Immobilization of (Aqueous) Cations in Low pH M-S-H Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Precipitation Experiments
2.3. Ex Situ Analytical Methods
3. Results
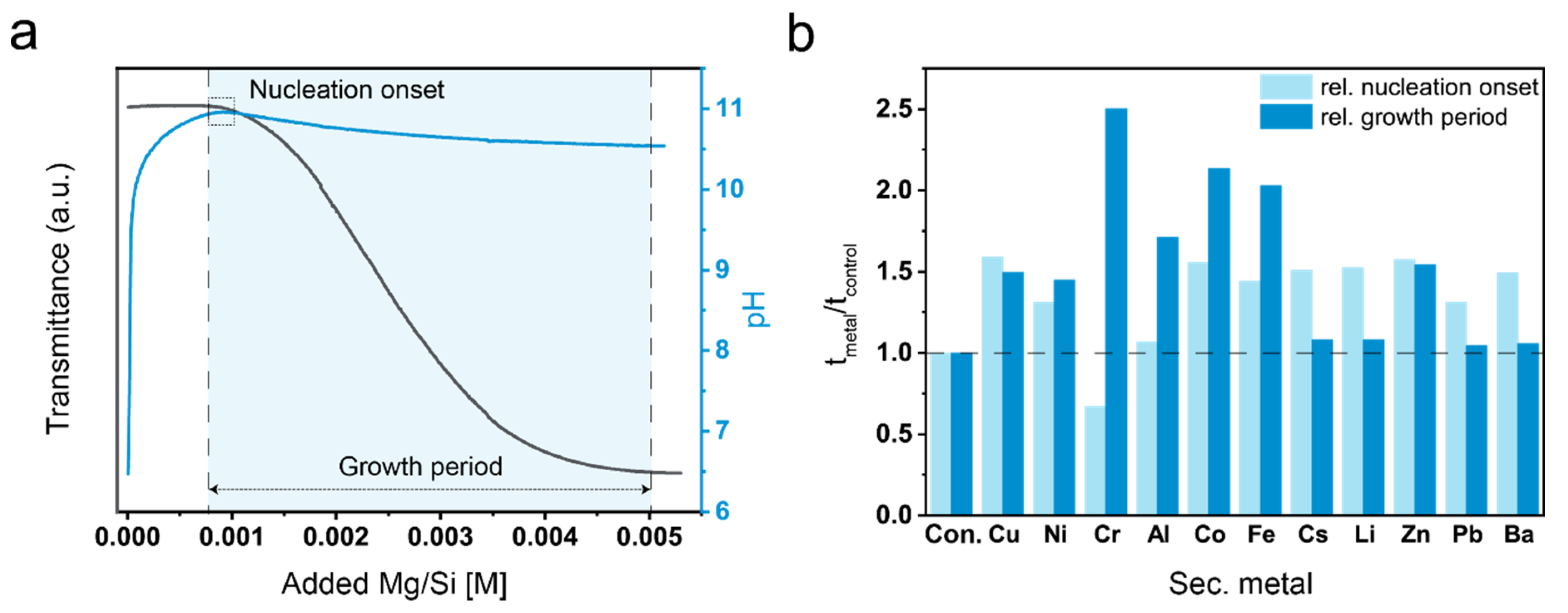
3.1. Precipitation Experiments
3.2. Ex Situ Characterization
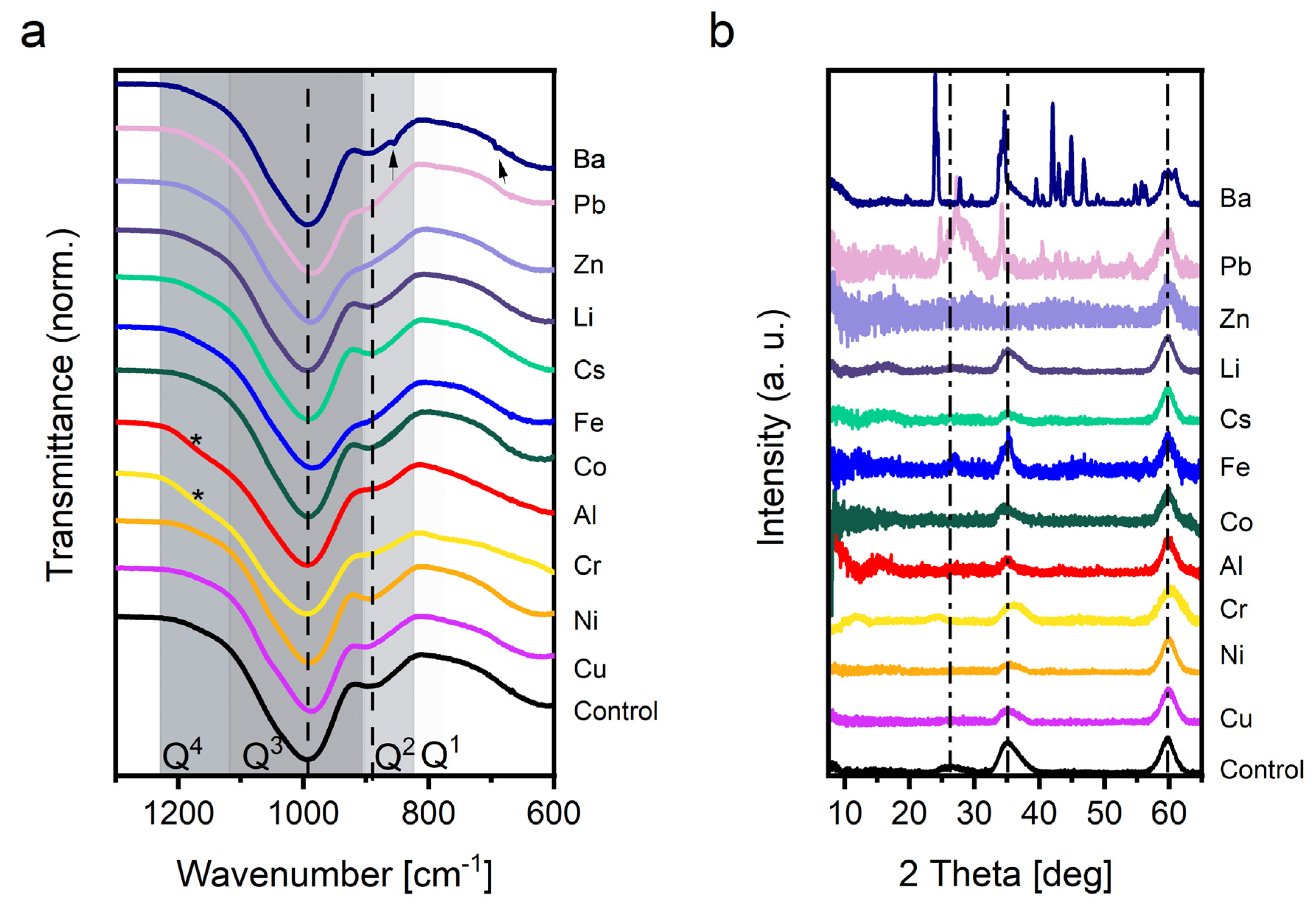
3.2.1. FTIR
3.2.2. PXRD
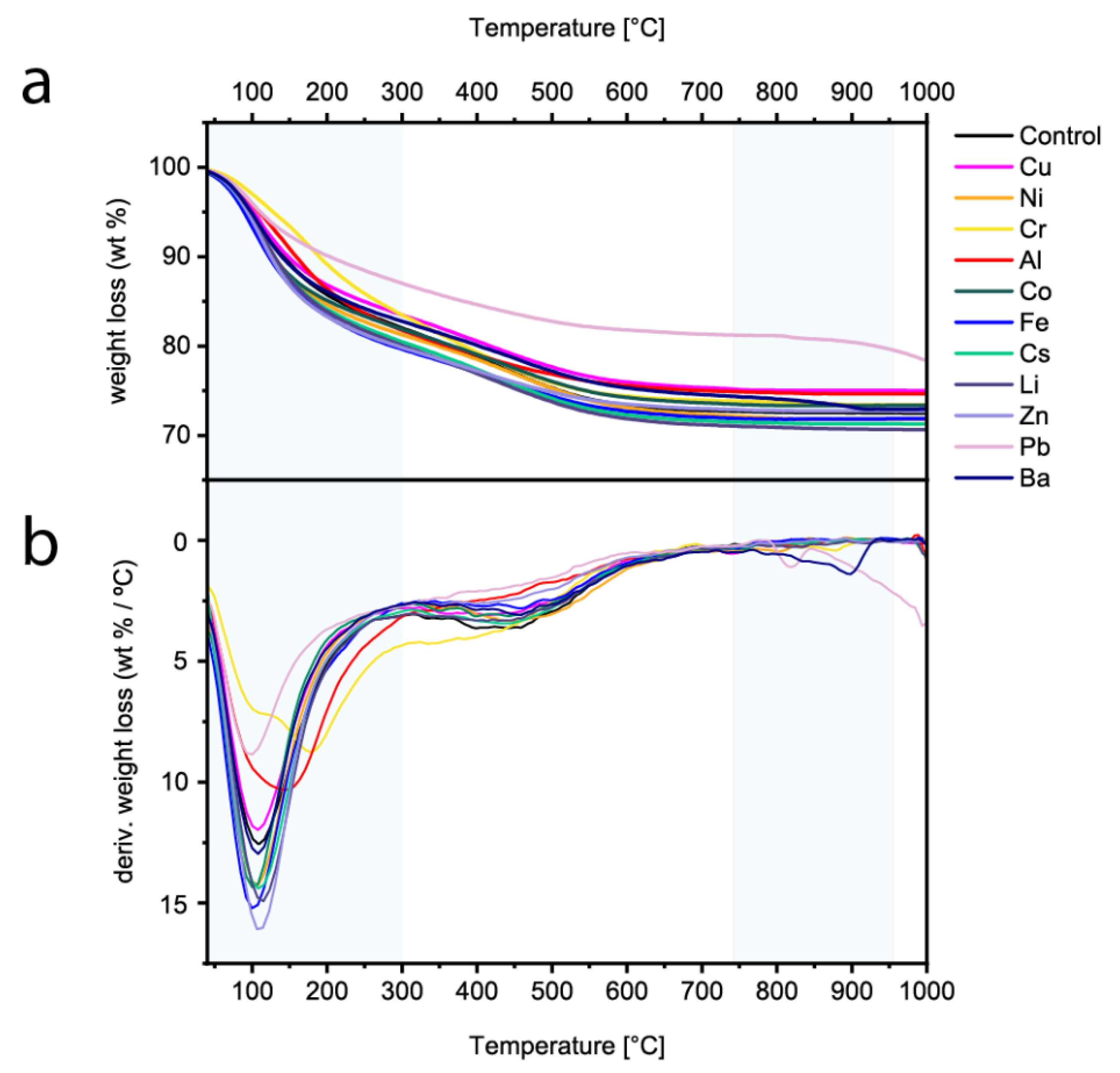
3.2.3. Thermogravimetric Analysis (TGA)
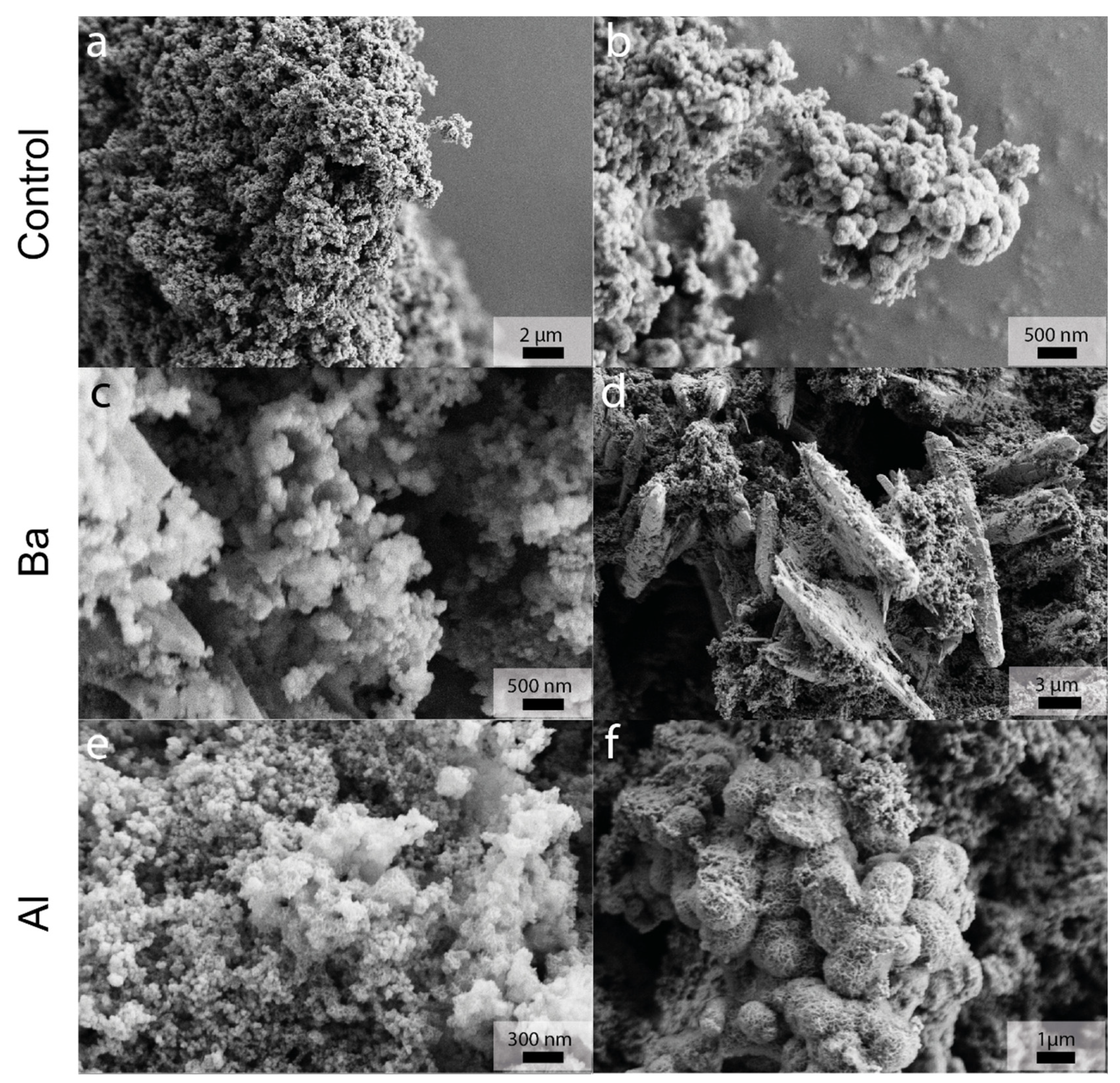
3.2.4. SEM
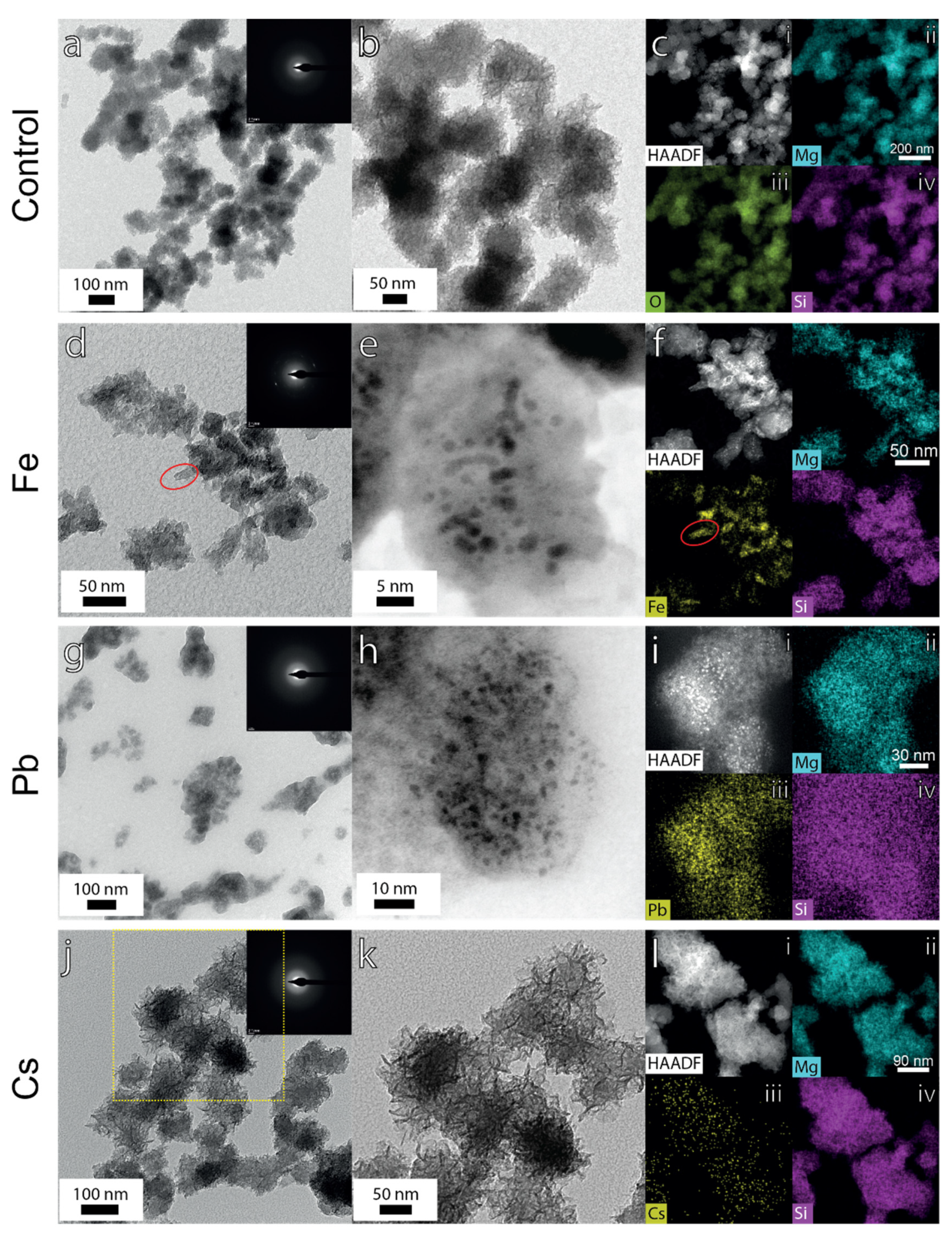
3.2.5. TEM
3.2.6. Compositional Analysis of the Precipitates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, H.; Huang, W.; Xing, Z.; Zuo, J.; Wang, Z.; Yang, K. Experimental study on removing heavy metals from the municipal solid waste incineration fly ash with the modified electrokinetic remediation device. Sci. Rep. 2019, 9, 8271. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.R. Chemical Fixation and Solidification of Hazardous Wastes; Van Nostrand Reinhold: New York, NY, USA, 1990. [Google Scholar]
