Featured Application
The results revealed that a piezoelectric low-voltage cold atmospheric plasma generator effectively inactivates foodborne pathogenic bacteria on stainless steel surfaces. This intervention shows great potential to be used in the food industry for the disinfection of equipment and processing surfaces.
Abstract
Cold atmospheric pressure plasma (CAP) is a novel non-thermal technology that is gaining increasing importance as a decontamination method. Stainless steel is a widespread food contact surface used in food-processing environments. In this study, for the first time, a low-voltage piezoelectric CAP device that uses ambient air was assessed for its antimicrobial efficiency against Salmonella and Listeria monocytogenes. These inoculated on stainless steel at different exposure times (0–300 s), two different distances (10 and 20 mm), and two different cleanliness levels (clean and protein-soiled). Two inactivation models were compared to study the inactivation kinetics of the pathogens. The results showed that CAP treatment effectively reduced L. monocytogenes and Salmonella levels. The Weibull + tail model showed better goodness of fit than the Weibull model. Protein-soiled coupons showed a protective effect to cold plasma inactivation achieving lower reductions compared to clean stainless-steel coupons for both L. monocytogenes and Salmonella. Longer distances from the plasma source decreased the decontamination efficiency of CAP; however, the difference in pathogen reduction was less pronounced at longer exposure times. This study demonstrates the capacity of a low-voltage piezoelectric CAP device to effectively reduce the levels of both foodborne pathogens on stainless-steel surfaces and the potential to adopt this technology by the food industry as a disinfection process of surfaces to reduce cross-contamination and thus increase safety.
1. Introduction
Safety is one of the main challenges the food industry is facing. Foodborne diseases with a bacterial origin, including Salmonella, Campylobacter, Enterohaemorrhagic Escherichia coli, Listeria, and Vibrio cholera, have an important socioeconomic impact. According to the World Health Organization (2015), it is estimated that 600 million cases and 420,000 deaths are caused by unsafe food [1]. Foodborne disease may cause severe symptoms and can be life-threatening if not treated properly. In particular, L. monocytogenes and Salmonella are foodborne pathogens of great importance due to their high prevalence and fatality rates. Salmonellosis caused by S. Typhimurium was reported to have a high prevalence of 21.2 per 100,000 people and a fatality rate of 0.24%, whereas Listeriosis, caused mainly by L. monocytogenes, has a lower prevalence (0.46/100,000 people) but a high fatality rate of 17.7% [2].
One of the common routes of food contamination is the direct contact of foodstuff with contaminated food-processing surfaces. It has been demonstrated that conventional procedures for disinfection and sanitization are not always enough to eliminate foodborne pathogens from processing surfaces, especially when biofilms are allowed to form [3,4,5,6]. Cross-contamination represents an important food safety issue; therefore, the food industry is looking for novel methods that can be incorporated into automated food-processing environments to tackle it.
Different novel methods are being tested for food contact surface decontamination, such as UV light, dry ice cleaning, ultrasound, and ozone, each one with its own advantages and limitations [7,8]. Cold atmospheric plasma (CAP) is a non-thermal decontamination technology suitable for treating material or foodstuffs that are sensitive to heat and other disinfection methods [9]. CAP is considered the fourth state of matter and can be generated by applying an electric field to air, nitrogen, or other working gases. The air molecules are then excited, and reactive species from oxygen and nitrogen (RONS) with antibacterial activity are generated, including NO, NO2, CO, CO2, H2O2, and O3 [10]. RONS act independently or in synergy through different mechanisms when in contact with the cell, including oxidative damage to the membrane, structural proteins, and DNA. Altogether, these mechanisms lead to cell-membrane disruption and ultimately to cell inactivation [11,12].
Different cold plasma technology sources have been studied for the inactivation capacity against foodborne pathogens, including dielectric barrier discharge (DBD), corona discharges, plasma jet (CDPJ), and glow discharge [13]. These technologies have been applied in the food industry for disinfection of processing equipment, foodstuff, and packaging materials; for instance, disinfection of milking machines [14], a DBD device for the sterilization of post-packaging ready-to-eat food [15], and grain for food and seed-purposed disinfection by a negative high-voltage corona discharge [16]. Also, DBD systems fed with different gases have been studied against spore-forming bacteria on wheat [17], and against Salmonella, E. coli, and L. monocytogenes, on glass slides, in-shell pecan, and on cherry tomato surfaces [18], with promising results.
