Stability of Bituminous Emulsion Induced by Waste Based Bio-Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparations
Asphaltene Determination
2.3. Rheological Characterization
2.4. NMR Measurement
2.5. Bitumen Acidic Number Determination Method
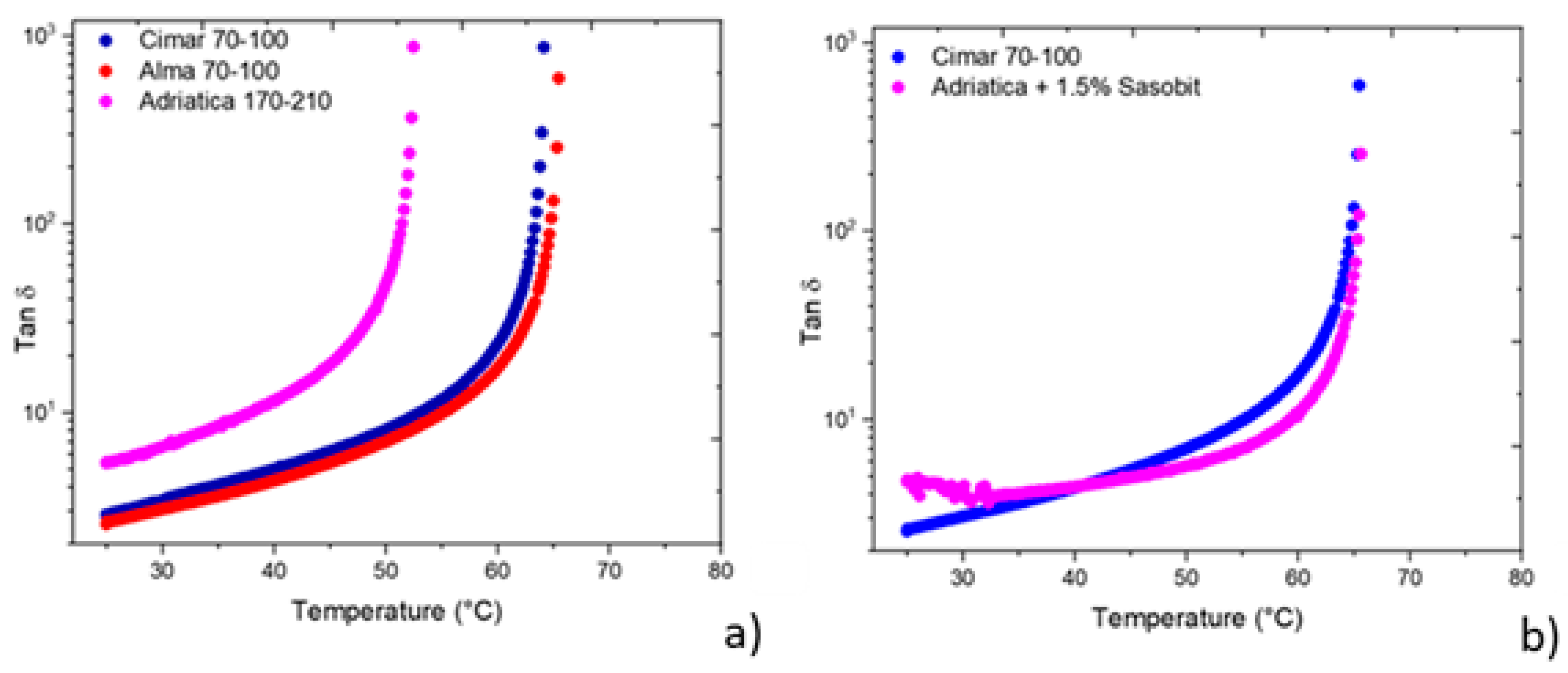
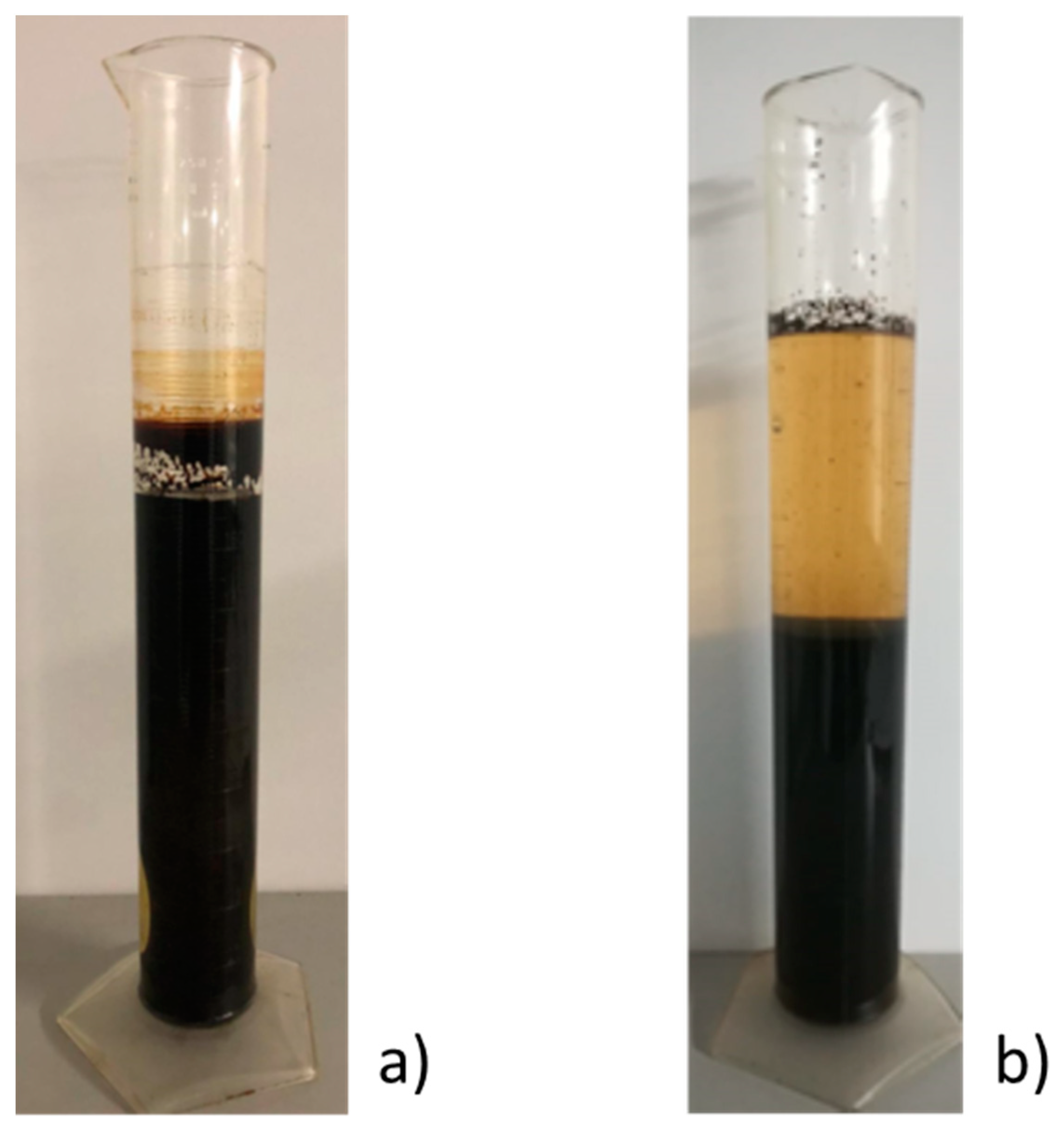
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leal-Calderon, F.; Schmitt, V.; Bibette, J. Emulsion Science: Basic Principles, 2nd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Salanger, J.L.; Màrquez, L.; Mira, I.; Pena, A.; Tyrode, E.; Zambran, N.B. Principles of Emulsion Formulation Engineering. In Adsorption and Aggregation of Surfactants in Solution; Mittal, K.L., Shah, D.O., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 501–523. [Google Scholar]
- Kabalnov, A. Thermodynamic and theoretical aspects of emulsions and their stability. Curr. Opin. Colloid Interface Sci. 1998, 3, 270–275. [Google Scholar] [CrossRef]
- Acevedo, S.; Gutierrez, X.; Rivas, H. Bitumen-in-Water Emulsions Stabilized with Natural Surfactants. J. Colloid Interface Sci. 2001, 242, 230–238. [Google Scholar] [CrossRef]
- Villalba, G.U.; Máximo, G.S. Effect of non-homogeneous spatial distributions of surfactants on the stability of high-content bitumen-in-water emulsions. Interciencia 2000, 25, 415–422. [Google Scholar]
- Babchin, A.J.; Schramm, L.L. Osmotic repulsion force due to adsorbed surfactants. Colloids Surf. B Biointerfaces 2012, 91, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ignatavicius, S.; Kavanagh, A.; Colleran, D.; Brennan, M.J.; Newell, S. Experimental investigation of optimum properties and conditions for use of anionic emulsions in road maintenance applications. In Proceedings of the Civil Engineering Research in Ireland 2020 (CERI 2020), Cork Institute of Technology, Cork, Ireland, 27–28 August 2020; ISBN 978-0-9573957-4-9. Available online: https://sword.cit.ie/monographs/1 (accessed on 6 April 2021).
