Fermented Rice Germ Extract Ameliorates Abnormal Glucose Metabolism via Antioxidant Activity in Type 2 Diabetes Mellitus Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sample
2.2. Animals and Diets
2.3. Fasting Blood Glucose Level
2.4. Oral Glucose Tolerance Test (OGTT)
2.5. Hepatic Lipid Level
2.6. Lipid Peroxidation
2.7. Hepatic Catalase
2.8. SOD Activity
2.9. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Body Weight, Food Intake, and Organ Weight
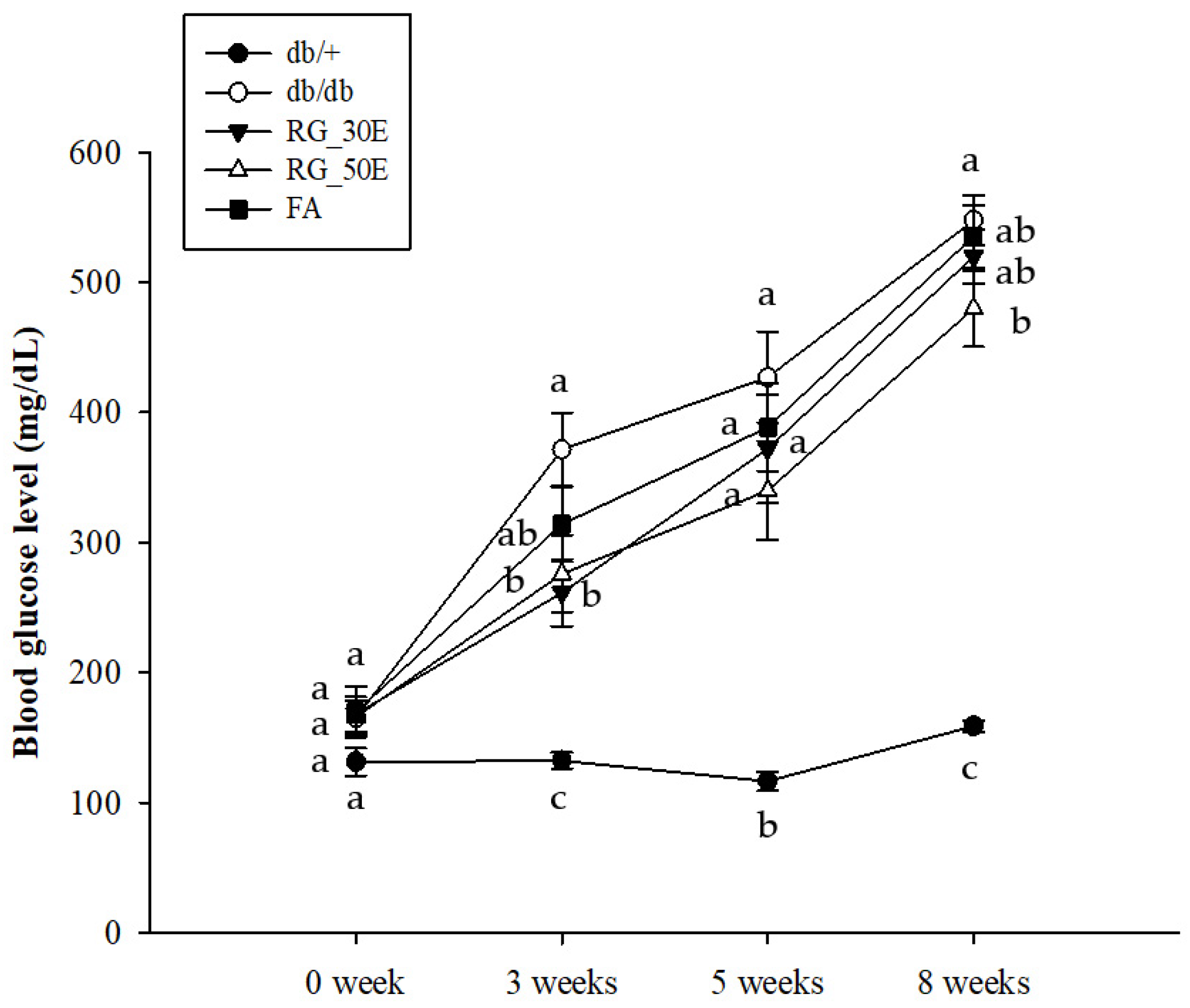
3.2. Fasting Blood Glucose Levels
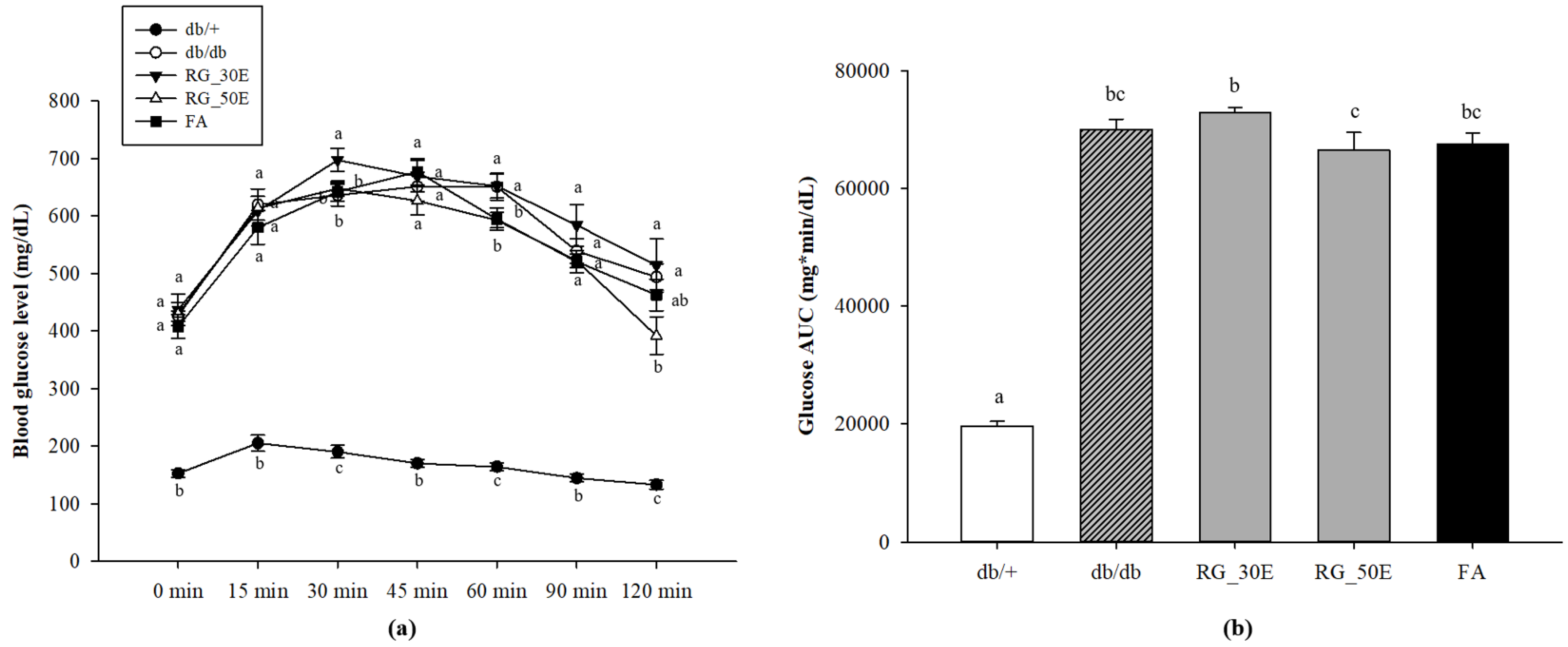
3.3. OGTT
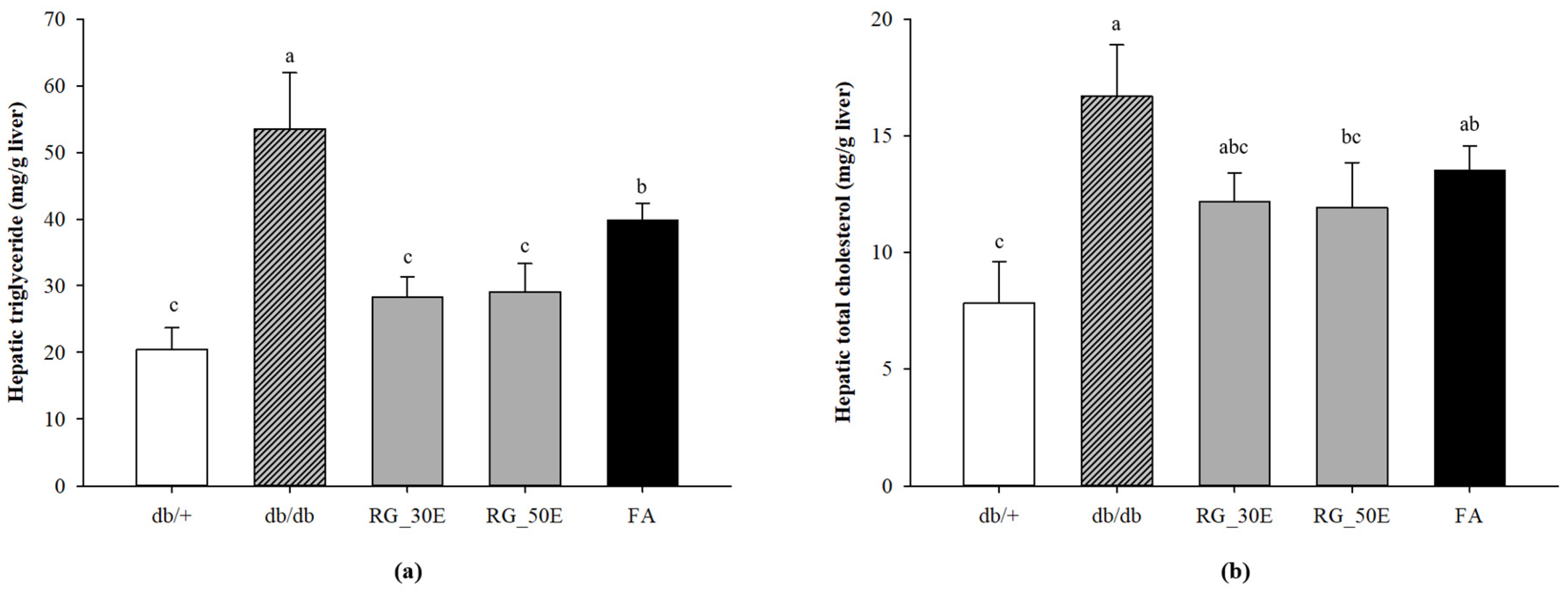
3.4. Hepatic TG and TC Concentration
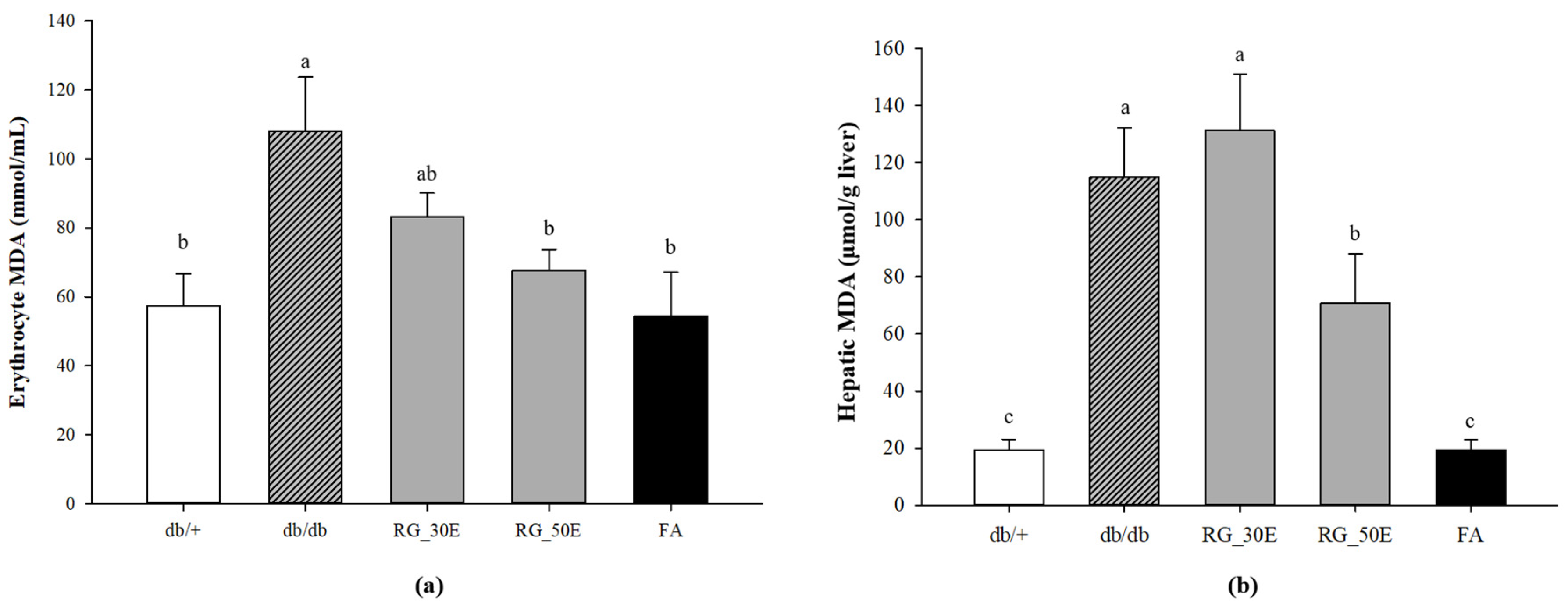
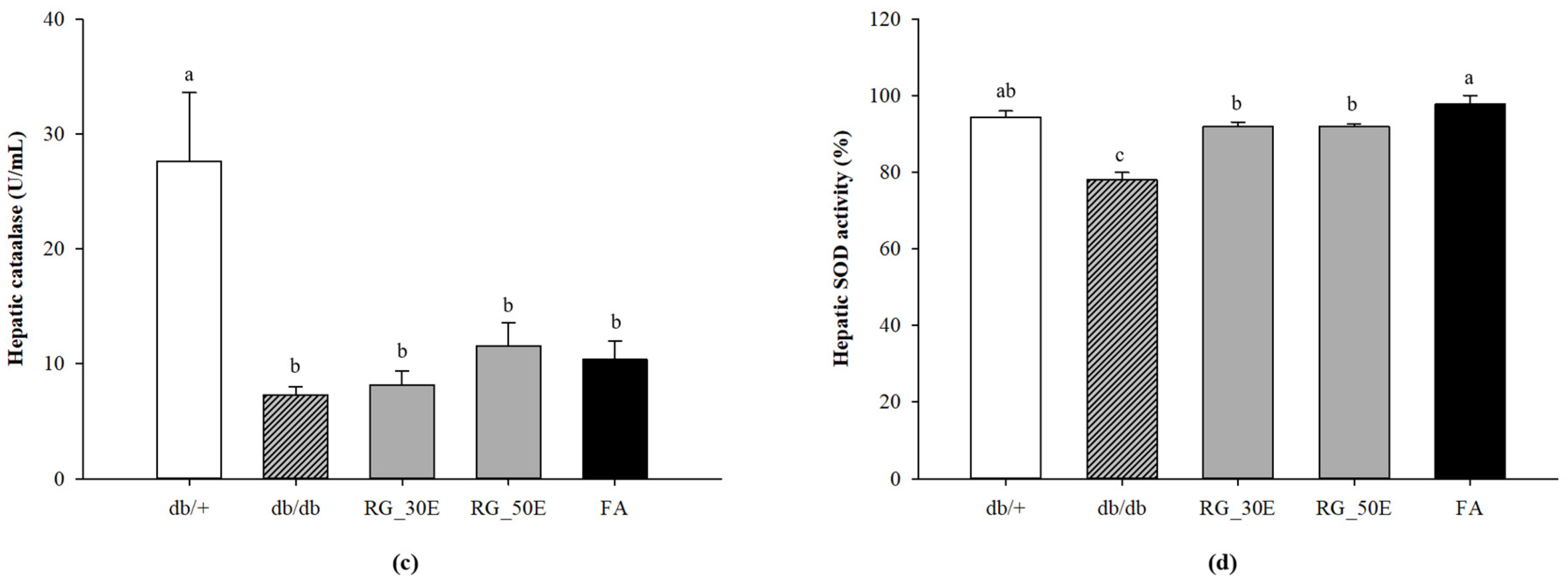
3.5. Oxidative Stress-Related Biomarkers
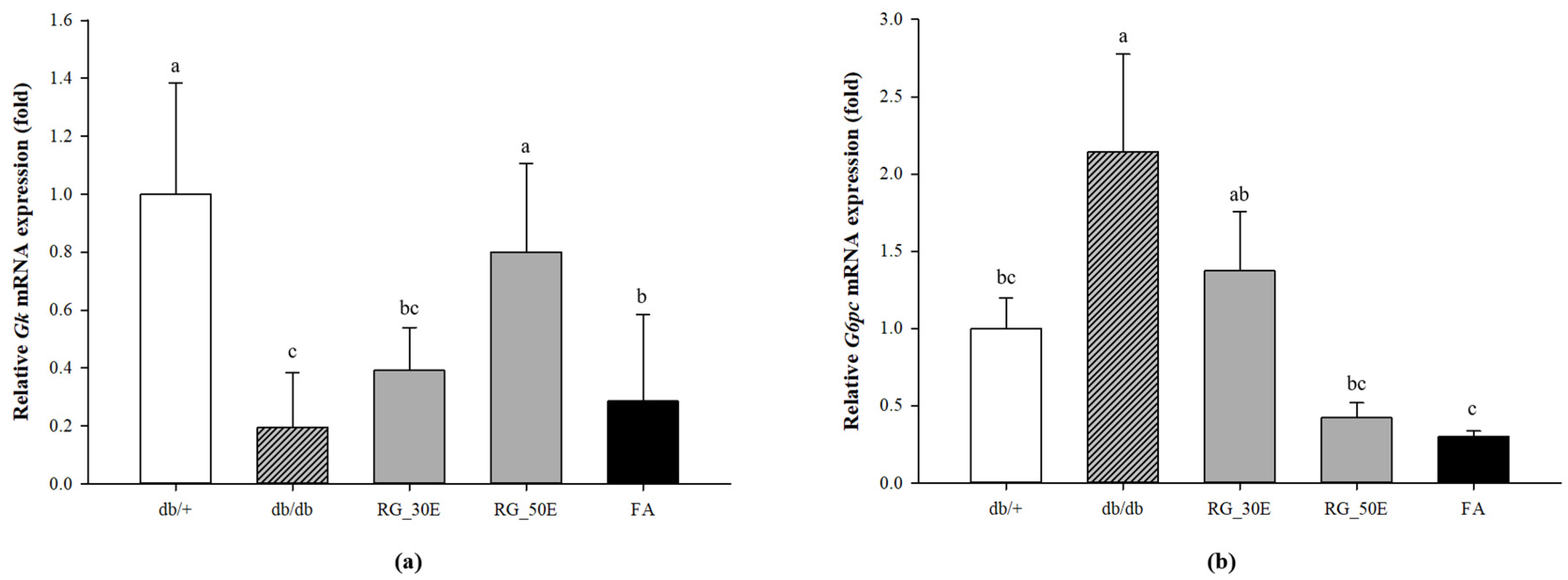
3.6. Expression of Genes Related Glycolysis and Gluconeogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RG_30E | fermented rice germ extract with 30% ethanol |
| RG_50E | fermented rice germ extract with 50% ethanol |
| FA | ferulic acid |
| T2DM | type 2 diabetes mellitus |
| GK | glucokinase |
| G6PC | glucose-6-phosphatase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| ROS | reactive oxygen species |
| GSH | glutathione |
| AUC | area under the curve |
| TG | triglyceride |
| TC | total cholesterol |
| OGTT | oral glucose tolerance test |
| NPY | neuropeptide Y |