CAP has also been tested for its ability to disinfect stainless steel, which provided that it is the most used material on the surfaces of food industry equipment and appliances. For instance, a custom-made high-voltage generator cold plasma device effectively reduced L. monocytogenes and Salmonella on stainless steel [3], and a high radio frequency CAP showed antimicrobial activity against E. coli K12 on stainless steel and polyethylene [19]. In general, the reductions reported fall within the range of 1 to 5 log depending on different factors such as the time of treatment, input voltage, frequency, type of plasma source, environmental humidity, working gas, and distance from the source [3,20].
In order to generate plasma in atmospheric air, an electric field higher than 30 kV/cm is required [21]. However, high-voltage devices imply higher costs and constraints in portability due to safety issues. Piezoelectric transformers amplify a low-voltage input to generate breakdown in air through electromechanical resonance [21]. Low-voltage CAP sources have been demonstrated to effectively inactivate bacteria and have shown potential for clinical use due to their low operating cost and low safety risks [22]. Specifically, an air-fed piezoelectric plasma pen showed antimicrobial activity against, Staphylococcus (S.) aureus, S. epidermis, E. coli, P. aeruginosa, and C. albicans on growth media [22].
In this study, the aim was to evaluate for the first time the effectiveness of a piezoelectric CAP device that used ambient air to produce plasma for the inactivation of Gram-positive L. monocytogenes and Gram-negative Salmonella deposited on stainless steel considering the plasma exposure time and distance from the plasma source as variables. Moreover, BSA was used as a soil accumulation model on stainless steel to simulate real-life conditions; thus, the effectiveness of this technology on clean or protein-soiled surfaces was also studied. To the best of our knowledge, this is the first study investigating the inactivation efficiency and inactivation kinetics of a piezoelectric CAP against foodborne pathogens on a food contact surface.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Salmonella enterica serovar Typhimurium DT104 and Listeria monocytogenes NCTC 7973 were used for this study. For each of the strains, a loopful of fresh Tryptone soya agar (TSA; Oxoid, UK) slope culture was inoculated into 10 mL of tryptone soya broth (TSB; Oxoid, UK) and incubated at 37 °C for 24 h. Subsequently, 100 μL of a 10−4 dilution of this broth culture in maximum recovery diluent (MRD, Oxoid, UK) was inoculated into another 10 mL TSB broth and incubated at 37 °C until the stationary phase of growth was reached. The final 10 mL cultures were harvested by centrifuging at 3600× g for 30 min, washed twice in PBS, and the bacterial pellet was subsequently resuspended in a final volume of 10 mL PBS to give approximately 109 CFU/mL.
2.2. Cold Plasma System
In this study, a handheld CAP system Piezobrush® PZ2 (Relyon Plasma, Regensburg, Germany), which uses a piezoelectric direct discharge (PDD®) technology, was employed. This technology is based on the direct electrical discharge of a piezo-ceramic transformer (PZT) into a working gas (air) to transform a low input voltage into high electric field forces. Specifically, the PZT with a voltage transformation ratio of more than 1000 can achieve high output voltage at low input voltage. The input voltage of DC power was 15 V and at a frequency of 50 kHz. In this study, a multigas nozzle with an inner electrode formed as a needle, as shown in Figure 1, was used. The inducer gas used was ambient air, which was fed into the CAP system by an internal axial fan, producing a corona-like plasma jet [22].
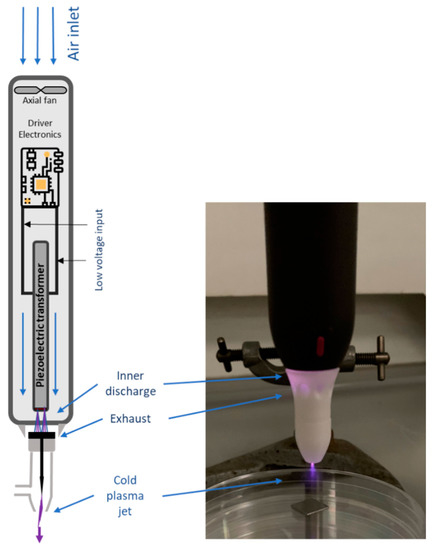
Figure 1.
Schematic view of the handheld CAP system Piezobrush® PZ2 with the multigas nozzle attached. Adapted from Timmermann et al. [22].
2.3. Inoculation and Treatment of Clean and Protein-Soiled Stainless Steel
Stainless-steel (SS) 304 samples were used as food contact surfaces (1 × 1 cm), as this SS type is very commonly used in the food industry due to its physicochemical stability and resistance to corrosion. Before inoculation, the coupons were thoroughly cleaned following the procedure described by Kostaki et al. (2012). The study included two surfaces of different cleanliness (clean and protein-soiled) to determine the inactivation efficiency of cold plasma before and after food factory cleaning. Bovine serum albumin (BSA) was used as a natural organic food contaminant to prepare the protein-soiled food contact surface. A stock BSA solution (6 mg/mL) was prepared with sterile deionized water and sterilized by filtration (0.2 µm pore size). The choice to use BSA was based on the EN 13697:2015 protocol for the evaluation of chemical disinfectants, which adopts BSA as a way to simulate realistic conditions typically found in a food-processing facility [23].
