Water-Induced Inverse Correlation between Temperature and Flux Changes in Vertical Vapor-Phase Diffusive Transport of Volatile Organic Compounds in Near-Surface Soil Environments
Abstract
1. Introduction
2. Materials and Methods
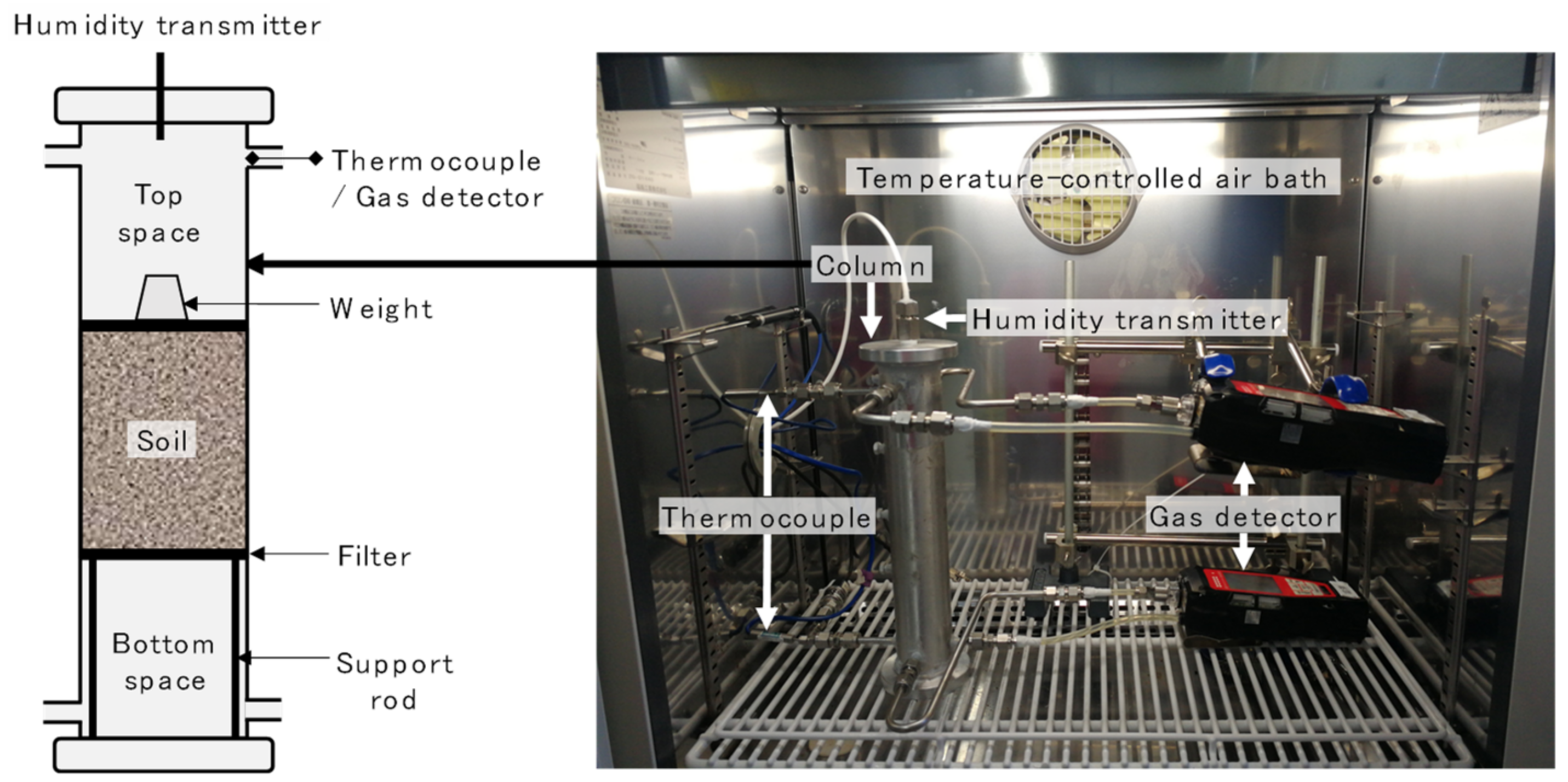
2.1. Experimental System
2.2. Experimental Procedures
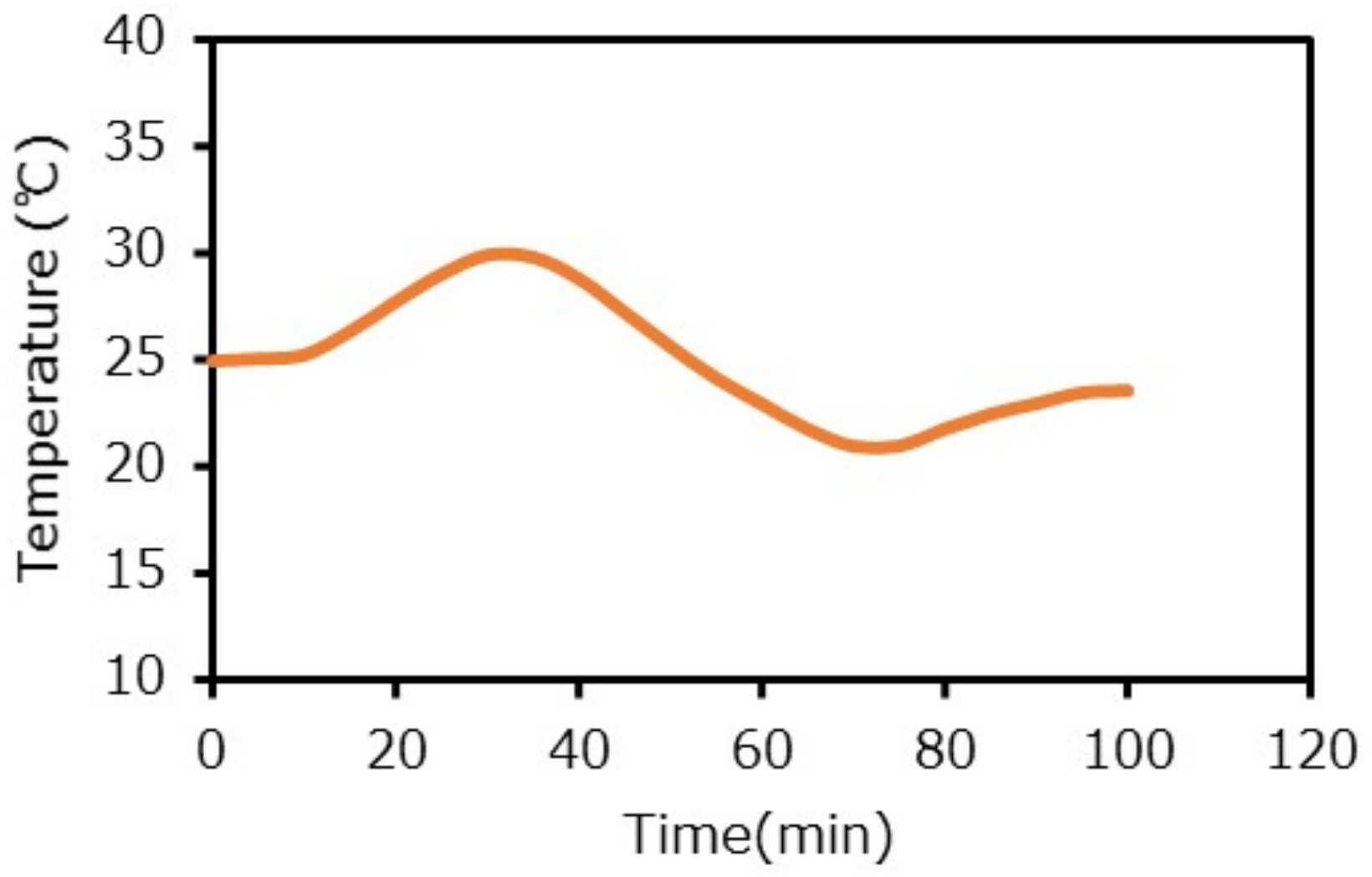
2.3. Experimental Conditions
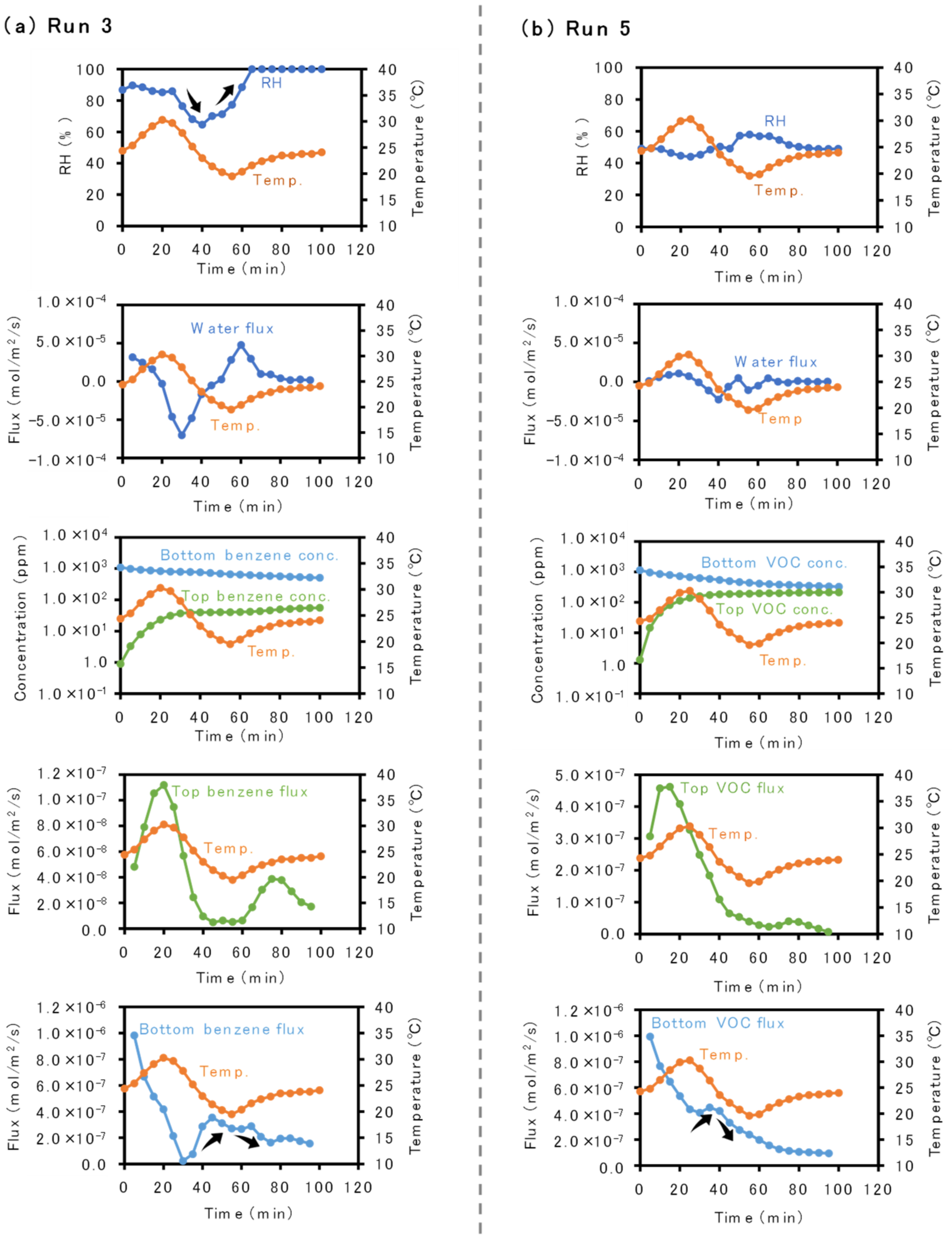
3. Results
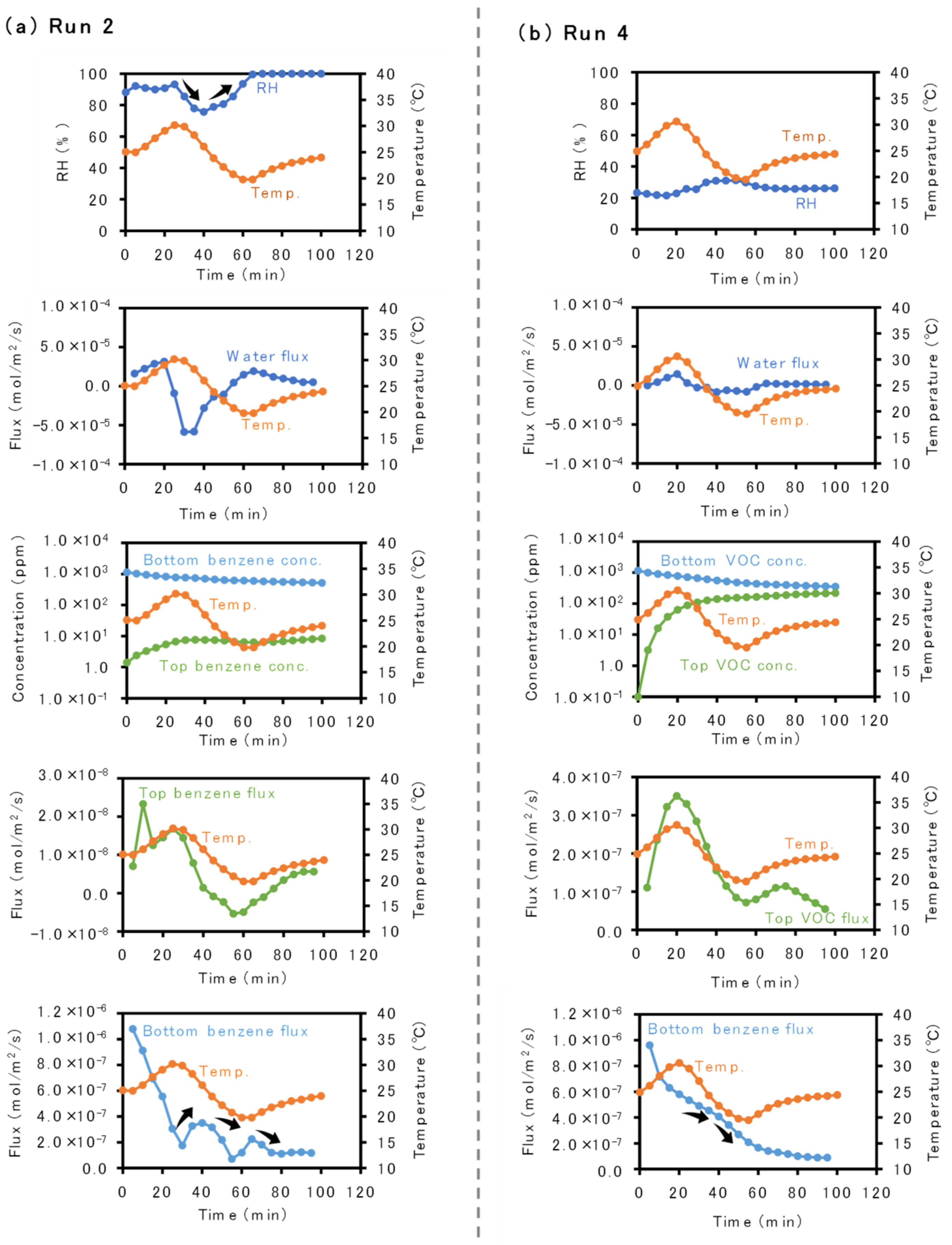
3.1. Inverse Correlation between Temperature and Benzene Flux Changes
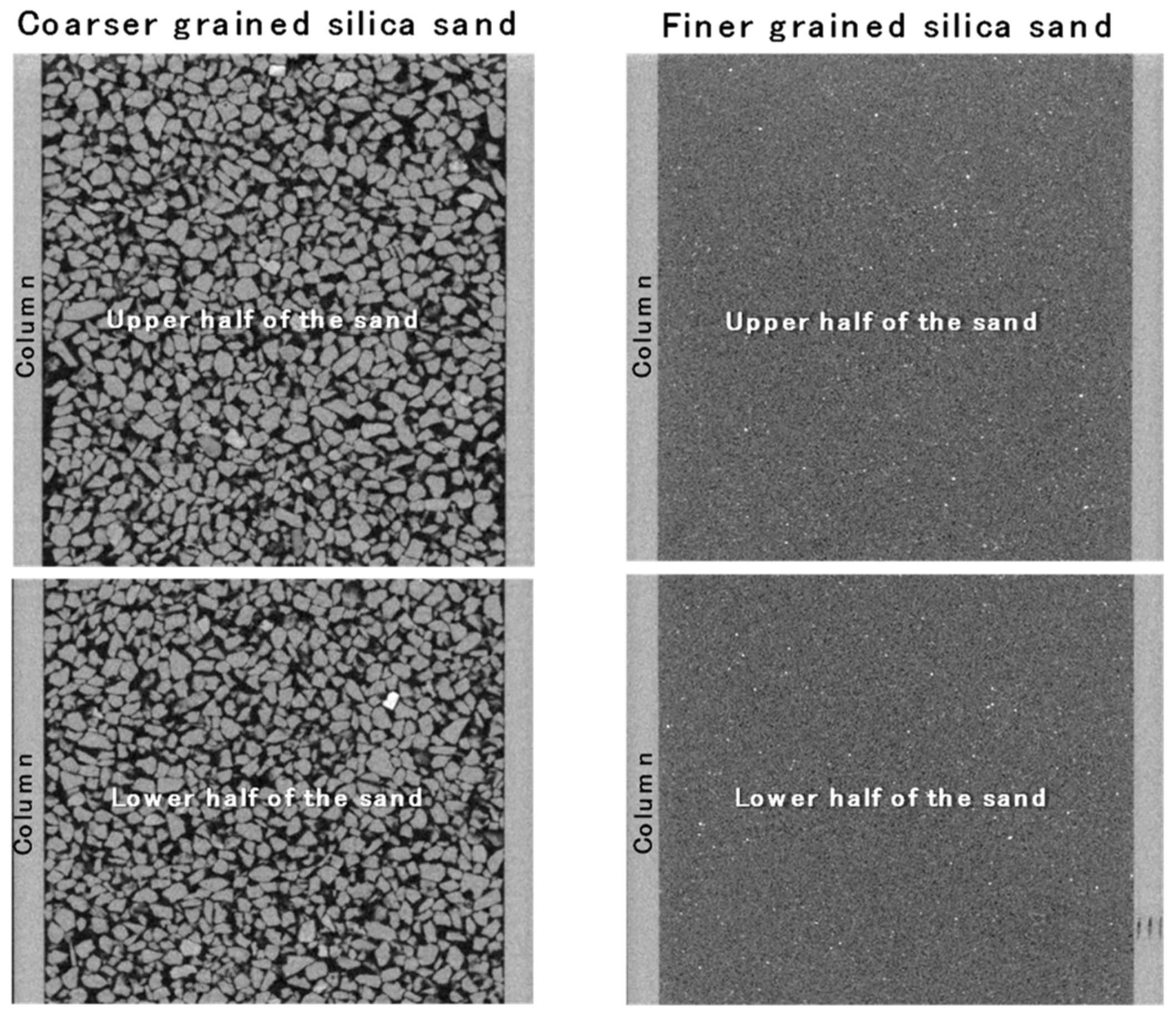
3.2. Influences of Sand Grain Size and Water Content on Inverse Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roveda, L.; Polvara, E.; Invernizzi, M.; Capelli, L.; Sironi, S. Definition of an emission factor for VOC emitted from Italian and European refineries. Atmosphere 2020, 11, 564. [Google Scholar] [CrossRef]
- Labianca, C.; De Gisi, S.; Picardi, F.; Todaro, F.; Notarnicola, M. Remediation of a petroleum hydrocarbon-contaminated site by soil vapor extraction: A full-scale case study. Appl. Sci. 2020, 10, 4261. [Google Scholar] [CrossRef]
