Abstract
The objective of this work was to investigate the advantages of using dichloro bisphenol A-glycidyl methacrylate (dCl-BisGMA) as a potential matrix for dental resin composites. A series of model composites containing 65 wt% resin (urethane dimethacrylate/triethylene glycol dimethacrylate/BisGMA as 1:3:1) and 35 wt% silanated silica were prepared. Thus, BisGMA was replaced by dCl-BisGMA as 0, 25, 50, and 100 wt% to obtain UTBC0, UTBC25, UTBC50, and UTBC100, respectively. The composites’ rheological properties, degree of double-bond conversion (DC), water sorption (WSP), and water solubility (WSL) were examined. The data revealed a statistically significant reduction in the complex viscosity of composites containing dCl-BisGMA, compared with UTBC0. No significant differences between DCs were detected (p < 0.05). A significant enhancement in the reduction of the dCl-BisGMA composite WSP was also detected, and conversely, WSL was increased. Although the viscosity, DC, and WSP characters were enhanced, a WSL increase is an undesirable development. However, WSL is supposedly caused by cyclization of small flexible chains, which is more likely to occur in the presence of hydrophobic monomers such as dCl-BisGMA and more prone to leaching than are crosslinked networks. We concluded that dCl-BisGMA is a monomer that could potentially be used as an alternative or in combination with traditional monomers, including BisGMA, in resin-based dental composites, and it deserves further investigation.
1. Introduction
Since the early 1960s, 2,2-bis[p-(20-hydroxy-30-methacryloxypropoxy) phenyl] propane, known as bisphenol A glycidyl methacrylate (BisGMA) or “Bowen’s resin,” has been the most widely used resin in the field of resin-based dental materials, including restorative composites, adhesives, and prophylactic sealants. Structurally, BisGMA has a high molecular weight of 512.6 g/mol with two terminal methacrylate functionalities, a stiff central core of two phenyl rings, and two hydroxyl groups. Such a structure facilitates a strong intermolecular interaction through hydrogen bonding and π–π interactions, leading to the extremely high viscosity of the monomer (910 Pa·s) [1,2]. The advantage of using BisGMA in dental composites is its low volatility and diffusivity into tissues (i.e., lesser toxicity), low volumetric shrinkage, rapid hardening, and aesthetic properties [3]. However, its high viscosity raises handling issues, decreases the degree of vinyl double-bond conversion (DC) upon curing, and restricts the addition of an adequate amount of reinforcing materials required for better mechanical properties of the restoratives [1,4]. To deal with viscosity issues, the incorporation of reactive, low-viscosity dimethacrylate monomers (diluents) within the resin matrices of dental composites is necessary. The most commonly used diluent is triethylene glycol dimethacrylate (TEGDMA); however, some other dimethacrylates, including ethoxylated bisphenol A dimethacrylate (BisEMA), urethane dimethacrylate (UDMA), and ethylene glycol dimethacrylate (EGDMA), are used in combination with BisGMA [4]. Owing to the linear structure associated with triethylene oxide spacers, the addition of TEGDMA commonly enhances the hydrophilicity characteristics of the resins and increases water sorption and microcavities, which further affect the longevity of the composites.
Indeed, the viscosity issue of BisGMA necessitates material development; thus, BisGMA structural modification including the replacement of its OH groups with low hydrophilic substituents may be one solution. In this context, various derivatives of BisGMA have been synthesized by different researchers, in which the OH functional groups were partially or fully replaced with targeted substituents, including alkyls, aryls, ethers, and esters with small or bulky groups [3,5,6,7,8,9,10,11,12]. Regardless of the type of substituent used, the viscosity of BisGMA was greatly reduced after modification, confirming that H-bonding is the major cause of its high viscosity character [2,6,10,12,13]. Adversely, substitution may affect the hygroscopic, mechanical, DC, and some other critical physicochemical properties of the composites. For example, modification of the BisGMA structure with less hydrophilic, small-sized substituents, such as methyl [14], methoxy [6], and chloride [2], resulted in low viscous analogs (η = 8.4, 3.6, and 7.2 Pa·s, respectively, at ~22–25 °C). The incorporation of modified-BisGMA resin in dental composites showed enhanced properties, e.g., lower volumetric shrinkage, better mechanical properties, and lower water uptake [6,14]. However, regulation of the degree of OH substitution may result in tailored properties [8], e.g., when diluents were not used.