For each pathogen, two different solutions were prepared to inoculate the SS surfaces. For the protein-soiled solution, 1 mL of BSA solution was transferred into a sterile container, and 1 mL of the microbial suspension was subsequently added (3 mg/mL final BSA concentration). For the clean surface solution, 1.0 mL of PBS was transferred into a container, and 1.0 mL of the microbial suspension was added. The solutions were 10-fold serially diluted and spread plated on TSA plates and incubated at 37 °C for 24 h to determine the levels of each pathogen before SS surface inoculation. A volume of 100 μL of either solution was placed on the SS surface and spread to cover the whole surface area. Subsequently, all SS surfaces were air-dried for 60 min under aseptic conditions before treatment. Salmonella and L. monocytogenes were inoculated onto the SS surfaces separately.
2.4. Recovery and Quantification of Salmonella and Listeria monocytogenes Cells
The cold plasma treatments were performed in atmospheric conditions (at approximately 22 °C and relative humidity of 60%) by placing the SS surfaces on a Petri dish at a distance of 10 or 20 mm from the plasma emission and treated for 0 (control), 60, 120, 180, 240, and 300 s. The quantification of pathogenic bacteria before and after CAP treatment on SS surfaces was performed by aseptically transferring each surface into a sterile falcon tube with 10 mL of PBS and a layer of glass beads (diameter 1.5 mm). The falcon tubes were then placed in a shaking incubator for 15 min. When appropriate, 10-fold dilutions were prepared in 9 mL MRD. An aliquot of 100 μL of each of the 10-fold dilutions was spread plated on TSA for both Salmonella and L. monocytogenes and left for incubation at 37 °C for 24 h followed by colony counting. Six replicate samples were analyzed, and each sample was plated in duplicate.
2.5. Statistical Analysis
The descriptive statistics and analysis of variance were carried out using SPSS v. 26.0. The ANOVA was performed considering treatment time, distance, cleanliness, and strains as factors, with log CFU/mL as the dependent variable. A Tukey-test was performed as a post hoc analysis among the different groups of treatment time. A p-value of <0.05 was considered significant. Six replicates were carried out for each of the experiments.
2.6. Inactivation Modeling
Inactivation models were fitted in R (version 3.6.1) [24], using the nlsMicrobio package [25]. As inactivation data are frequently nonlinear, for example, often exhibiting either a tail or a shoulder, a Weibull model and a Weibull + tail models were compared to describe the microbial inactivation curve [26,27,28]. For the Weibull model, the concentration of microorganisms at time , is given by:
For the Weibull + tail model is:
where, is the initial concentration of microorganisms, and Nres is the residual microbial cell density at the end of the study period while and are related to the shape and scale of the curve, respectively. Here is the time for the first decimal reduction, and gives the shape of the curve, where indicates a straight line, corresponds to an upward concave survival curve, and indicates a downward concave curve. Thus, in the context of microbial inactivation, indicates that the rate of inactivation is slowing over time, as the remaining cells are potentially harder to kill, whereas suggests that the remaining cells are becomingly increasing susceptible (van Boekel, 2002).
The fit of the two models (Weibull and Weibull + tail) was compared using leave one out cross-validation. That is, each observation was removed from the dataset in turn, and both models were fitted to the remaining data and used to predict the value for the removed observation. The predictive ability of the two models was compared using root mean square error and R-squared values (Table 1).

Table 1.
Log reduction of L. monocytogenes and Salmonella after CAP treatment from 0 to 300 s, at two different distances (10 and 20 mm), and inoculated on clean stainless-steel (SS) or protein-soiled SS. The results are expressed as the mean ± SD. The superscript letters indicate different subsets for each treatment; the same letter indicates no significant difference when α = 0.05 and n = 6.
Separate inactivation curves were fitted for each combination of the treatment types, that is, for the two bacteria (L. monocytogenes and Salmonella), the distances from the plasma source (10 or 20 mm), and the cleanliness of the steel (clean or protein-soiled). For each treatment combination, the final concentration at the end of the study period (i.e., after 300 s) was predicted under both models (Equations (1) and (2), respectively) to enable the log reduction to be calculated. Parameter estimates and the log reduction achieved during the study period were compared between the treatments.
