Abstract
Photodynamic therapy (PDT) is a minimally invasive cancer therapy that combines the accumulation of photosensitizers such as porphyrins in cancer cells with laser irradiation. I have previously reported that mitochondrially derived reactive oxygen species (ROS) regulate the expression of a porphyrin transporter, heme carrier protein 1 (HCP1), and increase porphyrin accumulation in cancer cells. Tumors that contain activated macrophages, referred to as tumor-associated macrophages (TAMs), have been reported to have increased malignancy. TAMs produce nitric oxide (NO), via the expression of inducible NO synthase (iNOS), and the highly reactive nitrogen species, peroxynitrite, which is produced by the reaction of NO with superoxide. Here, I examined the relationship between peroxynitrite, HCP1 expression, and intracellular porphyrin uptake in the murine macrophage cell line RAW264. RAW264 cells were activated by lipopolysaccharide (LPS) treatment which resulted in increased iNOS expression and NO production. Additional X-ray irradiation resulted in the generation of ROS and the subsequent generation of peroxynitrite. Importantly, LPS and X-ray co-treatment significantly enhanced HCP1 expression and porphyrin accumulation in cells, suggesting that the peroxynitrite upregulates the porphyrin transporter, HCP1. Therefore, TAMs may be effectively targeted with PDT, and tumor progression may be suppressed in general by agents that target the activation of macrophages.
1. Introduction
Society is continuing to age, and as it does so, the number of cancer patients is increasing worldwide. Surgery is a primary method used for cancer therapy; however, in the elderly population there are a large number who take anticoagulants to treat thrombosis, which leaves them at risk of severe bleeding during any surgical procedure.
Photodynamic therapy (PDT) is a cancer therapy that uses laser irradiation and a photosensitizer that has tumor-specific accumulation, such as porphyrins [1]. Irradiation with low energy of a specific wavelength tuned to the photosensitizer initiates a photochemical reaction that produces a reactive oxygen species (ROS), namely, singlet oxygen, which leads to the death of cancer cells via apoptosis or necrosis [2]. Thus, PDT is a non-invasive and promising therapy for the treatment of patients with cancer. However, the mechanism underlying the tumor-selective accumulation of porphyrins has not been completely elucidated.
In order to address this, I focused on a proton-coupled folate transporter, referred to as heme carrier protein 1 (HCP1), which has been identified as a heme transporter. It has previously been reported that both heme and porphyrins are transported into cells via HCP1 [3,4,5]. HCP1 is also reported to be regulated by ROS derived from mitochondria [6]. ROS, especially superoxide, are generated from mitochondria, as well as by the actions of NADPH oxidase (NOX) and xanthine oxidase. However, mitochondria are the main producers of superoxide because electrons that leak from the mitochondrial electron transport chain regularly react with oxygen. It is well known that increased oxidative stress can act as a signaling event that leads to the development of a variety of chronic diseases [7]. Overgeneration of ROS has been observed in cancer cells, and it has been shown to be related to cancer progression [8]. In recent years, macrophages have been reported to infiltrate tumor tissues, where they are referred to as tumor-associated macrophages (TAMs) and are associated with increased tumor malignancy [9]. Activated macrophages express inducible nitric oxide synthase (iNOS), which produces nitric oxide (NO) [10], a reactive compound that mediates many types of signaling events. In addition to activating signaling pathways, NO is able to react with superoxide to produce peroxynitrite, a highly reactive nitrogen-containing compound [11]. In this study, I used the murine macrophage cell line RAW264 treated with lipopolysaccharide (LPS) and irradiated with X-rays to mimic activated TAMs and examined the relationship among peroxynitrite levels, HCP1 expression, and cellular porphyrin uptake. LPS is an activator of macrophages and can induce iNOS expression [12]. In addition, cells irradiated with X-rays often overproduce ROS, especially superoxide derived from mitochondria [13]. Therefore, these systems were used to generate peroxynitrite, and the effect of porphyrin accumulation was studied.