- Liang, Q.; Bao, X.; Sun, Q.; Zhang, Q.; Zou, X.; Huang, C.; Shen, C.; Chu, Y. Imaging VOC distribution in cities and tracing VOC emission sources with a novel mobile proton transfer reaction mass spectrometer. Environ. Pollut. 2020, 265, 114628. [Google Scholar]
- Johnston, J.E.; Gibson, J.M. Spatiotemporal variability of tetrachloroethylene in residential indoor air due to vapor intrusion: A longitudinal, community-based study. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 564–571. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). OSWER Technical Guide for Assessing and Mitigating the Vapor Intrusion Pathway from Subsurface Vapor Sources to Indoor Air; EPA Office of Solid Waste and Emergency Response: Washington, DC, USA, 2015; pp. 2–154. [Google Scholar]
- Kponee, K.Z.; Nwanaji-Enwerem, J.C.; Fu, X.; Kakulu, I.I.; Weisskopf, M.G.; Jia, C. Elevated indoor volatile organic compound exposure in the Niger Delta Region of Nigeria. Int. J. Environ. Res. Public Health 2018, 15, 1939. [Google Scholar]
- Stefanakis, A.I. The fate of MTBE and BTEX in constructed wetlands. Appl. Sci. 2020, 10, 127. [Google Scholar]
- Bekele, D.N.; Naidu, R.; Chadalavada, S. Influence of spatial and temporal variability of subsurface soil moisture and temperature on vapour intrusion. Atmos. Environ. 2014, 88, 14–22. [Google Scholar] [CrossRef]
- Barnes, D.L.; McRae, M.F. The predictable influence of soil temperature and barometric pressure changes on vapor intrusion. Atmos. Environ. 2017, 150, 15–23. [Google Scholar]
- Song, C.; Liu, B.; Dai, Q.; Li, H.; Mao, H. Temperature dependence and source apportionment of volatile organic compounds (VOCs) at an urban site on the north China plain. Atmos. Environ. 2019, 207, 167–181. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Song, C.; Liu, J. Impact of temperature and microstructure on the emission characteristics of VOC in porous building materials. Procedia Eng. 2015, 121, 1067–1075. [Google Scholar] [CrossRef]