3. Results and Discussion
3.1. Inactivation of Salmonella and Listeria monocytogenes
This study aimed to assess the inactivation efficiency against L. monocytogenes and Salmonella inoculated on clean or protein-soiled stainless steel (SS), using an air-fed low-voltage piezoelectric CAP device at exposure times up to 300 s, and different distances between the plasma source and the contaminated surface. CAP treatment was able to significantly reduce the levels of both pathogens. The log reduction results in Table 1 demonstrate that pathogen inactivation on the SS surface is time-dependent. The analysis of variance indicates that the treatment time was the factor with the greatest influence (F > 1000, p < 0.001), followed by the cleanliness of the surface (F > 900, p < 0.001) and finally, the distance from the source (F > 152, p < 0.001) for both strains.
Our results are in agreement with previous studies, where higher levels of inactivation were achieved as a function of treatment time [3]. In a previous study, a high-voltage input CAP device at 13.7 kHz showed reductions of >3.55 log CFU/mL on L. monocytogenes and 2.06 log CFU/mL on S. Typhimurium, after 175 and 105 s, respectively, on SS when the initial inoculum was approximately 8 log CFU/mL. Another study that used an RF plasma system (13.6 MHz) reported a time-dependent antimicrobial activity against E. coli K12 inoculated on SS, achieving up to 5 log reduction after 30 min of treatment, with an initial inoculum of log 7 [19]. Another report using a high-voltage (10 kV) CAP device with a frequency of 2 kHz also showed 2.80, 3.43, and 4.17 log bacterial reductions after 5, 10, and 20 min, respectively, against E. coli inoculated on SS [29]. The present study showed that similar reductions of 3.51 and 3.59 log CFU/mL were achieved for Salmonella and L. monocytogenes, respectively, on SS after 5 min of treatment using the piezoelectric CAP device. It has been found that the air-fed CAP system employed in this study produces NO2, O3, and NO at temperatures below 35 °C [22]. A primary target for the cold plasma reactive species is the cell membrane of bacteria, resulting in oxidative damage, affecting its permeability and loss of membrane potential [30,31], denaturing functional proteins [32], damaging DNA [11,33,34], and causing accumulative injury in a time-dependent manner surpassing the anti-oxidative stress capacity of the bacteria [35].
The food-processing facility’s conditions can affect the effectiveness of cell inactivation by CAP. In this study, BSA was used to simulate soiling on inoculated SS surfaces. According to our results, CAP treatment of protein-soiled samples achieved a lower reduction compared to clean SS. After 300 s of CAP treatment, a difference of 0.80–0.85 log CFU/mL for L. monocytogenes and 0.84–1.22 log CFU/mL for Salmonella was observed between clean and protein-soiled samples. Similarly, Katsigiannis et al. observed that inactivation levels were higher using a high-voltage CAP in clean conditions compared to a protein-soiled surface achieving a log CFU difference of 1.72 log CFU/mL for L. monocytogenes after 175 s [3]. It has also been found that pathogenic bacteria covered with BSA and subsequently inoculated on stainless steel also showed reduced inactivation levels after cold plasma treatment. This was attributed to the protective effect of serum albumin on the bacterial cells against the reactive species generated by CAP [29].
The current results show that a shorter distance from the plasma source, i.e., 10 mm, resulted in higher inactivation levels compared to a 20 mm distance. For example, this can be shown by the reduction achieved for L. monocytogenes after 60 s plasma exposure at 10 mm (0.81–1.51 log CFU/mL) and 20 mm (0.48–1.01 log CFU/mL). A similar trend was observed for Salmonella as well. However, it is noteworthy that the difference in the achieved pathogen reductions between the two different treatment distances decreased with longer CAP exposure times. This phenomenon could be attributed to the higher accumulation of reactive species due to longer CAP exposure.
Spatiotemporal studies have shown that plasma reactive species (e.g., NO•, OH•) can be transported several millimeters beyond the plasma source during treatment [36,37]. However, the distance from the plasma source can affect the antimicrobial effectiveness due to changes in the mass transport of the different reactive species from the discharge layer to the target surface. Comparably to this study, Katsigiannis et al. and Niemira et al. reported a negative correlation between the distance from the plasma source and the inactivation rate on SS inoculation [3,38]. However, Yong et al. achieved an optimum inactivation at 20 mm of spacing compared to 10 and 30 mm using a pressure plasma jet on raw chicken breast and a mixture of N2 + O2 as working gas [39]. These differences could be due to differences in the topography of the surface and the plasma source. In general, a shorter distance would enhance bacterial inactivation by increasing the concentration of the reactive species and their contact with the cell wall.