2. Materials and Methods
2.1. Cell Culture
The mouse macrophage cell line, RAW264, was provided by the Riken Cell Bank (Ibaraki, Japan). RAW264 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 4 mM L-glutamine, 3.7 g/L sodium bicarbonate, 4.5 g/L glucose, and 1.0 mM sodium pyruvate (Sigma-Aldrich Co. LLC, St. Louis, MO, USA); 10% fetal bovine serum (Cytiva, Marlborough, MA, USA); and 1% penicillin/streptomycin (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37 °C in a humidified atmosphere containing 5% CO2.
2.2. Detection of LPS-Induced NO Production
RAW264 cells were activated by LPS to induce iNOS expression, and the intracellular production of NO was confirmed using a fluorescence-microscopy-based method. Briefly, cells were seeded in a 35 mm glass-bottom dish and incubated overnight. Cells were then treated with 0 or 1 µg/mL LPS for 24 h at 37 °C. Next, the cells were incubated with 10 µM diaminofluorescein-FM diacetate (DAF-FM DA; Goryo Chemical Inc., Hokkaido, Japan) for 30 min at 37 °C to detect intracellular NO production. The DAF-FM DA fluorescence was observed using a CSU-10 confocal laser scanning unit (Yokogawa Electric Co., Tokyo, Japan) coupled to an Eclipse Ti-U inverted microscope with a PlanAPO 20 objective lens (Nikon Co., Tokyo, Japan) and a C5810-01 color chilled 3CCD camera (Hamamatsu Photonics K.K., Shizuoka, Japan). The excitation and emission wavelengths for DAF-FM DA were 488 and 515 nm, respectively. Prior to DAF-FM DA treatment, the cells were incubated with 100 µM 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, sodium salt (carboxy-PTIO) (Dojindo Laboratories, Kumamoto, Japan), an NO scavenger, for 2 h at 37 °C.
2.3. Detection of Intracellular ROS Production After X-ray Irradiation
Intracellular ROS production after X-ray irradiation was measured using a fluorescence-microscopy-based method. Cells were seeded in a 35 mm glass-bottom dish and incubated overnight. Cells were irradiated with X-rays using an MBR-1505R X-ray irradiation unit (Hitachi Medical Corporation, Tokyo, Japan). The machine was operated at 120 kV and 3.2 mA with a 0.5 mm aluminum filter. The dose rate was 1 Gy/min at a focus-to-surface distance of 30 cm, and the total dose was 20 Gy. After further incubation for 0.5, 1.5, 3.5, and 24 h, cells were incubated with modified Hanks’ balanced salt solution (HBSS) containing 10 mM HEPES, 1 mM MgCl2, 1 mM CaCl2, 8.3 mM glucose, and 10 µM hydroxyphenyl fluorescein (HPF) (Goryo Chemical Inc.) for 30 min at 37 °C, and the HPF fluorescence was observed using the same method as that described above. HPF is a detector of intracellular highly ROS and can explicitly demonstrate intracellular ROS generation [14].
2.4. Measurement of Intracellular Peroxynitrite
Intracellular peroxynitrite production was measured after LPS treatment and X-ray irradiation. RAW264 cells were seeded in 96-well cell culture plates at a density of 2 × 104 cells/well and incubated overnight. Cells were treated with 0 or 1 µg/mL LPS for 24 h at 37 °C and subjected to Cell MeterTM Fluorimetric Intracellular Peroxynitrite Assay Kit *Green Fluorescence* (AAT Bioquest, Inc., Sunnyvale, CA, USA). This kit contains a specific fluorescent probe for peroxynitrite detection that emits green fluorescence upon reaction with peroxynitrite. Briefly, the cells were irradiated with 20 Gy X-rays as described above. Four hours after X-ray irradiation, cells were washed with phosphate-buffered saline (PBS) and a fluorometric analysis was performed using an Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The excitation and emission wavelengths were 490 and 530 nm, respectively.