Recently, Al-Odayni et al. synthesized a chlorinated analog of BisGMA (dichloro-BisGMA; termed dCl-BisGMA) with lower viscosity (7.22 Pa·s), compared with that of BisGMA (910 Pa·s) [2]. Then, dCl-BisGMA was incorporated within unfilled resins, and its properties, including DC, water sorption (WSP), water solubility (WSL), and biocompatibility were evaluated. A structural modification of BisGMA with one chlorine (termed mCl-BisGMA) was also synthesized with a viscosity value of 8.3 Pa·s at 25 °C [15]. The substantial reduction in the viscosity values of mCl- and dCl-BisGMA (about 65- and 126-fold, respectively, lower than BisGMA) may indicate their potential use as alternatives for BisGMA in the dental resin matrix. The overall remarkable enhancements in the BisGMA properties after modification with mono-Cl and di-Cl are of great importance in the field of dental materials and thus deserve further investigation, e.g., to explore its potential as a resin matrix for filled resin-based dental composites.
The aim of this study was to investigate the effect of replacing BisGMA by dCl-BisGMA on the properties (rheological, DC, WSP, and WSL) of experimental dental composites. Therefore, a series of dental composites containing UDMA, TEGDMA, and BisGMA/dCl-BisGMA as the base resin (35 wt%) and organic fillers (silanated Aerosil fumed silica, SA200) were prepared, in which the amount of BisGMA was gradually replaced by dCl-BisGMA.
2. Materials and Methods
2.1. Materials
The monomeric materials BisGMA (>98%), TEGDMA (>95%), and UDMA (>97%), as well as the photoinitiator camphorquinone (CQ; 97%), the curing accelerator 2-(N,N-dimethylamino)ethyl methacrylate (DMAEMA; 98%), the coupling agent γ-methacryloxypropyltrimethoxysilane (γ-MPS; 98%), and the chlorine source carbon tetrachloride (CCl4; >99.8%), were purchased from Sigma–Aldrich (Taufkirchen, Germany). Appel reaction catalyst triphenylphosphine (Ph3P; >98%) was obtained from Cica-Reagent (Kanto Chemical, Tokyo, Japan). The solvents n-hexane (n-H; >97%), ethyl acetate (EA; 99%), and dichloromethane (DCM; 99.6%) were procured by Fisher Scientific (Loughborough, UK). Th model filler Aerosil-200 fumed silica (A200) was purchased from Evonik (Evonik Industries, Essen, Germany) and was modified with γ-MPS. All chemicals were used as received unless stated otherwise.
2.2. Methods
2.2.1. Synthesis of dCl-BisGMA
dCl-BisGMA was synthesized using a previously described method [2] with slight modification. Thus, a solution of BisGMA in DCM (0.3 g/mL) was prepared with stirring and purged for 10 min with N2 gas. To this solution, an equimolar amount of CCl4 (0.039 mol) was added and homogenized with stirring for 15 min, and thereafter, 18 g of the catalyst Ph3P was added. The reaction was refluxed for 2 h and cooled to room temperature, and then to facilitate precipitation of the Ph3P-oxide by product, an adequate amount of an EA/n-H (1:1 v/v) solvent mixture was added and left to settle in a dark environment overnight. Then, the supernatant was decanted, and the solvent was reduced using a rotary evaporator at 30 °C. The obtained product was purified as described elsewhere [2]. After purification, a low viscosity, light-yellow resin with an isolated yield of 64% was achieved, which was kept in a dark container at about 8°C until use.
2.2.2. Modification of Silica Nanoparticles
The hydrophilic, amorphous fumed silica (A200) with an average particle size of 12 nm, 200 ± 25 m2/g Brunauer–Emmett–Teller (BET) specific surface area, ≤1.5 mass loss on drying, and 3.7–4.5 pH [16] was modified with 10 wt% of organosilane (γ-MPS), following previously described methods [16,17,18,19]. A200 is a commercially available, low-cost, and modifiable silica, with applicable properties for use in dental composites [20]. Owing to its high surface area, 35% was the highest applicable load. However, nanofillers typically need a high amount of resins for complete surface wetting.
2.2.3. Preparation of Resin Composites
A series of dental resin composites containing 65 wt% organic-based matrix (of which 20 wt% is BisGMA) and 35 wt% inorganic fillers were prepared. The amount of BisGMA in the composite was replaced sequentially with 0, 25, 50, and 100 wt% dCl-BisGMA (denoted as UTBCx, in which x is the amount (%) of dCl-BisGMA with respect to the total BisGMA and dCl-BisGMA), as shown in Table 1. Composites were prepared as follows: the UDMA/TEGDMA blend was first prepared, and then the predetermined amounts of BisGMA and dCl-BisGMA were added. The initiator was completely dissolved in the monomer mixture before the addition of the SA200 filler. Next, composites were manually homogenized using a stainless-steel spatula, sonicated for 3 min, and degassed under reduced pressure for 10 min. Then, the composites were mechanically mixed, three times at 3000 rpm for 1 min each with 2 min rest in between, in a dual asymmetric centrifugal mixing system (Speed Mixer TM DAC 150 FVZ; Hauschild &Co., Hamm, Germany). To assure similar conditions, one batch of each resin was prepared and subsequently used throughout all the experiments.

Table 1.