Although, in general, L. monocytogenes appeared to be more resistant to CAP treatment, no significant differences in reduction levels (i.e., p > 0.1) were found between the two pathogens used in this study. These findings differ from the results observed by Lis et al., 2018, where Gram-positive strains such as Klebsiella pneumoniae have shown more resistance to CAP treatment compared to the Gram-negative Y. enterocolitica [29]. This difference was attributed to the thickness of the outer cell wall, which is thicker in Gram-positive bacteria. However, other factors such as the shape of bacteria, where rods are more sensitive than cocci [40], and the type of plasma source [22], could introduce differences in the susceptibility of bacteria.
3.2. Modeling Inactivation Kinetics
The Weibull model has been proven suitable to describe inactivation curves when applying CAP [9,35,41] or other non-thermal systems for bacterial inactivation such as essential oils and high hydrostatic pressure freezing [40,42]. The Weibull model is based on the response observed on the cellular tolerance to lethal stress on the cells. This allows the model to represent linear and nonlinear curved survival kinetics with upward and downward concavity, with or without lag phase [40]. Moreover, a modified Weibull with a tail model was proposed by Albert and Mafart (2005), which has the advantage of fitting the most typical survivor curves and considers a new parameter, Nres, when the tail is assumed [28]. The survival curves in our results showed that the inactivation rate slowed down between 240 and 300 s, indicating a tail shape (i.e., p > 0.1). Hence, the Weibull and Weibull + tail models were compared for best fit.
According to the parameters of the goodness of fit in Table 2, both models showed similar accuracy. However, the values of the RMSE estimated for Weibull + tail were slightly smaller than those of the Weibull model. Furthermore, the R2 values were slightly higher for Weibull + tail indicating higher accuracy. More recent studies have reported a better fit using a Weibull + tail model when a corona discharge plasma jet (CDPJ) device was used against E. coli, S. Typhimurium, and S. aureus inoculated on slide glasses obtaining RMSE values < 0.1 and R2 values > 0.98 [43]. Thus, we selected the Weibull + tail model as it showed better goodness of fit.

Table 2.
Goodness of fit (RMSE and R2) for the Weibull and Weibull + tail inactivation curves.
It is important to note that the δ parameter of the Weibull + tail model is close to the D value used in the log-linear inactivation curve for thermal sterilization [28]. Therefore, lower δ values indicate higher inactivation efficiency during the first minutes. According to Table 3, the lowest δ indicates that clean SS at a distance of 10 mm was the most efficient condition for inactivation with a similar response for both strains, 33.29 and 32.48 s, whereas the protein-soiled SS coupons showed higher δ values (75.57 and 82.88 s) for L. monocytogenes and Salmonella, respectively. This is 30–40 s of longer plasma exposure to achieve the first log reduction for the protein-soiled SS coupons, indicating that due to the protective effect protein has on the bacterial cells, a longer CP treatment time is needed to reach similar log reduction levels. Similar δ values (13.96–48.84) were reported for S. Typhimurium using a CDPJ with different levels of relative humidity% (RH%; 41–62%), achieving the lowest value when RH% = 51% [43]. Moreover, Kilonzo-Nthenge et al. reported δ values of 12.73, 22, 12.26, and 46.7 s for S. Typhimurium, S. Choleraesuis, E. coli ATCC 25922, and E. coli ATCC 11775, respectively, using a high-voltage DBD plasma on apples [9]. The authors used a Weibull model, which does not consider a residual concentration of cells (Nres), and the δ values were generally lower than those predicted by the Weibull + tail model [9]. However, previous studies employing the Weibull model using a high-voltage output Argon/O2 (98:2) plasma jet device reported δ values of 2.266–2.447 min for L. monocytogenes [41]. Another study using a high-voltage DBD against a multiple antibiotic-resistant strain of S. aureus showed δ values of 2.9, 1.98, and 0.95 min at 8, 6, and 4 mm of distance, respectively, also employing a Weibull model [35]. Since the antimicrobial efficiency of cold plasma is greatly influenced by the cleanliness of the treated surfaces, it is suggested that a cleaning stage should be applied prior to cold plasma treatment.

Table 3.
Comparison between Weibull and Weibull + tail model parameters and predicted log reduction achieved during the study period (300 s).
Figure 2 and Figure 3 for L. monocytogenes and Salmonella, respectively, showed a concave shape (p < 1) with the 10 mm distance indicating a quick inactivation rate during the first minutes, which slows down as time progresses, forming a tail shape after 240 s. The experiments carried out at a 20 mm distance showed less pronounced antimicrobial efficiency compared to 10 mm. Interestingly, for most of the conditions at 20 mm, there is a stepwise log decrease from 180 to 240 s. We hypothesize that this effect implies an accumulation of critical concentrations of reactive compounds necessary to kill a more resistant subpopulation.