2.5. Hematoporphyrin Dihydrochloride (HpD) Uptake
Intracellular porphyrin accumulation was estimated by detecting porphyrin fluorescence after LPS treatment and X-ray irradiation. RAW264 cells were seeded in 35 mm cell culture dishes at a density of 5 × 105 cells/well and incubated overnight. Cells were treated with 0 or 1 µg/mL LPS for 24 h at 37 °C and then irradiated with 20 Gy X-rays by the method described above. After further incubation for 24 h, the cells were treated with 20 µM hematoporphyrin dihydrochloride (HpD) (Santa Cruz Biotechnology Inc., Dallas, TX, USA) in the culture medium for 6 h at 37 °C, and the cells were washed twice with PBS. Cells were dissolved in 200 µL of radioimmunoprecipitation assay (RIPA) cell lysis buffer, and the fluorescence of the cell lysate was measured using an Infinite M200 microplate reader (Tecan Group Ltd.). The excitation and emission wavelengths were 405 and 625 nm, respectively.
2.6. Western Blotting
Both the expression of intracellular iNOS following LPS treatment and changes in the expression levels of HCP1 after LPS and X-ray irradiation were examined by Western blot analysis. To assess iNOS expression, RAW264 cells were treated with 0 or 1 µg/mL LPS for 24 h; then, cells were harvested and proteins were extracted using RIPA cell lysis buffer. For HCP1 detection, the cells were irradiated with 20 Gy X-rays after LPS treatment, and cell lysates were prepared after a further 24 h incubation. The cell lysis protein samples were mixed with 2× Western blotting sample buffer containing 100 mM Tris-HCl (pH 6.8), 4% (w/v) sodium dodecyl sulfate (SDS), 12% (v/v) 2-mercaptoethanol, 20% (v/v) glycerol, and 0.01% (w/v) bromophenol blue, and heated at 95 °C for 5 min. The proteins were separated by electrophoresis on 10% (w/v) polyacrylamide gels at 80 V for 90 min. The separated proteins were then electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Burlington, MA, USA) at 2 mA/cm2 for 70 min. The membranes were blocked by immersion for 60 min in 5% (w/v) skim milk dissolved in PBS-Tween (PBS-T). After blocking, the membranes were incubated with the following primary antibodies overnight at 4 °C: mouse anti-NOS2 antibody (Santa Cruz Biotechnology Inc.) diluted at 1:200 or mouse anti-HCP1 antibody (Santa Cruz Biotechnology Inc.) diluted at 1:100. After incubation with the primary antibodies, the membranes were washed three times with PBS-T for 10 min and incubated with mouse IgGκ light chain binding protein conjugated to horseradish peroxidase (mIgGκ BP-HRP) (Santa Cruz Biotechnology Inc.) diluted at 1:1000 for 3 h at 25 °C as a secondary antibody. The secondary antibody solution was removed, and the membranes were washed three times with PBS-T. A luminescence substrate solution, namely, Immobilon Forte Western HRP substrate (Merck Millipore), was applied to the membranes, and luminescence was detected using a FluorChemFC2 (Alpha Innotech Co., San Leandro, CA, USA). An anti-β-actin antibody (Santa Cruz Biotechnology Inc.) was used to detect the β-actin protein as the sample loading control.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS Statistics 24 software (International Business Machines Corporation, Armonk, NY, USA). Scheffe’s test or Tukey’s test was used to compare more than two data sets, and Student’s t-test was used to compare two data sets. p < 0.05 and p < 0.01 were considered to indicate statistically significant differences. All data are presented as mean ± standard deviation.