- Gingras, J.-P.; Tanguy, P.A.; Mariotti, S.; Chaverot, P. Effect of process parameters on bitumen emulsions. Chem. Eng. Process. Process. Intensif. 2005, 44, 979–986. [Google Scholar] [CrossRef]
- Jair, M. Bitumen Emulsions. In The Shell Bitumen Handbook, 6th ed.; Hunter, R.N., Self, A., Read, J., Eds.; ICE Publishing: Westminster/London, UK, 2018; pp. 185–214. [Google Scholar]
- International Bitumen Emulsion Federation Publication, Asphalt Emulsions: Beyond Pavement Preservation. Available online: http://www.ibef.net/download/1155/ (accessed on 6 November 2020).
- Yuliestyan, A.; García-Morales, M.; Moreno, E.; Carrera, V.; Partal, P. Assessment of modified lignin cationic emulsifier for bitumen emulsions used in road paving. Mater. Des. 2017, 131, 242–251. [Google Scholar] [CrossRef]
- Dołżycki, B.; Jaskuła, P. Review and evaluation of cold recycling with bitumen emulsion and cement for rehabilitation of old pavements. J. Traffic Transp. Eng. 2019, 6, 311–323. [Google Scholar] [CrossRef]
- Day, D.; Lancaster, I.M.; McKay, D. Emulsion cold mix asphalt in the UK: A decade of site and laboratory experience. J. Traffic Transp. Eng. 2019, 6, 359–365. [Google Scholar] [CrossRef]
- Ronald, M.; Pumarejo Luis, F. Asphalt emulsions formulation: State-of-the-art and dependency of formulation on emulsions properties. Constr. Build. Mater. 2016, 123, 162–173. [Google Scholar] [CrossRef]
- Yan, Z.; Elliott, J.A.W.; Masliyah, J.H. Roles of Various Bitumen Components in the Stability of Water-in-Diluted-Bitumen Emulsions. J. Colloid Interface Sci. 1999, 220, 329–337. [Google Scholar] [CrossRef]
- Abdelfatah, E.; Chen, Y.; Berton, P.; Rogers, R.D.; Bryant, S.L. Tuning Ionic Liquids for Simultaneous Dilution and Demulsification of Water-In-Bitumen Emulsions at Ambient Temperature. SPE J. 2020, 25, 759–770. [Google Scholar] [CrossRef]
- Ziyani, L.; Gaudefroy, V.; Ferber, V.; Deneele, D.; Hammoum, F. Chemical reactivity of mineral aggregates in aqueous solution: Relationship with bitumen emulsion breaking. J. Mater. Sci. 2014, 49, 2465–2476. [Google Scholar] [CrossRef]
- Rossi, O.; Caputo, P.; de Luca, G.; Maiuolo, L.; Eskandarsefat, S.; Sangiorgi, C. 1H-NMR Spectroscopy: A Possible Approach to Advanced Bitumen Characterization for Industrial and Paving Applications. Appl. Sci. 2018, 8, 229. [Google Scholar] [CrossRef]
- Caputo, P.; Porto, M.; Calandra, P.; de Santo, M.P.; Rossi, C.O. Effect of epoxidized soybean oil on mechanical properties of bitumen and aged bitumen. Mol. Cryst. Liq. 2018, 675, 68–74. [Google Scholar] [CrossRef]
- Gafonova, V.O.; Yarranton, H.W. The Stabilization of Water-in-Hydrocarbon Emulsionsby Asphaltenes and Resins. J. Colloid Interface Sci. 2001, 241, 469–478. [Google Scholar] [CrossRef]
- Samieadel, A.; Fini, E.H. Interplay between wax and polyphosphoric acid and its effect on bitumen thermomechanical properties. Constr Build Mater. 2020, 243, 118194. [Google Scholar] [CrossRef]
- Zieliński, K.; Babiak, M.; Ratajczak, M.; Kosno, J. Impact of chemical and physical modification on thermoplastic characteristics of bitumen. Procedia Eng. 2017, 172, 1297–1304. [Google Scholar] [CrossRef]