References
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, D.; Kang, J.; Meng, X.; Yang, J.; Yang, L.; Xue, N.; Gao, Q.; Han, S.; Gou, X. Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Uto, H.; Ueki, K.; Takamura, T.; Kohgo, Y.; Kawata, S.; Yasui, K.; Park, H.; Nakamura, N.; Nakatou, T.; et al. Clinicopathological features of liver injury in patients with type 2 diabetes mellitus and comparative study of histologically proven nonalcoholic fatty liver diseases with or without type 2 diabetes mellitus. J. Gastroenterol. 2013, 48, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dang, Y.; Li, Q.; Zhou, W.; Zuo, J.; Yao, Z.; Zhang, L.; Ji, G. Berberine alleviates hyperglycemia by targeting hepatic glucokinase in diabetic db/db mice. Sci. Rep. 2019, 9, 8003. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2018, 829, 112–120. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine Int. J. Phytother. Phytopharm. 2009, 16, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, H.; Zhu, C.; Deng, J.; Fan, D. Ginsenoside Rg5 relieves type 2 diabetes by improving hepatic insulin resistance in db/db mice. J. Funct. Foods 2020, 71, 104014. [Google Scholar] [CrossRef]
- Goedeke, L.; Perry, R.J.; Shulman, G.I. Emerging Pharmacological Targets for the Treatment of Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Type 2 Diabetes. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 65–87. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Goncalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Nisa, K.; Rosyida, V.T.; Nurhayati, S.; Indrianingsih, A.W.; Darsih, C.; Apriyana, W. Total phenolic contents and antioxidant activity of rice bran fermented with lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012020. [Google Scholar] [CrossRef]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Kamil, D.; Justyna, R.K.; Ewa, N.; Gabruela, K. Characterisrics and biological properites of ferulic acid. Biotechnol. Food Sci. 2019, 83, 71–85. [Google Scholar]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. BioFactors 2004, 21, 315–319. [Google Scholar] [CrossRef]
- Gohil, K.J.; Kshirsagar, S.B.; Sahane, R.S. Ferulic acid—A comprehensive pharmacology of an important bioflavonoid. Int. J. Pharm. Sci. Res. 2012, 3, 700–710. [Google Scholar]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. Ptr 2004, 18, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Tamaya, K.; Matsui, T.; Toshima, A.; Noguchi, M.; Ju, Q.; Miyata, Y.; Tanaka, T.; Tanaka, K. Suppression of blood glucose level by a new fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves in disaccharide-loaded Sprague-Dawley rats. J. Sci. Food Agric. 2010, 90, 779–783. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Yildirim, A.; Gumus, M.; Dalga, S.; Sahin, Y.N.; Akcay, F. Dehydroepiandrosterone improves hepatic antioxidant systems after renal ischemia-reperfusion injury in rabbits. Ann. Clin. Lab. Sci. 2003, 33, 459–464. [Google Scholar]