3. Results
3.1. LPS-Induced NO Production and iNOS Expression in RAW264 Macrophage Cells
Intracellular production of nitric oxide (NO) was detected using the fluorescent probe DAF-FM DA. Non-fluorescent DAF-FM DA, which permeates the cell membrane, accumulates in cells; the ester bonds are hydrolyzed by intracellular esterase, generating membrane-impermeable DAF-FM, which emits a green fluorescent signal on reacting with NO. In this experiment, LPS-only treatment was performed to confirm cellular activation by LPS-induced iNOS and subsequent NO production in RAW264 cells. The left panel in Figure 1a shows the DAF-FM fluorescence in cells treated with 0 or 1 µg/mL LPS for 24 h. Clear DAF-FM fluorescence, which appeared as discrete dots, was apparent in RAW264 macrophage cells following treatment with 1 µg/mL LPS. Notably, treatment with the NO scavenger carboxy-PTIO suppressed the increase in NO generation. The bar graph in Figure 1a represents a quantitation of the fluorescent signals and shows that there was a significant increase in fluorescence intensity in 1 µg/mL LPS-treated cells compared to 0 µg/mL treated cells. Figure 1b shows the expression levels of endogenous inducible nitric oxide synthase (iNOS) in RAW264 macrophages treated with 0 or 1 µg/mL LPS for 24 h, as assessed by Western blotting. There was a significant increase in iNOS levels following treatment with 1 µg/mL LPS. These results indicate that treatment of the mouse macrophage cell line RAW264 with LPS induces iNOS expression and subsequent NO production.
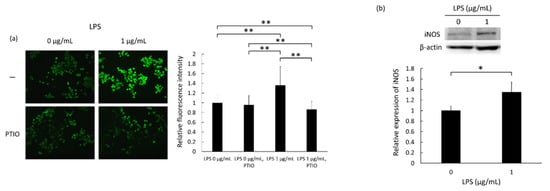
Figure 1.
Intracellular nitric oxide (NO) and inducible NO synthase (iNOS) levels in RAW264 macrophages following treatment with 0 or 1 µg/mL lipopolysaccharide (LPS) for 24 h. (a) Intracellular NO production was assessed using the fluorescence probe diaminofluorescein-FM diacetate (DAF-FM DA). Carboxy-PTIO was used for NO scavenging. Statistical significance was assessed using Scheffe’s test. Data are shown as mean ± standard deviation. ** p < 0.01. (b) Expression of iNOS assessed by Western blotting. Statistical significance was determined using Student’s t-test. Data are shown as mean ± standard deviation; n = 3. * p < 0.05.
3.2. Intracellular ROS Production Following X-ray Irradiation
To confirm the increase in ROS production in RAW264 macrophages following irradiation by X-rays and to optimize conditions for further experiments, cells were irradiated with 0 or 20 Gy of X-rays. Intracellular ROS levels were then assessed using the fluorescent probe HPF, which emits a green fluorescent signal following its reaction with ROS. Figure 2a shows fluorescence microscopy images of cells irradiated with either 0 Gy or 20 Gy of X-rays for the indicated times. There was a clear increase in fluorescent signal in cells irradiated with 20 Gy of X-rays at all the assessed time points. The ratio of fluorescence intensity was then normalized to the fluorescence intensity of the 0 Gy control at each respective time point. The relative intensity was found to be the highest at 4 h after irradiation (Figure 2b). These results indicate that 20 Gy X-ray irradiation causes the generation of intracellular ROS in RAW264 cells, with the highest ROS levels being observed 4 h after irradiation. These incubation and irradiation times were used for the subsequent experiments.
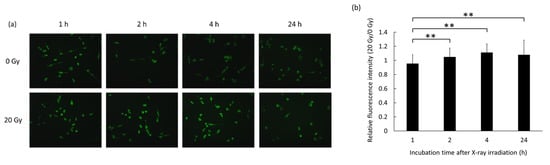
Figure 2.
Production of intracellular reactive oxygen species (ROS) in RAW264 macrophages following X-ray irradiation as assessed using the fluorescent probe hydroxyphenyl fluorescein (HPF). (a) Representative fluorescence images of cells treated with either 0 Gy or 20 Gy of X-rays over time. (b) Ratio of the fluorescence signals (20 Gy/0 Gy) at each respective time point. Statistical significance was assessed using Scheffe’s test. Data are shown as mean ± standard deviation. ** p < 0.01.