- Xia, T.; Chen, X.; Xu, J.; Chen, W.; Zhang, A. Effect of annealing method and chemical reaction on the structure and properties of polyethylene/polyethylene glycol modified bitumen. Constr. Build. Mater. 2020, 269, 121228. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Partal, P.; Navarro, F.J.; García-Morales, M.; Gallegos, C. Bitumen chemical modification by thiourea dioxide. Fuel 2011, 90, 2294–2300. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; de Filpo, G.; Rossi, C.O.; Calandra, P. Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior. Appl. Sci. 2019, 9, 5564. [Google Scholar] [CrossRef]
- Caputo, P.; Porto, M.; Loise, V.; Teltayev, B.; Rossi, C.O. Analysis of mechanical performance of bitumen modified with waste plastic and rubber (SBR) additives by rheology and PGSE NMR experiments. Eurasian Chem. Technol. J. 2019, 21, 235–239. [Google Scholar] [CrossRef]
- Sengoz, B.; Isikyakar, G. Evaluation of the properties and microstructure of SBS and EVA polymer modified bitumen. Constr. Build. Mater. 2008, 22, 1897–1905. [Google Scholar] [CrossRef]
- Pérez-Lepe, A.; Martínez-Boza, F.J.; Gallegos, C.; González, O.; Muñoz, M.E.; Santamaría, A. Influence of the processing conditions on the rheological behaviour of polymer-modified bitumen. Fuel 2003, 82, 1339–1348. [Google Scholar] [CrossRef]
- Edwards, Y. Influence of Waxes on Bitumen and Asphalt Concrete Mixture Performance. Road Mater. Pavement Des. 2009, 10, 313–335. [Google Scholar] [CrossRef]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Fan, W.; Wang, H.; Erkens, S. Rheological properties, compatibility, and storage stability of SBS latex-modified asphalt. Materials 2019, 12, 3683. [Google Scholar] [CrossRef]
- Liang, M.; Sun, C.; Yao, Z.; Jiang, H.; Zhang, J.; Ren, S. Utilization of wax residue as compatibilizer for asphalt with ground tire rubber/recycled polyethylene blends. Constr. Build. Mater. 2020, 230, 116966. [Google Scholar] [CrossRef]
- Lin, P.; Liu, X.; Apostolidis, P.; Erkens, S.; Ren, S.; Scarpas, S.X.T.; Huang, W. On the rejuvenator dosage optimization for aged SBS modified bitumen. Constr. Build. Mater. 2021, 271, 121913. [Google Scholar] [CrossRef]
- Qin, Q.; Farrar, M.J.; Pauli, A.T.; Adams, J.J. Morphology, thermal analysis and rheology of Sasobit modified warm mix asphalt binders. Fuel 2014, 115, 416–425. [Google Scholar] [CrossRef]
- Solouki, A.; Muniandy, R.; Hassim, S.; Kheradmand, B. Rheological Property Investigation of Various Sasobit-modified Bitumen. Pet. Sci. Technol. 2015, 33, 773–779. [Google Scholar] [CrossRef]
- Yousefi, A.; Nowruzi, A.B.A.; Haghshenas, H. Performance evaluation of asphalt mixtures containing warm mix asphalt (WMA) additives and reclaimed asphalt pavement (RAP). Constr. Build. Mater. 2020, 268, 121200. [Google Scholar] [CrossRef]
- Borrego, A.G.; Blanco, C.G.; Prado, J.G.; Díaz, C.; Guillén, M.D. 1H NMR and FTIR Spectroscopic Studies of Bitumen and Shale Oil from Selected Spanish Oil Shales. Energy Fuel 1996, 10, 77–84. [Google Scholar] [CrossRef]
- Caputo, P.; Loise, V.; Ashimova, S.; Teltayev, B.; Vaiana, R.; Rossi, C.O. Inverse Laplace Transform (ILT)NMR: A powerful tool to differentiate a real rejuvenator and a softener of aged bitumen. Colloids Surf. A 2019, 574, 154–161. [Google Scholar] [CrossRef]
- Rossi, C.O.; Caputo, P.; Loise, V.; Ashimova, S.; Teltayev, B.; Sangiorgi, C. A New Green Rejuvenator: Evaluation of Structural Changes of Aged and Recycled Bitumens by Means of Rheology and NMR. In RILEM 252-CMB 2018, RILEM Bookseries 20; Poulikakos, L.D., Falchetto, A.C., Wistuba, M., Hofko, B., Porot, L., Di Benedetto, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 177–182. [Google Scholar]