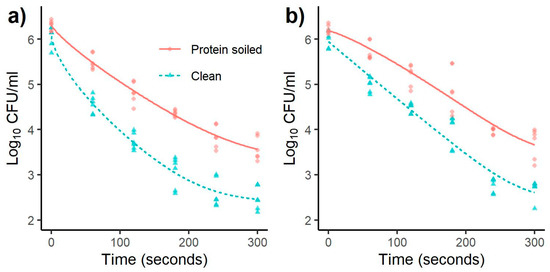
Figure 2.
Effect of treatment time on inactivation of L. monocytogenes with a distance of (a) 10 and (b) 20 mm. Models were fitted using a Weibull + tail inactivation curve (Equation (2)) (n = 6).
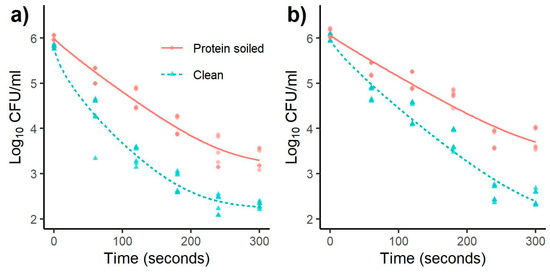
Figure 3.
Effect of treatment time on inactivation of Salmonella with a distance of (a) 10 and (b) 20 mm. Models were fitted using a Weibull + tail inactivation curve (Equation (2)) (n = 6).
Moreover, the p-value that correlates with the scale and shape of the survival curve is similar in both models. In nearly all cases, p is <1, indicating a concave upward survival curve, except for L. monocytogenes at 20 mm with protein-soiled surfaces where p > 1, showing a concave downward curve shape in Figure 2b. The inactivation curve models clearly show a difference in the CAP antimicrobial effectiveness between the clean and the protein-soiled SS surface, indicating a protective effect of BSA. This is closer to real conditions in the food industry where the surfaces can be soiled with residual protein pertaining to foodstuff [3]. It is important to note that the inactivation was tested on dried cells, and biofilms were not allowed to form. This would add an extra protective barrier to the cells, which was not investigated as it was beyond the aim of this study. Previous studies have shown other CAP systems’ capabilities, such as DBD, to inactivate bacterial biofilms [38,44]. Further research is needed to assess the effectiveness of the piezoelectric CAP on biofilms.
The predicted log reductions in both models were similar to the experimental values obtained after CAP treatment. A different study using a surface barrier discharge (SBD) configuration of a DBD plasma cold plasma device achieved a >3.55 and 2.06 log CFU/mL reduction of Listeria monocytogenes and Salmonella Typhimurium, respectively, at 5 mm distance within 3 min [3]. In addition, Kim et al. reported a reduction of 3.65 log for S. Typhimurium inoculated on glass slides after 300 s exposure time using a high-voltage CDPJ device that used air as inducer gas at 51% relative humidity [43]. These results are similar to those achieved with the studied low-voltage CAP device; however, since a high-voltage power supply is not required, it could facilitate scaling up and adoption by the food industry.
4. Conclusions
The aim of this study was to determine the antimicrobial efficiency of an easy-to-use, low-voltage piezoelectric cold plasma using ambient air as an inducer gas against foodborne pathogens inoculated on stainless-steel surfaces. Results showed that this technology was able to achieve a high reduction for L. monocytogenes and Salmonella in a matter of minutes. Results also showed that the exposure time to cold plasma, the distance from the plasma source, and surface cleanliness significantly influence the antimicrobial efficiency of the treatment. The Weibull + tail model showed higher accuracy in estimating the log reduction achieved during the treatment timeframe studied. Thus, this study demonstrated that a low-voltage CAP source that uses environmentally friendly ambient air could potentially be introduced in highly automated food-processing environments for decontaminating stainless-steel surfaces as means of controlling foodborne pathogens and minimizing cross-contamination. Although this study focused on L. monocytogenes and Salmonella, other Gram-negative and Gram-positive pathogens could be inactivated using this technology; however, further studies are needed to conclusively state this. In addition, further work against food spoilage microorganisms, other types of food contact surfaces, and the potential influence of different relative humidity values on the antimicrobial efficiency of this technology is needed to fully elucidate the efficiency and suitability of this technology in the food-processing environment.