3.3. Generation of Peroxynitrite Following Co-treatment with LPS and X-rays
Peroxynitrite is a highly reactive oxidant that is synthesized by the reaction of NO and superoxide [15]. The production of peroxynitrite was assessed after LPS treatment and X-ray irradiation using a fluorescent-probe-based assay. Figure 3 shows that cellular peroxynitrite generation was significantly increased by LPS and X-ray irradiation. In addition, LPS treatment induced iNOS expression to generate elevated levels of NO in RAW264 cells, and ROS production was enhanced by X-ray irradiation, as mentioned above. Indo et al. reported that X-ray irradiation induced cellular ROS production derived from mitochondria and that the primary source of ROS is superoxide [16]. Thus, I hypothesized that peroxynitrite production was increased by the reaction of NO and superoxide from mitochondria caused by X-ray irradiation.
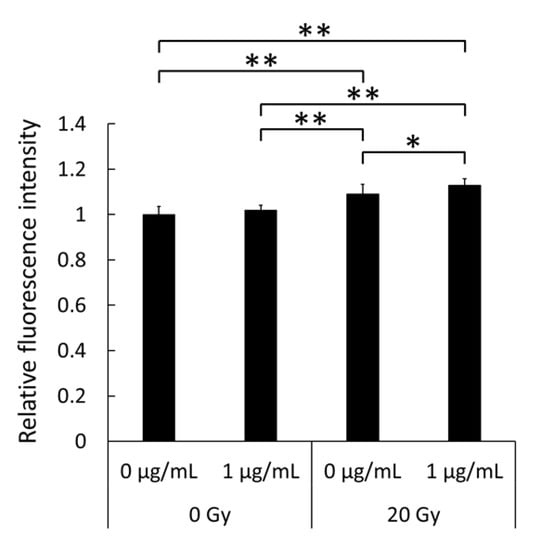
Figure 3.
Intracellular levels of peroxynitrite in RAW264 macrophages treated with 0 or 1 µg/mL lipopolysaccharide (LPS) for 24 h followed by irradiation with 0 Gy or 20 Gy X-rays. Statistical significance was assessed using Tukey’s test. Data are shown as mean ± standard deviation; n = 12. * p < 0.05, ** p < 0.01.
3.4. Elevated Accumulation of Hematoporphyrin in Cells
Intracellular porphyrin accumulation was evaluated in RAW264 macrophages following co-treatment with LPS and X-rays. Cells were exposed to HpD for 6 h after LPS treatment and X-ray irradiation, and the fluorescence derived from porphyrin in the cells was measured. Figure 4 shows that porphyrin fluorescence was enhanced by 1 µg/mL LPS treatment compared to the untreated control, and X-ray irradiation at 20 Gy also increased porphyrin fluorescence in cells, though it was not significant in the present study. Furthermore, co-treatment with LPS and X-rays significantly augmented the fluorescence compared to non-LPS-treated, 1 µg/mL LPS-treated, or 20 Gy X-ray non-LPS-treated cells. These results suggest that the peroxynitrite produced by LPS and X-ray co-treatment enhances the accumulation of intracellular porphyrin.
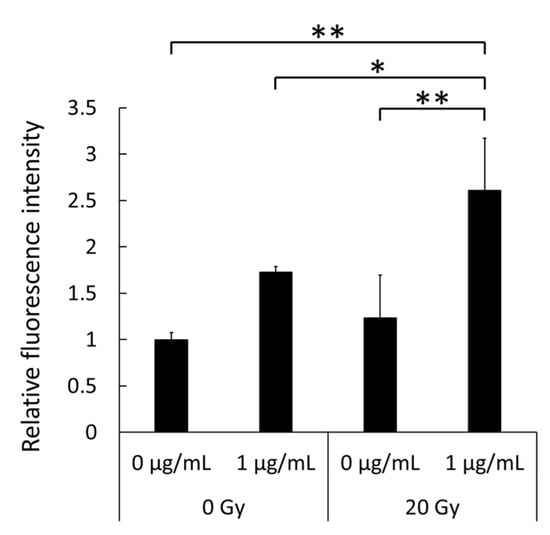
Figure 4.