- Glasser, F.P. Fundamental aspects of cement solidification and stabilisation. J. Hazard. Mater. 1997, 52, 151–170. [Google Scholar] [CrossRef]
- Conner, J.R.; Hoeffner, S.L. A Critical Review of Stabilization/Solidification Technology. Crit. Rev. Environ. Sci. Technol. 1998, 28, 397–462. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. 2006, 137, 267–276. [Google Scholar] [CrossRef]
- Sobiecka, E. Investigating the chemical stabilization of hazardous waste material (fly ash) encapsulated in Portland cement. Int. J. Environ. Sci. Technol. 2013, 10, 1219–1224. [Google Scholar] [CrossRef][Green Version]
- Hyatt, N.C.; Ojovan, M.I. Special Issue: Materials for Nuclear Waste Immobilization. Materials 2019, 12, 3611. [Google Scholar] [CrossRef]
- Osmanlioglu, A. Immobilization of radioactive waste by cementation with purified kaolin clay. Waste Manag. 2002, 22, 481–483. [Google Scholar] [CrossRef]
- Macphee, D.E.; Glasser, F.P. Immobilization Science of Cement Systems. MRS Bull. 1993, 18, 66–71. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, X.; Wang, L.; Sun, X. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar] [CrossRef]
- Gitari, W.M.; Key, D.L.; Petrik, L.F.; Okujeni, C. Partitioning of major and trace inorganic contaminants in fly ash acid mine drainage derived solid residues. Int. J. Environ. Sci. Technol. 2010, 7, 519–534. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.; de Schutter, G.; Guo, X.; Ye, G. Preparation of alinite cement from municipal solid waste incineration fly ash. Cem. Concr. Compos. 2012, 34, 322–327. [Google Scholar] [CrossRef]
- Ojovan, M.I. (Ed.) Handbook of Advanced Radioactive Waste Conditioning Technologies: Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Glasser, F. Application of inorganic cements to the conditioning and immobilisation of radioactive wastes. In Handbook of Advanced Radioactive Waste Conditioning Technologies: Woodhead Publishing Series in Energy; Ojovan, M.I., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 67–135. [Google Scholar]
- Soundararajan, R. An overview of present day immobilization technologies. J. Hazard. Mater. 1990, 24, 199–212. [Google Scholar] [CrossRef]