- Bai, Y.; Huo, L.; Zhang, Y.; Liu, J.; Shao, H.; Wu, C.; Guo, Z. A spatial fractional diffusion model for predicting the characteristics of VOCs emission in porous dry building material. Sci. Total Environ. 2020, 704, 135342. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Sagehashi, M.; Terada, A.; Hosomi, M. Characterization of benzene emission to the atmosphere from contaminated soil. Environ. Sci. 2010, 23, 467–475. [Google Scholar]
- Voudrias, E.A.; Li, C. Benzene vapor transport in unsaturated soil: Adequacy of the diffusion equation. J. Hazard. Mater. 1993, 34, 295–311. [Google Scholar] [CrossRef]
- Lin, T.F.; Little, J.C.; Nazaroff, W.W. Transport and sorption of volatile organic compounds and water vapor within dry soil grains. Environ. Sci. Technol. 1994, 28, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.F.; Loy, M.D.; Nazaroff, W.W. Gas-phase transport and sorption of benzene in soil. Environ. Sci. Technol. 1996, 30, 2178–2186. [Google Scholar] [CrossRef]
- Kim, J.Y.; Edil, T.B.; Park, J.K. Volatile organic compound (VOC) transport through compacted clay. J. Geotech. Geoenviron. 2001, 127, 126–134. [Google Scholar] [CrossRef]
- Osagie, E.I.; Cn, O. Investigation of mixture effects on the adsorption rates of aromatics from aqueous solution on clay and sandy soil. Chem. Sci. J. 2015, 6, 1–5. [Google Scholar]
- Cabbar, C.H.; Bostanci, A. Moisture effect on the transport of organic vapors in sand. J. Hazard. Mater. 2001, 82, 313–322. [Google Scholar] [CrossRef]
- Cotel, S.; Schäfer, G.; Barthes, V.; Baussand, P. Effect of density-driven advection on trichloroethylene vapor diffusion in a porous medium. Vadose Zone J. 2011, 10, 565–581. [Google Scholar] [CrossRef]
- Du, P.; Sagehashi, M.; Tarada, A.; Zhou, S.; Li, F.; Hosomi, M. Adequacy of a simple diffusion model to predict benzene behavior in soil. Soil Sci. Soc. Am. J. 2011, 75, 2147–2157. [Google Scholar] [CrossRef]
- Bekele, D.N.; Naidu, R.; Chadalavada, S. Influence of soil properties on vapor-phase sorption of trichloroethylene. J. Hazard. Mater. 