Composition of the investigated experimental resin composites.
2.3. Analytical Experiments
2.3.1. Rheological Test
The rheological properties of the UTBC model composites were measured on an MCR 72 rheometer (Anton Paar, Graz, Austria) using a 25 mm diameter parallel-plate geometry and a measuring gap of 0.25 mm. The experimental test was performed at the oscillatory shear mode over a frequency sweep range of 0.1 to 100 (ω, rad/s) at 25 °C. At least four replicate measurements (n = 4) were made per sample and averaged.
2.3.2. Degree of Conversion
The degree of DC of UTBCs due to photo-irradiation was determined using the Fourier transform infrared–attenuated total reflection (FTIR–ATR) method. Samples were pressed in stainless-steel disks (5 mm diameter and 2 mm thickness, n = 4), and their FTIR spectra were recorded (denoted as uncured) using a Nicolet iS10 FTIR spectrometer (Thermo Scientific, Madison, WI, USA). Subsequently, the specimens were covered with plastic strips and glass slides, irradiated for 40 s using a light-curing unit (3M ESPE Elipar S10, LED curing light; wavelength of 430–480 nm; the intensity of approximately 1200 mW∙cm−2), and then their FTIR spectra were analyzed (cured). By comparison of the peak areas of vinylic C=C at 1638 cm−1 before and after curing with respect to the unaffected aromatic C=C band at 1608 cm−1 as an internal reference, the DC can be quantified, as in Equation (1). Generally, the FTIR peak integration (the area, A) is proportional to its mole fraction in the spectra and, when bonds are of the same properties, their mole ratios can be adequately compared.
2.3.3. Water Sorption and Water Solubility
To assess the swelling behavior of the prepared composites in oral fluid, which resulted in water sorption (WSP) and water solubility (WSL), experiments were conducted in distilled water to simulate the oral environment and in accordance with ISO 4049 [21]. The specimens were cast in fabricated stainless-steel disk-shaped molds that were 15 mm in diameter and with 2 mm thickness (n = 5). They were photo-irradiated from both sides in four overlapped areas for 40 s each. After disk preparations, samples were put in a dry silica-containing desiccator at 37 °C ± 1 °C and weighed with a Mettler Toledo analytical balance with an accuracy of 0.1 mg every 24 h. Prior to each recording, samples were cooled in a new desiccator at room temperature for around 1 h. Drying–weighing processes were repeated until a constant weight was obtained (dry specimen, m1). Specimens were then transferred into a 30 mL closed vial containing 15 mL distilled water and maintained at 37 °C ± 1 °C. Every 24 h, samples were removed from the water, carefully swabbed, waved in air for 20 s, weighed, and returned to the water; this process was repeated until no change in the disk weight was observed (swelled specimen, m2). Finally, disks were removed from the water and dried again as above until a constant weight was obtained (m3). The WSP and WSL can be calculated as in Equations (2) and (3), respectively.
2.4. Statistical Analysis
Data were analyzed using SPSS 21. Variables were presented as the mean ± standard deviation (SD). Analysis of variance and repeated-measures analysis of variance were used to compare four groups and three cycles with Bonferroni correction as post hoc test. A p-value of <0.05 was considered significant.
3. Results and Discussion
3.1. dCl-BisGMA Properties
The chemical structure of the monomer under investigation, dCl-BisGMA, was reported elsewhere [2]. The substitution generally occurs through a catalytic Appel reaction with an SN2 mechanism, resulting in an inverted configuration [15,22,23] (Figure 1). Al-Odayni et al. [2] reported some of the monomer physicochemical properties, including viscosity and biocompatibility. Viscosity was found to be 126-fold lower than that of BisGMA at 22.1 °C (i.e., 909.9 and 7.2 Pa·s for BisGMA and dCl-BisGMA, respectively). The dCl-BisGMA biocompatibility was also evaluated within resin mixtures of TEGDMA/BisGMA against immortalized human bone marrow stromal cells, using dCl-BisGMA-free resin as a reference. In this study, dCl-BisGMA was incorporated in experimental dental composites. Then, the composite physicochemical properties, including complex viscosity, DC, WSP, WSL, were investigated.
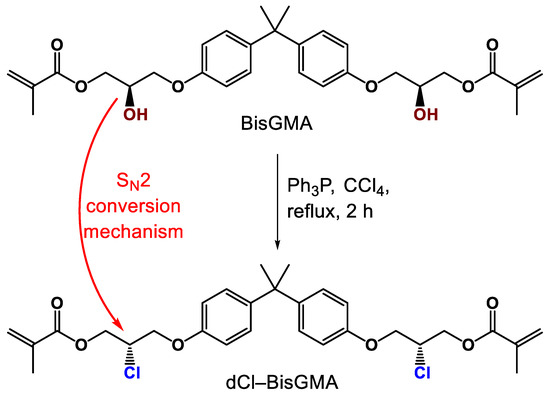
Figure 1.