- Noda, A.; Susan, M.A.B.H.; Kudo, K.; Mitsushima, S.; Hayamizu, K.; Watanabe, M. Brønsted Acid−Base Ionic Liquids as Proton-Conducting Nonaqueous Electrolytes. J. Phys. Chem. B 2003, 107, 4024–4033. [Google Scholar] [CrossRef]
- Uraguchi, D.; Terada, M. Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation. J. Am. Chem. Soc. 2004, 126, 5356–5357. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Tan, J.; Liu, G.; Xu, J.; Sun, D. Pickering Emulsions Stabilized by a Lipophilic Surfactant and Hydrophilic Platelike Particles. Langmuir 2010, 26, 5397–5404. [Google Scholar] [CrossRef]
- Kiran, S.K.; Acosta, E.J.; Moran, K. Evaluating the hydrophilic-lipophilic nature of asphaltenic oils and naphthenic amphiphiles using microemulsion models. J. Colloid Interface Sci. 2009, 336, 304–313. [Google Scholar] [CrossRef]
- Calandra, P.; Caputo, P.; de Santo, M.P.; Todaro, L.; Liveri, V.T.; Rossi, C.O. Effect of additives on the structural organization of asphaltene aggregates in bitumen. Constr. Build. Mater. 2019, 119, 288–297. [Google Scholar] [CrossRef]
- Johansson, I.; Svensson, M. Surfactants based on fatty acids and other natural hydrophobes. Curr. Opin. Colloid Interface Sci. 2011, 6, 178–188. [Google Scholar] [CrossRef]
- Holmberg, K. Natural surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 148–159. [Google Scholar] [CrossRef]


| Measured Properties | Standard | Unit | Cimar | Adriatica | Alma |
|---|---|---|---|---|---|
| 70/100 | 170/210 | 70/100 | |||
| Penetration at 25 °C | EN 1426 | 0.1 mm | 66 ± 1 | 185 ± 1 | 68 ± 1 |
| Softening point | EN 1427 | °C | 47.8 ± 0.2 | 42.6 ± 0.2 | 47.2 ± 0.2 |
| Flash point | EN 2592 | °C | ≥230 | ≥220 | ≥230 |
| Solubility | EN 12592 | % (m/m) | ≥99 | ≥99 | ≥99 |
| Bitumen | Acid Number (mg/g KOH) |
|---|---|
| Cimar | 22.7 |
| Alma | 13.5 |
| Adriatica | 14.7 |
| Bitumen | Transition Temperature ± 0.1 (°C) | Asphaltene ± 1 (%) |
|---|---|---|
| Cimar | 64.1 | 17 |
| Alma | 64.4 | 31 |
| Adriatica | 52.4 | 19 |
| Sample | Hydrogen Distribution ± 0.05 | |||
|---|---|---|---|---|
| Har | Hα | Hβ | Hγ | |
| Cimar Bitumen | 3.88 | 12.26 | 56.95 | 26.91 |
| Adriatica Bitumen | 4.17 | 12.46 | 53.50 | 29.87 |
| Alma Bitumen | 5.75 | 14.46 | 55.52 | 24.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, M.; Caputo, P.; A. Abe, A.; Loise, V.; Oliviero Rossi, C. Stability of Bituminous Emulsion Induced by Waste Based Bio-Surfactant. Appl. Sci. 2021, 11, 3280. https://doi.org/10.3390/app11073280
Porto M, Caputo P, A. Abe A, Loise V, Oliviero Rossi C. Stability of Bituminous Emulsion Induced by Waste Based Bio-Surfactant. Applied Sciences. 2021; 11(7):3280. https://doi.org/10.3390/app11073280
Chicago/Turabian StylePorto, Michele, Paolino Caputo, Abraham A. Abe, Valeria Loise, and Cesare Oliviero Rossi. 2021. "Stability of Bituminous Emulsion Induced by Waste Based Bio-Surfactant" Applied Sciences 11, no. 7: 3280. https://doi.org/10.3390/app11073280
APA StylePorto, M., Caputo, P., A. Abe, A., Loise, V., & Oliviero Rossi, C. (2021). Stability of Bituminous Emulsion Induced by Waste Based Bio-Surfactant. Applied Sciences, 11(7), 3280. https://doi.org/10.3390/app11073280