- Deng, N.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. IRS-1/PI3K/Akt pathway and miRNAs are involved in whole grain highland barley (Hordeum vulgare L.) ameliorating hyperglycemia of db/db mice. Food Funct. 2020, 11, 9535–9546. [Google Scholar] [CrossRef]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Ternero, C.P.; Sotomayor, M.A.; Herrea, M.D. Contribution of ferulic acid, γ-oryzanol and tocotrienols to the cardiometabolic protective effects of rice bran. J. Funct. Foods 2017, 32, 58–71. [Google Scholar] [CrossRef]
- Sindhu, S.C.; Khetarpaul, N. Effect of probiotic fermentation on antinutrients and in vitro protein and starch digestibilities of indigenously developed RWGT food mixture. Nutr. Health 2002, 16, 173–181. [Google Scholar] [CrossRef]
- Abdul Qadir, M.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of Phenolic Compounds and Antioxidant and Antimicrobial Activities of Some Common Herbs. Int. J. Anal. Chem. 2017, 2017, 3475738. [Google Scholar] [CrossRef] [Green Version]
- Phuriyakorn, S.; Seechmnanturakit, V.; Wichienchot, S. Antioxidant and prebiotics gut-microbiota effects of dierary phenolic compounds in Etlingera elatior extracts. Intern. Food Res. J. 2019, 26, 1751–1761. [Google Scholar]