Synthesis of the dCl-BisGMA monomer.
3.2. Rheological Properties of UTBC Composites
Figure 2 shows the complex viscosity (η*, kPa·s) of the tested composites as a function of the frequency (ω, rad/s), and Table 2 presents its evolution at 1.0 rad/s. It is obvious that all the tested model composites were viscoelastic, non-Newtonian materials and showed a shear-thinning behavior, i.e., as the shear rate increased, the η* value decreased dramatically. Through the series of UTBCs, the η* value decreased, whereas the amount of dCl-BisGMA increased. The shear-thinning behavior with a frequency increase was most likely due to rearrangement of the filler particles [24]. Furthermore, shearing may also cause disentanglement of matrix molecules, particularly at a higher applied force [25], thus weakening the physical interaction between the components leading to an increase in the flowability of the composite. Generally, the viscosity of a resin composite is influenced by the type and amount of both the organic matrix and the reinforcing inorganic materials, as well as the surrounding conditions, including temperature. Since the preparation analysis conditions of all the tested composites were similar, except for the amount of BisGMA/dCl-BisGMA, the η* variation is due to the respective chemical properties of each of these two monomers. When dCl-BisGMA increases, η* decreases. This was predominantly due to the low viscosity of dCl-BisGMA (η = 7.2 Pa·s), compared with that of BisGMA (η = 910 Pa∙s), which is the property that is reflected in the η* values of the studied experimental composites.
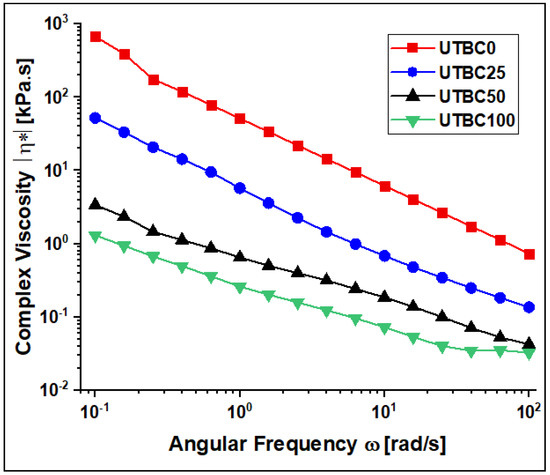
Figure 2.
The complex viscosity (η*) of the UTBC resin composites at 25 °C.

Table 2.
Degree of conversion (DC), complex viscosity (η*, at 25 °C), water sorption (WSP), and solubility (WSL) measured over three cycles of sorption–desorption.
The structure–property relationships of the monomers, including the symmetricity, hydrophobicity–hydrophilicity, and H-bonding, was reflected in the viscosity value of the composites [26]. A previous study reported that the viscosity of BisGMA reduces when the −OH functional group is replaced by chlorine in dCl-BisGMA [2]. The low viscosity of dCl-BisGMA was due to the absence of the −OH groups and reduced H-bonding that is largely responsible for the strong intermolecular interactions. For instance, the complex viscosity (η*) values were decreased from 47.29 to 0.30 (kPa·s) for dCl-BisGMA-free (UTBC0) and BisGMA-free (UTBC100) samples, respectively. Indeed, the reduced viscosity of the samples containing dCl-BisGMA in the prepared model composites was also reflected in the other properties, including the DC, water uptake (WSP), and water solubility (WSL).
Figure 3 illustrates the storage (G’) and loss (G”) moduli of the composites under investigation. In all the cases and at any frequency, the value of G’ is greater than that of G” of the same composite, reflecting that the elastic nature is prevailing and composites are stable even at higher frequency [19]. It is well known that G’ and G” are very sensitive to the variation in the molecular structure of matrix resins and the structural network of the composite [27,28]. The decrease in the moduli values with the dCl-BisGMA increase may imply there is less interaction between the components, reflecting the effect of the resin type. As the hydrophilicity of the matrix decreases with the addition of dCl-BisGMA, the physical interaction caused by H-bonding decreased, and thus, the elasticity increased. Matrices containing BisGMA are more tightly and closely packed than dCl-BisGMA-containing ones, i.e., G’ is higher for UTBC0 than for the others. Moreover, the results indicated frequency-dependent moduli of UTBC50 and UTBC100, i.e., G’ and G” increased with frequency and increased in the whole sweeping frequency range, revealing less structural networking when the dCl-BisGMA amount exceeded that of BisGMA [29].