Intracellular accumulation levels of hematoporphyrin dihydrochloride (HpD) in RAW264 macrophages after treatment with lipopolysaccharide (LPS) and X-rays. Cells were exposed to HpD for a total of 6 h. Statistical significance was assessed by Tukey’s test. Data are shown as mean ± standard deviation; n = 4. * p < 0.05, ** p < 0.01.
3.5. Enhancement of the Expression of the Porphyrin Transporter
The expression levels of the porphyrin transporter HCP1 were then evaluated by Western blotting. LPS treatment or X-ray irradiation alone increased expression levels of HCP1 compared to that in non-treated cells, although these differences were not significant (Figure 5). However, the combination treatment with both LPS and X-rays significantly enhanced HCP1 expression compared to non-treated and LPS or X-ray solely treated cells. These results indicate that the NO produced by LPS treatment and the ROS induced by X-ray irradiation react together to generate the reactive nitrogen species (RNS) peroxynitrite, which then increases the expression levels of HCP1.
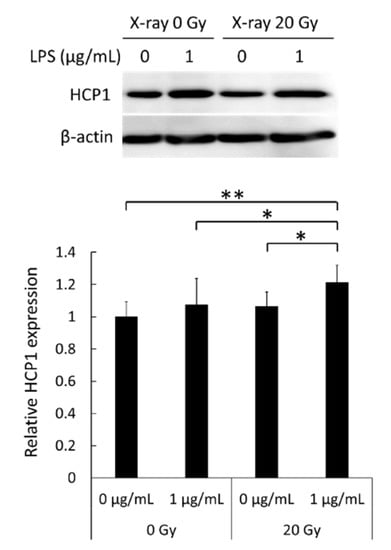
Figure 5.
Levels of heme carrier protein 1 (HCP1) in RAW264 macrophages after treatment with lipopolysaccharide (LPS) and X-rays as assessed by Western blotting. Statistical significance was tested by Tukey’s test. Data are shown as mean ± standard deviation; n = 12. * p < 0.05, ** p < 0.01.
4. Discussion
PDT is a technique that is already used for the clinical treatment of cancer. However, the mechanism underlying the cancer-specific accumulation of porphyrin remains unclear. Endocytosis via the low-density lipoprotein (LDL) receptor and the inactivation of ferrochelatase, which is an enzyme that inserts iron ions into porphyrin and synthesizes heme, have been reported to be associated with the accumulation of intracellular porphyrin [17,18]. I previously reported that mitochondrial ROS upregulate the expression of HCP1 and enhance the effect of cancer therapy by PDT [6]. I also reported that HCP1 is a downstream target of hypoxia-inducible factor (HIF)–1α, and that NO stabilizes HIF–1α by inhibiting prolyl hydroxylase, thereby regulating HCP1 expression [19]. In this study, I showed that the NO induced by LPS treatment and the ROS generated by X-ray irradiation together upregulated HCP1 expression levels and increased cellular porphyrin uptake. It is likely that the effect on HCP1 levels is mediated by peroxynitrite, which is an RNS produced by the reaction with NO and superoxide.