- Chung, S.I.; Rico, C.W.; Kang, M.Y. Comparative study on the hypoglycemic and antioxidative effects of fermented paste (doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients 2014, 6, 4610–4624. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.R.; Lee, S.H.; Jang, G.Y.; Lee, Y.J.; Kim, M.Y.; Kim, Y.B.; Lee, J.; Jeong, H.S. Antioxidative and antidiabetic effects of germinated rough rice extract in 3T3-L1 adipocytes and C57BLKS/J-db/db mice. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Park, S.; Lee, H.G. Hypocholesterolmic action of fermented brown rice supplement in cholesterol-fed rats: Cholesterol-lowering action of fermented brown rice. J. Food Sci. 2005, 70, s527–s531. [Google Scholar]
- Xiong, W.T.; Gu, L.; Wang, C.; Sun, H.X.; Liu, X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef]
- Mooradian, A. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and gamma-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef] [Green Version]
- Iynedjian, P.B.; Gjinovci, A.; Renold, A.E. Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J. Biol. Chem. 1988, 263, 740–744. [Google Scholar] [CrossRef]
- Friedman, J.E.; Sun, Y.; Ishizuka, T.; Farrell, C.J.; McCormack, S.E.; Herron, L.M.; Hakimi, P.; Lechner, P.; Yun, J.S. Phosphoenolpyruvate carboxykinase (GTP) gene transcription and hyperglycemia are regulated by glucocorticoids in genetically obese db/db transgenic mice. J. Biol. Chem. 1997, 272, 31475–31481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Chowdhury, S.; Sarkar, P.; Sil, P.C. Ameliorative role of ferulic acid against diabetes associated oxidative stress induced spleen damage. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 118, 272–286. [Google Scholar] [CrossRef]
- Sri Balasubashini, M.; Rukkumani, R.; Menon, V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidnat properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Gerin, F.; Erman, H.; Erboga, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The Effects of Ferulic Acid Against Oxidative Stress and Inflammation in Formaldehyde-Induced Hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef]
- Mabunga, D.F.; Gonzales, E.L.; Kim, H.J.; Choung, S.Y. Treatment of GABA from Fermented Rice Germ Ameliorates Caffeine-Induced Sleep Disturbance in Mice. Biomol. Ther. 2015, 23, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.; Jain, M.R.; Horvath, T.L.; Diano, S.; Kalra, P.S.; Kalra, S.P. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: A morphological and pharmacological analysis. Endocrinology 1999, 140, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Nehus, Z.T.; Badger, T.M.; Fang, N. Quantification of vitamin E and gamma-oryzanol components in rice germ and bran. J. Agric. Food Chem. 2007, 55, 7308–7313. [Google Scholar] [CrossRef]
- Masuzaki, H.; Kozuka, C.; Okamoto, S.; Yonamine, M.; Tanaka, H.; Shimabukuro, M. Brown rice-specific gamma-oryzanol as a promising prophylactic avenue to protect against diabetes mellitus and obesity in humans. J. Diabetes Investig. 2019, 10, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]

| Dietary Group (g/kg Diet) | ||||
|---|---|---|---|---|
| Ingredients (g/kg) | Normal Diet | FA Diet | RG_30E Diet | RG_50E Diet |
| Casein | 200 | 200 | 200 | 200 |
| L-Cystine | 3 | 3 | 3 | 3 |
| Corn starch | 384.81 | 384.81 | 384.81 | 384.81 |
| Maltodextrin | 157.36 | 157.36 | 118.02 | 118.02 |
| Sucrose | 87.32 | 87.32 | 87.32 | 87.32 |
| Soybean Oil | 70 | 70 | 70 | 70 |
| Cellulose | 50 | 50 | 50 | 50 |
| AIN-93G-MX | 35 | 35 | 35 | 35 |
| AIN-93-VX | 10 | 10 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| TBHQ, antioxidant | 0.014 | 0.014 | 0.014 | 0.014 |
| RG_30E | 39.34 | |||
| RG_50E | 39.34 | |||
| Ferulic acid | 0.008 | |||
| Total (g/kg) | 1000.004 | 1000.012 | 1000.004 | 1000.004 |
| Group (1) | |||||
|---|---|---|---|---|---|
| db/+ | db/db | FA | RG_30E | RG_50E | |
| Body weight (g) | |||||
| Initial weight (g) | 22.49 ± 0.76 b | 37.30 ± 0.33 a | 37.20 ± 0.70 a | 38.28 ± 1.15 a | 36.34 ± 1.77 a |
| Final weight (g) | 27.66 ± 0.49 c | 50.78 ± 1.04 ab | 48.34 ± 3.82 b | 52.60 ± 1.53 a | 51.98 ± 1.15 a |
| Food intake (g/day) | 2.87 ± 0.10 d | 18.14 ± 0.58 a | 15.56 ± 0.09 c | 16.83 ± 0.12 b | 15.92 ± 0.02 c |