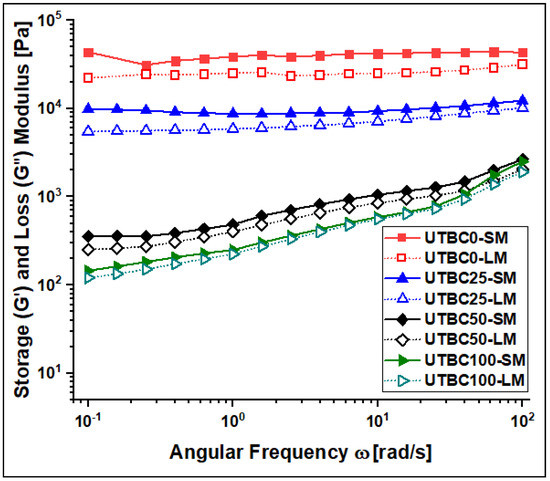
Figure 3.
Storage modulus (G’, solid markers) and loss modulus (G”, open markers) of UTBC resin composites at 25 °C.
3.3. DC Analysis
The DC of UTBCs due to photo-irradiation was assessed using FTIR. The peak area of the aliphatic curable vinyl group at about 1638 cm−1 (A1638) was compared with that of the internal standard aromatic C=C peak (A1608) before and after light curing (Equation (1)). The results of DC measurements were reported as the average and standard deviation (SD) of four independent replicates of each resin composite (Figure 4 and Table 2). The DC was higher for composites containing dCl-BisGMA than for the reference dCl-BisGMA-free one. However, the difference was statistically insignificant. The slight increases in DC of dCl-BisGMA to 79.01% for UTBC100, compared with 74.18% of UTBC0, is likely due to the lower viscosity of dCl-BisGMA, allowing better mobility of the particles (molecules and radicals) and thus facilitating higher conversion. Generally, incomplete vinylic DC is due to rapid solidification and gelation that may hamper radical mobility. Since the experimental conditions are the same, and dCl-BisGMA has a structure similar to that of BisGMA, except for the secondary hydroxyl groups, the altered properties of UTBC100 could be attributed to the absence of −OH and the presence of −Cl. Furthermore, dCl-BisGMA allows enhancement of the hydrophobicity characteristic and thus reduced the intermolecular interaction, leading to lower viscosity compared to BisGMA. According to literature [30,31,32], many factors can influence the DC of composites, including resin matrix composition, filler type and geometry, and their ratios and curing conditions. The effect of filler loading and degree of salinization on the DC has also been investigated [30], reporting lower conversion with increasing filler percentage but no visible differences between the DC of silane treated and untreated fillers, whereas DC decreases with silane amount increase. Compared with filled composites, the DC of the unfilled resin matrix is higher due to mobility restriction caused by fillers [33]. This negative impact of filler on the DC is independent of whether or not the filler was silanized [30]. In this regard, the DC of unfilled composites containing dCl-BisGMA was found to increase with dCl-BisGMA amount increase [2]. For instance, the DC of resins containing 70 wt% of BisGMA or dCl-BisGMA (and 30 wt% TEGDMA) were 62.0 and 70.6%, respectively, which is reasonably due to the lower viscosity of dCl-BisGMA, compared with that of BisGMA. In this study, the DC of UTBCs was in the range of 74.18–79.01% and increased with dCl-BisGMA amount increased. However, the apparent higher values of DC of UTBCs are due to the high amount used of diluents TEGDMA and UDMA (50% and 30% with respect to the total resin weight, respectively). Generally, the percentage of DC for adequate clinical performance has not yet been determined [34]. Nevertheless, for both filled and unfilled dCl-BisGMA-based composites, the obtained DC was above the minimum acceptable values for clinical use (>55%) [35]. The DC of dental composite commonly varies between 40% and 75% [36]; however, other ranges were also reported [37,38] with higher values closer to 85% [39]. The difference in the DC of various systems can be due to several reasons, including the variation in composition, viscosity, curing conditions, curing times, etc. In this work, the resin matrix of UTBC0 consists of conventional monomers (TEGDMA, TEGDMA, and BisGMA) commonly used in dental composites. Thus, this dCl-BisGMA-free composite was used as a control during the evaluation of the tested properties of dCl-BisGMA containing composites (UTBC25–UTBC100).
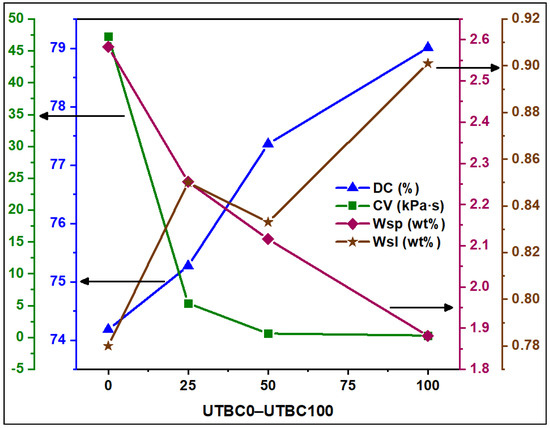
Figure 4.