2016, 306, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Shonnard, D.R.; Bell, R.L. Benzene emissions from a contaminated air-dry soil with fluctuations of soil temperature or RH. Environ. Sci. Technol. 1993, 27, 2909–2913. [Google Scholar] [CrossRef]
- Ausma, S.; Edwards, G.C.; Gillespie, T.J. Laboratory scale measurement of trace gas fluxes from land farm soils. J. Environ. Qual. 2003, 32, 8–22. [Google Scholar] [CrossRef]
- Huang, C.W. Study on Particle Breakage of Sands Subjected to Various Confining Stress and Shear Strain Levels. Ph.D. Thesis, Tokyo Metropolitan University, Tokyo, Japan, 30 September 2015. [Google Scholar]
- Nakarai, K.; Yoshida, T. Effect of carbonation on strength development of cement treated Toyoura silica sand. Soils Found. 2015, 55, 857–865. [Google Scholar] [CrossRef]
- Simatupang, M.; Sukri, A.S.; Nasrul; Sulha; Putri, T.S. Effect of confining pressures on the shear modulus of sand treated with enzymatically induced calcite precipitation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 615, 012042. [Google Scholar] [CrossRef]
- Kojima, T.; Saito, H.; Fukuda, K.; Takuma, Y.; Kato, S.; Satokawa, S. The influence of soil and organic matter on trichloroethylene decomposition by Fenton reaction. J. Chem. Eng. Jpn. 2009, 42, s23–s28. [Google Scholar] [CrossRef][Green Version]
- Kimura, S.; Kaneko, H.; Ito, T.; Minagawa, H. Investigation of fault permeability in sands with different mineral compositions (evaluation of gas hydrate reservoir). Energies 2015, 8, 7202–7223. [Google Scholar] [CrossRef]
- Yokura, K.; Yamamoto, H.; Wu, Y. Crushing tests of soil particles by high pressure true tri-axial compression apparatus. Jpn. Geotech. Soc. Spec. Publ. 2015, 1, 51–56. [Google Scholar] [CrossRef]
- Siegrist, R.L. Volatile organic compounds in contaminated soils: The nature and validity of the measurement process. J. Hazard. Mater. 1991, 29, 3–15. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). EPA On-Line Tools for Site Assessment Calculation. Available online: https://www3.epa.gov/ceampubl/learn2model/part-two/onsite (accessed on 12 February 2021).