NO and ROS together produce peroxynitrite, and the rate constant is quite fast [20]. In support of this, when RAW264 cells stimulated by LPS—which have increased iNOS levels and a resultant increase in NO levels—are irradiated with X-rays, there is an increase in the intracellular production of peroxynitrite (Figure 3). Although high levels of increase were not observed in the present study, the induction of intracellular peroxynitrite production stimulated by LPS or irradiation was recently reported using a novel detection probe [21]. Peroxynitrite is also formed in vivo and plays numerous roles as a signal mediator in the development of many diseases, such as chronic inflammatory diseases, carcinogenesis, and neurodegenerative disorders [11,22]. In the tumor microenvironment, TAMs expressing iNOS promote tumor growth, invasion, immune evasion, and the acquisition of drug resistance [23]. Activated macrophages also produce superoxide via NOX activation and form peroxynitrite in the presence of NO and superoxide. This peroxynitrite could result in the oxidation and nitration of both proteins and lipids, leading to changes in cellular signaling [24]. In PDT, NO- and H2O2-producing agents have been reported to enhance sensitization to PDT [25,26]. They are thought to directly enhance the effect of PDT, but the effect of the RNS peroxynitrite has not been reported. The non-steroidal anti-inflammatory drug indomethacin has been used as an ROS inducer; it has been reported to enhance the cancer-specific accumulation of porphyrin and the overexpression of manganese superoxide dismutase (MnSOD, a mitochondrial superoxide scavenging enzyme) decreased HCP1 expression and the subsequent PDT effect [27]. These results suggest that the removal of superoxide by MnSOD could prevent the synthesis of peroxynitrite and the accumulation of intracellular porphyrin. In numerous types of cancer cells, elevated expression of iNOS, increased NO production, and the overgeneration of ROS have been reported [28,29,30,31]. In addition, TAMs possibly help to promote the expression of HCP1 in tumor cells via the production of peroxynitrite, as shown in Figure 3 and Figure 5, and may therefore lead to effective phototherapy.
In conclusion, a highly reactive nitrogen species, peroxynitrite, is generated in the mouse macrophage cell line RAW264 through the production of NO, which is produced as a result of the upregulated expression of iNOS by LPS treatment, and ROS, which is mainly superoxide derived from mitochondria, produced by irradiation with X-rays. It was observed that the cellular production of peroxynitrite contributed more toward upregulating the expression of HCP1 and enhanced intracellular porphyrin accumulation. This macrophage-mediated mechanism may therefore enhance the effect of PDT to suppress tumor growth. This knowledge could be used to develop novel agents and therapies targeting the activation of macrophages. To confirm this in humans, further studies including photoirradiation should be conducted using in vivo models.
Funding
This research was funded by Japan Society for the Promotion of Science (JSPS) KAKENHI, grant number JP19K16751.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Laftah, A.H.; Latunde-Dada, G.O.; Fakih, S.; Hider, R.C.; Simpson, R.J.; McKie, A.T. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). Br. J. Nutr. 2009, 101, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, K.; Matsui, H.; Tamura, M.; Shimokawa, O.; Hiyama, M.; Kaneko, T.; Nagano, Y.; Hyodo, I.; Tanaka, J.; Miwa, Y.; et al. Cancer cells uptake porphyrins via heme carrier protein 1. J. Porphyr. Phthalocyanines 2013, 17, 36–43. [Google Scholar] [CrossRef]