- Myers, T.E.; Zappi, M.E. Laboratory Evaluation of Stabilization/Solidification Technology for Reducing the Mobility of Heavy Metals in New Bedford Harbor Superfund Site Sediment. In Stabilization and Solidification of Hazardous, Radioactive, and Mixed Wastes: 2nd Volume; Gilliam, T.M., Wiles, C.C., Eds.; ASTM International: West Conshohocken, PA, USA, 1992; pp. 304–319. [Google Scholar]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Mollah, A.; Vempati, R.K.; Lin, T.-C.; Cocke, D.L. The interfacial chemistry of solidification/stabilization of metals in cement and pozzolanic material systems. Waste Manag. 1995, 15, 137–148. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Zhang, T.; Vandeperre, L.J.; Cheeseman, C.R. Magnesium-silicate-hydrate cements for encapsulating problematic aluminium containing wastes. J. Sustain. Cem. Based Mater. 2012, 1, 34–45. [Google Scholar] [CrossRef]
- Taylor, H. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Dupuis, R.; Dolado, J.S.; Surga, J.; Ayuela, A. Doping as a Way to Protect Silicate Chains in Calcium Silicate Hydrates. ACS Sustain. Chem. Eng. 2018, 6, 15015–15021. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M.; Al-Tabbaa, A. MgO expansive cement and concrete in China: Past, present and future. Cem. Concr. Res. 2014, 57, 1–12. [Google Scholar] [CrossRef]
- Brew, D.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nied, D.; L’Hôpital, E.; Achiedo, G.; Dauzères, A. Magnesium and calcium silicate hydrates. Cem. Concr. Res. 2015, 77, 60–68. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (M-S-H): The relation with 2:1 Mg–Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D.; Pochard, I.; Dauzères, A. Formation of magnesium silicate hydrates (M-S-H). Phys. Chem. EarthParts A/B/C 2017, 99, 142–157. [Google Scholar] [CrossRef]
- Zhang, T.; Cheeseman, C.R.; Vandeperre, L.J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, J.; Wang, B.; Wu, Z.; Jia, Y.; Cheeseman, C.R. Characterization of Magnesium Silicate Hydrate (MSH) Gel Formed by Reacting MgO and Silica Fume. Materials 