- Mäki, M.; Krasnov, D.; Hellén, H.; Noe, S.M.; Bäck, J. Stand type affects fluxes of volatile organic compounds from the forest floor in hemiboreal and boreal climates. Plant Soil 2019, 441, 363–381. [Google Scholar] [CrossRef]
- Ingram, S.; Cai, C.; Song, Y.-C.; Glowacki, D.R.; Topping, D.O.; O’Meara, S.; Reid, J.P. Characterising the evaporation kinetics of water and semi-volatile organic compounds from viscous multicomponent organic aerosol particles. Phys. Chem. Chem. Phys. 2017, 19, 31634–31646. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Hunt, S.L.; Smith, J.A. Measurement of effective air diffusion coefficients for trichloroethene in undisturbed soil cores. J. Contam. Hydrol. 2002, 56, 193–208. [Google Scholar] [CrossRef]
- Gao, W.; Liu, F.; Chan, H. Gas phase effective diffusion coefficient of gasoline and characteristic components in dry fine sand. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009. [Google Scholar]
- Pandey, P.; Yadav, R. A review on volatile organic compounds (VOCs) as environmental pollutants: Fate and distribution. Int. J. Plant Environ. 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Delle Site, A. Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants: A review. J. Phys. Chem. Ref. Data 2001, 30, 187–439. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chaing, P.C.; Huang, C.P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Baioni, E.; Porta, G.M.; Mousavi Nezhad, M.; Guadagnini, A. Assessment of turbulence effects on effective solute diffusivity close to a sediment-free fluid interface. Stoch. Environ. Res. Risk Assess. 2020, 34, 2211–2228. [Google Scholar] [CrossRef]
- Schaper, J.L.; Posselt, M.; Bouchez, C.; Jaeger, A.; Nuetzmann, G.; Putschew, A.; Singer, G.; Lewandowski, J. Fate of trace organic compounds in the hyporheic zone: Influence of retardation, the benthic biolayer, and organic carbon. Environ. Sci. Technol. 2019, 53, 4224–4234. [Google Scholar] [CrossRef]


| Experiment | Silica Sand | Water Content (wt%) | Temperature |
|---|---|---|---|
| Run 1 | Coarser-grained | 10 | Static |
| Run 2 | Coarser-grained | 10 | Dynamic |
| Run 3 | Finer-grained | 10 | Dynamic |
| Run 4 | Coarser-grained | Air-dried (~0.3) | Dynamic |
| Run 5 | Finer-grained | Air-dried (~0.3) | Dynamic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlin, A.A.; Watanabe, N.; Nakamura, K.; Yamada, M.; Wang, J.; Komai, T. Water-Induced Inverse Correlation between Temperature and Flux Changes in Vertical Vapor-Phase Diffusive Transport of Volatile Organic Compounds in Near-Surface Soil Environments. Appl. Sci. 2021, 11, 3489. https://doi.org/10.3390/app11083489
Parlin AA, Watanabe N, Nakamura K, Yamada M, Wang J, Komai T. Water-Induced Inverse Correlation between Temperature and Flux Changes in Vertical Vapor-Phase Diffusive Transport of Volatile Organic Compounds in Near-Surface Soil Environments. Applied Sciences. 2021; 11(8):3489. https://doi.org/10.3390/app11083489
Chicago/Turabian StyleParlin, Asma Akter, Noriaki Watanabe, Kengo Nakamura, Mizuki Yamada, Jiajie Wang, and Takeshi Komai. 2021. "Water-Induced Inverse Correlation between Temperature and Flux Changes in Vertical Vapor-Phase Diffusive Transport of Volatile Organic Compounds in Near-Surface Soil Environments" Applied Sciences 11, no. 8: 3489. https://doi.org/10.3390/app11083489
APA StyleParlin, A. A., Watanabe, N., Nakamura, K., Yamada, M., Wang, J., & Komai, T. (2021). Water-Induced Inverse Correlation between Temperature and Flux Changes in Vertical Vapor-Phase Diffusive Transport of Volatile Organic Compounds in Near-Surface Soil Environments. Applied Sciences, 11(8), 3489. https://doi.org/10.3390/app11083489