- Ito, H.; Matsui, H.; Tamura, M.; Majima, H.J.; Indo, H.P.; Hyodo, I. Mitochondrial reactive oxygen species accelerate the expression of heme carrier protein 1 and enhance photodynamic cancer therapy effect. J. Clin. Biochem. Nutr. 2014, 55, 67–71. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.I.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeon, Y.J. Macrophage activation by polysaccharide isolated from Astragalus membranaceus. Int. Immunopharmacol. 2005, 5, 1225–1233. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, H.S.; Kim, D.J.; Noh, Y.H.; Yuk, D.Y.; Hong, J.T. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264.7 cells. Arch. Pharmacal Res. 2008, 31, 342–349. [Google Scholar] [CrossRef]
- Motoori, S.; Majima, H.J.; Ebara, M.; Kato, H.; Hirai, F.; Kakinuma, S.; Yamaguchi, C.; Ozawa, T.; Nagano, T.; Tsujii, H.; et al. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res. 2001, 61, 5382–5388. [Google Scholar]
- Indo, H.P.; Davidson, M.; Yen, H.C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Brock, T.A.; Chang, L.Y.; Crapo, J.; Briscoe, P.; Ku, D.; Bradley, W.A.; Gianturco, S.H.; Gore, J.; Freeman, B.A. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA 1994, 91, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Inanami, O.; Koumura, T.; Suenaga, S.; Yen, H.C.; Kakinuma, S.; Matsumoto, K.I.; Nakanishi, I.; Saint Clair, W.; Saint Clair, D.K.; et al. Roles of mitochondria-generated reactive oxygen species on X-ray-induced apoptosis in a human hepatocellular carcinoma cell line, HLE. Free Radic. Res. 2012, 46, 1029–1043. [Google Scholar] [CrossRef]
- Shibata, Y.; Matsumura, A.; Yoshida, F.; Yamamoto, T.; Nakai, K.; Nose, T.; Sakata, I.; Nakajima, S. Competitive uptake of porphyrin and LDL via the LDL receptor in glioma cell lines: Flow cytometric analysis. Cancer Lett. 2001, 166, 79–87. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, F.; Ohgari, Y.; Yamaki, N.; Kitajima, S.; Shimokawa, O.; Matsui, H.; Taketani, S. The role of nitric oxide in δ-aminolevulinic acid (ALA)-induced photosensitivity of cancerous cells. Biochem. Biophys. Res. Commun. 2007, 353, 541–546. [Google Scholar] [CrossRef]
- Kurokawa, H.; Ito, H.; Terasaki, M.; Matano, D.; Taninaka, A.; Shigekawa, H.; Matsui, H. Nitric oxide regulates the expression of heme carrier protein-1 via hypoxia inducible factor-1α stabilization. PLoS ONE 2019, 14, e0222074. [Google Scholar] [CrossRef]
- Huie, R.E.; Padmaja, S. The reaction of no with superoxide. Free Radic. Res. 1993, 18, 195–199. [Google Scholar] [CrossRef]
- Kang, S.H.; Chung, B.Y.; Park, J.E.; Jeon, J.; Park, Y.D. Activatable red emitting fluorescent probe for rapid and sensitive detection of intracellular peroxynitrite. Talanta 2020, 217, 121053. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Pryor, W.A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem. Biol. Interact. 1995, 96, 203–206. [Google Scholar] [CrossRef]
- Perrotta, C.; Cervia, D.; Di Renzo, I.; Moscheni, C.; Bassi, M.T.; Campana, L.; Martelli, C.; Catalani, E.; Giovarelli, M.; Zecchini, S.; et al. Nitric oxide generated by tumor-associated macrophages is responsible for cancer resistance to cisplatin and correlated with syntaxin 4 and acid sphingomyelinase inhibition. Front. Immunol. 2018, 9, 1186. [Google Scholar] [CrossRef]
- Prolo, C.; Álvarez, M.N.; Radi, R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. BioFactors 2014, 40, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Lazzarato, L.; Gazzano, E.; Blangetti, M.; Fraix, A.; Sodano, F.; Picone, G.M.; Fruttero, R.; Gasco, A.; Riganti, C.; Sortino, S. Combination of PDT and NOPDT with a tailored BODIPY derivative. Antioxidants 2019, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nano Micro Lett. 2020, 12, 15. [Google Scholar] [CrossRef]
- Ito, H.; Matsui, H.; Hirayama, A.; Indo, H.P.; Majima, H.J.; Hyodo, I. Reactive oxygen species induced by non-steroidal anti-inflammatory drugs enhance the effects of Photodynamic therapy in gastric cancer cells. J. Clin. Biochem. Nutr. 2016, 58, 180–185. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Tamura, M.; Matsui, H.; Tomita, T.; Sadakata, H.; Indo, H.P.; Majima, H.J.; Kaneko, T.; Hyodo, I. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J. Clin. Biochem. Nutr. 2014, 54, 12–17. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).