Degree of conversion (DC), complex viscosity (CV) at 1.0 rad/s angular frequency, water uptake (WSP), and water solubility (WSL) of the first cycle of sorption–-desorption versus the dCl-BisGMA amount in the investigated composites (UTBC0, UTBC25, UTBC50, and UTBC100).
3.4. Water Sorption and Solubility
The effect of replacing BisGMA by dCl-BisGMA on water uptake (WSP) and solubility (WSL) of resin-based dental composites was also investigated. The results are given as a percentage with respect to disk weight ((wt/wt)%; three cycles). As given in Table 2, the WSP of UTBC100 was significantly different from that of UTBC0 (p < 0.05), indicating great enhancement, even for the three cycles of the sorption–desorption process. However, a significant increase over the three cycles for both the control (UTBC0) and samples (UTBC25 to UTBC100) was observed, apparently due to void generation as a result of soluble materials exclusion. It is clear that increasing dCl-BisGMA decreases WSP but increases WSL. The value of WSP is generally associated with DC and the network structure, specifically the hydrophilicity–hydrophobicity characteristics, three-dimensional structure, and free volume [40]. Here, WSP decreased with increasing DC, as expected, owing to the low hydrophilicity of dCl-BisGMA.
Conversely, although WSL increased with the dCl-amount increase, WSP decreased. However, WSL depended on both the amount of water absorbed and the amount and hydrophilicity of the leachable materials, as well as the microstructure of the polymeric network. A previous study on the effects of dimethacrylate monomer hydrophilicity reported that the hydrophobic monomers are more flexible than hydrophilic ones and contribute to a higher free volume in the polymeric network, despite the value of DC. Moreover, flexible monomers tend to undergo primary and secondary cyclization, which may cause gelation, forming short chains, and leachable species (monomers and oligomers) that contribute to the increased solubility of the final composite [41]. Thus, the increase in WSL with increasing dCl-BisGMA concentrations, despite increasing WSP and DC, may be attributed to the formation of small fragments upon polymerization as a result of the cyclization of more flexible monomers (i.e., dCl-BisGMA and TEGDMA) [40]. This was further supported by the lower WSL for UTBC0, compared with that for UTBC100, despite the lower DC and WSP values. The WSP and WSL behavior, evaluated after three cycles of the sorption–desorption process, as shown in Table 2 and Figure 5, revealed a slight increase in WSP and decrease in WSL from cycle 1 to cycle 3. However, WSL decreased to near zero after cycle 2, indicating that the release of leachable species ceased. The values of WSP and WSL may also be influenced by filler size in the composite. In this regard, previous studies highlighted higher WSP and WSL of nanocomposites compared with hybrid ones [42,43]. This behavior could be explained on the basis of the high surface area of the nanofillers, making them more prone to ion leaching and hydrolysis of the silane coupling agent.
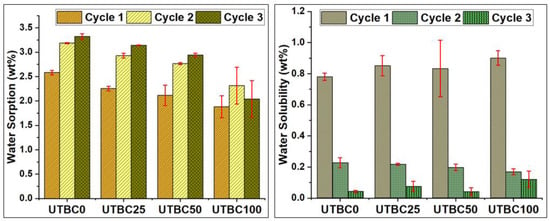
Figure 5.
Water upatake and water solubility of UTBC0–UTBC100 after three cycles of sorption–desorption processes.
4. Conclusions
Low-viscosity BisGMA derivatives are of great importance and could offer solutions to the disadvantage associated with its high viscosity toward property enhancement of the current resin-based dental composites. The present study was designed to evaluate the advantage of replacing BisGMA, owing to its low viscosity and low hydrophilicity, with an analog dCl-BisGMA monomer in resin-based dental composites. The study confirmed that dCl-BisGMA is a promising alternative monomer for BisGMA. The relevant supporting properties including complex viscosity, DC, and WSP were enhanced when BisGMA is replaced, even partially, by dCl-BisGMA in the matrix. The rheological investigations revealed a pseudoplastic, non-Newtonian, and shear thinning behavior, demonstrating a disproportional decrease in complex viscosity with increasing frequency at 25 °C. At all frequencies, the complex viscosity of composites containing dCl-BisGMA was significantly lower than the control (UTBC0). DC showed a slight, statically insignificant increase with the increase of dCl-BisGMA, e.g., from 74.18 ± 2.87 to 79.01 ± 4.61 for UTBC0 and UTBC100, respectively. The WSP was significantly enhanced, reduced almost to half when BisGMA was totally replaced by dCl-BisGMA (i.e., from 2.58% to 1.88%, respectively); however, an increase in the WSL values was observed, which is due to the cyclization process that occurred on the microstructure level as a result of the differences in the hydrophilicity of the polymeric matrix, which decreases as dCl-BisGMA increases. The overall findings will be of interest for the development of resin-based composites for various applications including dental materials, providing insight for future composite property enhancements. Therefore, this new material deserves further investigation for complete characterization as a potential matrix.