| Organ weight (g) | |||||
| Liver (g/100 g BW) | 0.93 ± 0.03 c | 2.89 ± 0.13 a | 2.58 ± 0.11 ab | 2.69 ± 0.16 ab | 2.40 ± 0.12 b |
| Epididymal adipose tissue (g/100 g BW) | 0.54 ± 0.05 b | 2.81 ± 0.09 a | 2.70 ± 0.11 a | 2.44 ± 0.07 a | 2.61 ± 0.23 a |
| Kidney (g/100 g BW) | 0.30 ± 0.00 a | 0.35 ± 0.02 a | 0.35 ± 0.02 a | 0.33 ± 0.03 a | 0.30 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, Y.J.; Kim, J.G.; Jung, S.K.; Kim, J.Y. Fermented Rice Germ Extract Ameliorates Abnormal Glucose Metabolism via Antioxidant Activity in Type 2 Diabetes Mellitus Mice. Appl. Sci. 2021, 11, 3091. https://doi.org/10.3390/app11073091
Hyun YJ, Kim JG, Jung SK, Kim JY. Fermented Rice Germ Extract Ameliorates Abnormal Glucose Metabolism via Antioxidant Activity in Type 2 Diabetes Mellitus Mice. Applied Sciences. 2021; 11(7):3091. https://doi.org/10.3390/app11073091
Chicago/Turabian StyleHyun, Ye Ji, Ju Gyeong Kim, Sung Keun Jung, and Ji Yeon Kim. 2021. "Fermented Rice Germ Extract Ameliorates Abnormal Glucose Metabolism via Antioxidant Activity in Type 2 Diabetes Mellitus Mice" Applied Sciences 11, no. 7: 3091. https://doi.org/10.3390/app11073091
APA StyleHyun, Y. J., Kim, J. G., Jung, S. K., & Kim, J. Y. (2021). Fermented Rice Germ Extract Ameliorates Abnormal Glucose Metabolism via Antioxidant Activity in Type 2 Diabetes Mellitus Mice. Applied Sciences, 11(7), 3091. https://doi.org/10.3390/app11073091







