 3614. https://doi.org/10.3390/app11083614
Filippini C, Cardone D, Perpetuini D, Chiarelli AM, Gualdi G, Amerio P, Merla A. Convolutional Neural Networks for Differential Diagnosis of Raynaud’s Phenomenon Based on Hands Thermal Patterns. Applied Sciences. 2021; 11(8):3614. https://doi.org/10.3390/app11083614
Chicago/Turabian StyleFilippini, Chiara, Daniela Cardone, David Perpetuini, Antonio Maria Chiarelli, Giulio Gualdi, Paolo Amerio, and Arcangelo Merla. 2021. "Convolutional Neural Networks for Differential Diagnosis of Raynaud’s Phenomenon Based on Hands Thermal Patterns" Applied Sciences 11, no. 8: 3614. https://doi.org/10.3390/app11083614
APA StyleFilippini, C., Cardone, D., Perpetuini, D., Chiarelli, A. M., Gualdi, G., Amerio, P., & Merla, A. (2021). Convolutional Neural Networks for Differential Diagnosis of Raynaud’s Phenomenon Based on Hands Thermal Patterns. Applied Sciences, 11(8), 3614. https://doi.org/10.3390/app11083614