Author Contributions
Conceptualization, A.-B.A.-O. and A.A.; data curation, A.-B.A.-O.; formal analysis, A.-B.A.-O., R.K. and W.S.S.; funding acquisition, A.A.; investigation, A.-B.A.-O., R.K. and K.A.; methodology, A.-B.A.-O. and T.A.; project administration, A.A. and A.A.-K.; supervision, A.A. and A.A.-K.; visualization, A.-B.A.-O. and W.S.S.; writing—original draft preparation, A.-B.A.-O.; writing—review and editing, A.-B.A.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs, Engineer Abdullah Bugshan research chair for Dental and Oral Rehabilitation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Odayni, A.-B.; Alfotawi, R.; Khan, R.; Saeed, W.S.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, M. Synthesis and characterization of novel dimethacrylates of different chain lengths as possible dental resins. Dent. Mater. 2010, 26, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, L.; Kim, C.; Cho, B.; Kim, O. Characteristics of novel dental composites containing 2, 2-bis [4-(2-methoxy-3-methacryloyloxy propoxy) phenyl] propane as a base resin. Biomacromolecules 2006, 7, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, T.; Melinte, V.; Jitaru, F.; Aldea, H.; Buruiana, E.C. Photopolymerization experiments and properties of some urethane/urea methacrylates tested in dental composites. J. Compos. Mater. 2012, 46, 371–382. [Google Scholar] [CrossRef]
- Melinte, V.; Buruiana, T.; Chibac, A.; Mares, M.; Aldea, H.; Buruiana, E.C. New acid BisGMA analogs for dental adhesive applications with antimicrobial activity. Dent. Mater. 2016, 32, e314–e326. [Google Scholar] [CrossRef]
- Rivera-Torres, F.; Vera-Graziano, R. Effects of water on the long-term properties of Bis-GMA and silylated-(Bis-GMA) polymers. J. Appl. Polym. Sci. 2008, 107, 1169–1178. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, M.; Bao, S.; Liu, F.; Jiang, X.; Zhu, M. Synthesis of two Bis-GMA derivates with different size substituents as potential monomer to reduce the polymerization shrinkage of dental restorative composites. J. Mater. Sci. Res. 2013, 2, 12. [Google Scholar] [CrossRef][Green Version]
- Jeon, M.; Yoo, S.; Kim, J.; Kim, C.; Cho, B. Dental restorative composites fabricated from a novel organic matrix without an additional diluent. Biomacromolecules 2007, 8, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Wolska, J.; Walkowiak-Kulikowska, J.; Koroniak, H.; Sun, Y. Fluorinated bis-GMA as potential monomers for dental restorative composite materials. Eur. Polym. J. 2017, 90, 334–343. [Google Scholar] [CrossRef]
- Al-Odayni, A.-B.; Saeed, W.S.; Ahmed, A.Y.B.H.; Alrahlah, A.; Al-Kahtani, A.; Aouak, T. New Monomer Based on Eugenol Methacrylate, Synthesis, Polymerization and Copolymerization with Methyl Methacrylate–Characterization and Thermal Properties. Polymers 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.G.; Osorio, R.; Toledano, M.; Cabrerizo-Vílchez, M.A.; Nunes, T.G.; Kalachandra, S. Novel light-cured resins and composites with improved physicochemical properties. Dent. Mater. 2007, 23, 1189–1198. [Google Scholar] [CrossRef]
- Alrahlah, A.; Al-Odayni, A.-B.; Al-Mutairi, H.F.; Almousa, B.M.; Alsubaie, F.S.; Khan, R.; Saeed, W.S. A Low-Viscosity BisGMA Derivative for Resin Composites: Synthesis, Characterization, and Evaluation of Its Rheological Properties. Materials 2021, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Atai, M.; Pahlavan, A.; Moin, N. Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent. Mater. 2012, 28, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kleczewska, J.; Bieliński, D.; Nowak, J.; Sokołowski, J.; Łukomska-Szymańska, M. Dental composites based on dimethacrylate resins reinforced by nanoparticulate silica. Polym. Polym. Compos. 2016, 24, 411–418. [Google Scholar] [CrossRef]
- Zanchi, C.H.; Ogliari, F.A.; e Silva, R.M.; Lund, R.G.; Machado, H.H.; Prati, C.; Carreño, N.L.V.; Piva, E. Effect of the silane concentration on the selected properties of an experimental microfilled composite resin. Appl. Adhes. Sci. 2015, 3, 27. [Google Scholar] [CrossRef]
- Alrahlah, A.; Khan, R.; Al-Odayni, A.-B.; Saeed, W.S.; Bautista, L.S.; Vohra, F. Evaluation of Synergic Potential of rGO/SiO2 as Hybrid Filler for BisGMA/TEGDMA Dental Composites. Polymers 2020, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, P.; Mirabedini, S.M.; Esfandeh, M. APS-silane modification of silica nanoparticles: Effect of treatment’s variables on the grafting content and colloidal stability of the nanoparticles. J. Coat. Technol. Res. 2014, 11, 651–660. [Google Scholar] [CrossRef]
- Standards, I. ISO 4049 Dentistry—Polymer based restorative materials. Int. Organ. Stand. 2009, 4, 1–28. [Google Scholar]
- Appel, R. Tertiary Phosphane/Tetrachloromethane, a Versatile Reagent for Chlorination, Dehydration, and P N Linkage. Angew. Chem. Int. Ed. Engl. 1975, 14, 801–811. [Google Scholar] [CrossRef]
- Castro, B.R. Replacement of alcoholic hydroxyl groups by halogens and other nucleophiles via oxyphosphonium intermediates. Org. React. 2004, 29, 1–162. [Google Scholar]
- Meier, R.; Kirdar, C.; Rudolph, N.; Zaremba, S.; Drechsler, K. Investigation of the shear thinning behavior of epoxy resins for utilization in vibration assisted liquid composite molding processes. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; pp. 458–462. [Google Scholar]
- Vlachopoulos, J.; Strutt, D. The Role of Rheology in Polymer Extrusion. In Proceedings of the New Technology for Extrusion Conference; pp. 20–21.