Author Contributions
Conceptualization, A.C.S.; methodology, C.R.G.-G., B.J.H., S.S. and A.C.S.; software, C.R.G.-G. and B.J.H.; validation, C.R.G.-G., B.J.H., S.S. and A.C.S.; formal analysis, C.R.G.-G., B.J.H. and A.C.S.; investigation, C.R.G.-G., B.J.H. and S.S.; resources, A.C.S.; data curation, C.R.G.-G. and B.J.H.; writing—original draft preparation, C.R.G.-G.; writing—review and editing, C.R.G.-G., B.J.H., S.S. and A.C.S.; supervision, A.C.S.; project administration, A.C.S.; funding acquisition, A.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a University of the West of England internal funding grant awarded to A.C.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Diseases Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Boelaert, F.; Amore, G.; Van der Stede, Y.; Nagy, K.; Rizzi, V.; Mirena, I.; Stoicescu, A.; Riolo, F.; Gervelmeyer, A.; Niskanen, T.; et al. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar] [CrossRef]
- Katsigiannis, A.S.; Bayliss, D.L.; Walsh, J.L. Cold plasma decontamination of stainless steel food processing surfaces assessed using an industrial disinfection protocol. Food Control 2021, 121, 107543. [Google Scholar] [CrossRef]
- Fink, R.; Oder, M.; Stražar, E.; Filip, S. Efficacy of cleaning methods for the removal of Bacillus cereus biofilm from polyurethane conveyor belts in bakeries. Food Control 2017, 80, 267–272. [Google Scholar] [CrossRef]
- Fagerlund, A.; Moretro, T.; Heir, E.; Briandet, R.; Langsrud, S.; Møretrø, T.; Heir, E.; Briandet, R.; Langsruda, S. Cleaning and Disinfection of Biofilms Composed of Listeria monocytogenes. Appl. Environ. Microbiol. 2017, 83, 1–21. [Google Scholar] [CrossRef]
- Corcoran, M.; Morris, D.; De Lappe, N.; O’Connor, J.; Lalor, P.; Dockery, P.; Cormican, M. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl. Environ. Microbiol. 2014, 80, 1507–1514. [Google Scholar] [CrossRef]
- Otto, C.; Zahn, S.; Rost, F.; Zahn, P.; Jaros, D.; Rohm, H. Physical Methods for Cleaning and Disinfection of Surfaces. Food Eng. Rev. 2011, 3, 171–188. [Google Scholar] [CrossRef]
- Skåra, T.; Rosnes, J.T. 6—Emerging Methods and Principles in Food Contact Surface Decontamination/Prevention. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Leadley, C.E., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 151–172. ISBN 978-1-78242-447-5. [Google Scholar]
- Kilonzo-Nthenge, A.; Liu, S.; Yannam, S.; Patras, A. Atmospheric Cold Plasma Inactivation of Salmonella and Escherichia coli on the Surface of Golden Delicious Apples. Front. Nutr. 2018, 5, 120. [Google Scholar] [CrossRef]
- Noriega, E.; Shama, G.; Laca, A.; Díaz, M.; Kong, M.G. Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua. Food Microbiol. 2011, 28, 1293–1300. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Denes, F.S.; Reinemann, D.J.; Manolache, S.O.; Helgren, J.M. Plasma-Assisted Disinfection of Milking Machines 2005. U.S. Patent US 7,536,975, 26 May 2009. [Google Scholar]
- Cheol, M.S.; Hyun, K.J. A Method of Sterilization of Post-Packaging of Ready-to-Eat Food 2020. Korea Patent KR102113831B1, 11 May 2020. [Google Scholar]
- Potoroko, I.Y.; Naumenko, V.N.; Lejvi, Y.A.; Kalinina, I.V. Method of Grain Disinfection 2019. Russia Patent RU2707944C1, 19 July 2019. [Google Scholar]
- Butscher, D.; Zimmermann, D.; Schuppler, M.; Rudolf von Rohr, P. Plasma inactivation of bacterial endospores on wheat grains and polymeric model substrates in a dielectric barrier discharge. Food Control 2016, 60, 636–645. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga toxin-producing Escherichia coli, and Listeria monocytogenes by a novel surface discharge cold plasma design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Sen, Y.; Mutlu, M. Sterilization of Food Contacting Surfaces via Non-Thermal Plasma Treatment: A Model Study with Escherichia coli-Contaminated Stainless Steel and Polyethylene Surfaces. Food Bioprocess Technol. 2013, 6, 3295–3304. [Google Scholar] [CrossRef]
- Lis, K.A.; Boulaaba, A.; Binder, S.; Li, Y.; Kehrenberg, C.; Zimmermann, J.L.; Klein, G.; Ahlfeld, B. Inactivation of Salmonella Typhimurium and Listeria monocytogenes on ham with nonthermal atmospheric pressure plasma. PLoS ONE 2018, 13, e0197773. [Google Scholar] [CrossRef]
- Johnson, M.J.; Go, D.B. Piezoelectric transformers for low-voltage generation of gas discharges and ionic winds in atmospheric air. J. Appl. Phys. 2015, 118, 243304. [Google Scholar] [CrossRef]
- Timmermann, E.; Bansemer, R.; Gerling, T.; Hahn, V.; Weltmann, K.D.; Nettesheim, S.; Puff, M. Piezoelectric-driven plasma pen with multiple nozzles used as a medical device: Risk estimation and antimicrobial efficacy. J. Phys. D Appl. Phys. 2021, 54, 025201. [Google Scholar] [CrossRef]
- BS EN 13697:2015. Chemical Disinfectants and Antiseptics. In Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants Used in food, Industrial, Domestic and Institutional Areas; BSI Standards Publications: Brussels, Belgium, 2015; ISBN 978-0-539-07394-2. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 29 March 2021).