- Lee, I.-B.; Son, H.-H.; Um, C.-M. Rheologic properties of flowable, conventional hybrid, and condensable composite resins. Dent. Mater. 2003, 19, 298–307. [Google Scholar] [CrossRef]
- Haddadi, H.; Famili, M.H.N.; Nazokdast, E.; Moradi, S. Chemorheological analyses of a reaction injection moulding polyurethane formulation. Iran. Polym. J. 2006, 15, 967–977. [Google Scholar]
- Kumar, S.; Zindani, D.; Bhowmik, S. Investigation of mechanical and viscoelastic properties of flax-and ramie-reinforced green composites for orthopedic implants. J. Mater. Eng. Perform. 2020, 29, 3161–3171. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Yadav, A.; Guo, Z. Rheological behaviors and electrical conductivity of epoxy resin nanocomposites suspended with in-situ stabilized carbon nanofibers. Polymer 2010, 51, 2643–2651. [Google Scholar] [CrossRef]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Turssi, C.; Ferracane, J.; Vogel, K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials 2005, 26, 4932–4937. [Google Scholar] [CrossRef]
- Rastelli, A.N.; Jacomassi, D.P.; Faloni, A.P.S.; Queiroz, T.P.; Rojas, S.S.; Bernardi, M.I.B.; Bagnato, V.S.; Hernandes, A.C. The filler content of the dental composite resins and their influence on different properties. Microsc. Res. Tech. 2012, 75, 758–765. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Krasowski, M.; Sokolowski, J. The influence of filler amount on selected properties of new experimental resin dental composite. Open Chem. 2018, 16, 905–911. [Google Scholar] [CrossRef]
- Lempel, E.; Czibulya, Z.; Kovács, B.; Szalma, J.; Tóth, Á.; Kunsági-Máté, S.; Varga, Z.; Böddi, K. Degree of conversion and BisGMA, TEGDMA, UDMA elution from flowable bulk fill composites. Int. J. Mol. Sci. 2016, 17, 732. [Google Scholar] [CrossRef] [PubMed]
- Silikas, N.; Eliades, G.; Watts, D. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent. Mater. 2000, 16, 292–296. [Google Scholar] [CrossRef]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Galvão, M.R.; Caldas, S.G.F.R.; Bagnato, V.S.; de Souza Rastelli, A.N.; de Andrade, M.F. Evaluation of degree of conversion and hardness of dental composites photo-activated with different light guide tips. Eur. J. Dent. 2013, 7, 86. [Google Scholar] [PubMed]
- Pałka, K.; Kleczewska, J.; Sasimowski, E.; Belcarz, A.; Przekora, A. Improved Fracture Toughness and Conversion Degree of Resin-Based Dental Composites after Modification with Liquid Rubber. Materials 2020, 13, 2704. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Young, J.S.; Kannurpatti, A.R.; Bowman, C.N. Effect of comonomer concentration and functionality on photopolymerization rates, mechanical properties and heterogeneity of the polymer. Macromol. Chem. Phys. 1998, 199, 1043–1049. [Google Scholar] [CrossRef]
- Silva, E.M.d.; Almeida, G.S.; Poskus, L.T.; Guimarães, J.G.A. Relationship between the degree of conversion, solubility and salivary sorption of a hybrid and a nanofilled resin composite. J. Appl. Oral Sci. 2008, 16, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sangi, L. Water sorption, solubility, and resultant change in strength among three resin-based dental composites. J. Investig. Clin. Dent. 2014, 5, 144–150. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).