- Baty, F.; Delignette-Muller, M.-L. nlsMicrobio: Data Sets and Nonlinear Regression Models Dedicated to Predictive Microbiology 2014. Available online: https://CRAN.R-project.org/package=nlsMicrobio (accessed on 13 April 2021).
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Acta Hortic. 2001, 566, 107–114. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef]
- Lis, K.A.; Kehrenberg, C.; Boulaaba, A.; von Köckritz-Blickwede, M.; Binder, S.; Li, Y.; Zimmermann, J.L.; Pfeifer, Y.; Ahlfeld, B. Inactivation of multidrug-resistant pathogens and Yersinia enterocolitica with cold atmospheric-pressure plasma on stainless-steel surfaces. Int. J. Antimicrob. Agents 2018, 52, 811–818. [Google Scholar] [CrossRef]
- Suwal, S.; Coronel-Aguilera, C.P.; Auer, J.; Applegate, B.; Garner, A.L.; Huang, J.-Y. Mechanism characterization of bacterial inactivation of atmospheric air plasma gas and activated water using bioluminescence technology. Innov. Food Sci. Emerg. Technol. 2019, 53, 18–25. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Takai, E.; Kitano, K.; Kuwabara, J.; Shiraki, K. Protein Inactivation by Low-temperature Atmospheric Pressure Plasma in Aqueous Solution. Plasma Process. Polym. 2012, 9, 77–82. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of Atmospheric Pressure Plasmas on Isolated and Cellular DNA—A Review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Liao, X.; Forghani, F.; Liu, D.; Ding, T. Cumulative Damages by Nonthermal Plasma (NTP) Exceed Defense Barrier of Multiple Antibiotic Resistant Staphylococcus aureus: A Key to Achieve Completed Inactivation. Food Qual. Saf. 2021, 1–14. [Google Scholar] [CrossRef]
- Hasan, M.I.; Walsh, J.L. Numerical investigation of the spatiotemporal distribution of chemical species in an atmospheric surface barrier-discharge. J. Appl. Phys. 2016, 119, 203302. [Google Scholar] [CrossRef]
- Dickenson, A.; Britun, N.; Nikiforov, A.; Leys, C.; Hasan, M.I.; Walsh, J.L. The generation and transport of reactive nitrogen species from a low temperature atmospheric pressure air plasma source. Phys. Chem. Chem. Phys. 2018, 20, 28499–28510. [Google Scholar] [CrossRef]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold Plasma Inactivation of Escherichia coli O157:H7 Biofilms. Front. Sustain. Food Syst. 2018, 2, 47. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Choe, W.; Oh, M.W.; Jo, C. Evaluation of the treatment of both sides of raw chicken breasts with an atmospheric pressure plasma jet for the inactivation of escherichia coli. Foodborne Pathog. Dis. 2014, 11, 652–657. [Google Scholar] [CrossRef]
- Maresca, P.; Ferrari, G. Modeling of the microbial inactivation by high hydrostatic pressure freezing. Food Control 2017, 73, 8–17. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.H.; Lv, X.; Sun, D.W. Assessing the inactivation efficiency of Ar/O2 plasma treatment against Listeria monocytogenes cells: Sublethal injury and inactivation kinetics. LWT 2019, 111, 318–327. [Google Scholar] [CrossRef]
- De Oliveira, T.L.C.; Soares, R.; de Araújo Soares, R.; Piccoli, R.H. A Weibull model to describe antimicrobial kinetics of oregano and lemongrass essential oils against Salmonella Enteritidis in ground beef during refrigerated storage. Meat Sci. 2013, 93, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, T.; Puligundla, P.; Mok, C. Effect of relative humidity on the inactivation of foodborne pathogens by corona discharge plasma jet (CDPJ). LWT 2020, 127, 109379. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Baka, M.; Ećimović, B.; Walsh, J.L.; Van Impe, J. Resistance of L. monocytogenes and S. Typhimurium towards cold atmospheric plasma as function of biofilm age. Appl. Sci. 2018, 8, 2702. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).