2018, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulou, E.; Ntinoudi, E.; Zouboulis, A.I.; Mitrakas, M.; Yiannoulakis, H.; Zampetakis, T. Heavy metal stabilization of industrial solid wastes using low-grade magnesia, Portland and magnesia cements. J. Mater. Cycles Waste Manag. 2020, 22, 975–985. [Google Scholar] [CrossRef]
- De Ruiter, L.; Austrheim, H. Formation of magnesium silicate hydrate cement in nature. J. Geol. Soc. 2018, 175, 308. [Google Scholar] [CrossRef]
- De Ruiter, L.; Gunnæs, A.E.; Dysthe, D.K.; Austrheim, H. Quartz dissolution associated with magnesium silicate hydrate cement precipitation. Solid Earth 2021, 12, 389–404. [Google Scholar]
- Dauzeres, A.; Achiedo, G.; Nied, D.; Bernard, E.; Alahrache, S.; Lothenbach, B. Magnesium perturbation in low-pH concretes placed in clayey environment—solid characterizations and modeling. Cem. Concr. Res. 2016, 79, 137–150. [Google Scholar] [CrossRef]
- Cole, W.F.; Demediuk, T. X-ray, thermal, and Dehydration studies on Magnesium oxychlorides. Aust. J. Chem. 1955, 8, 234–251. [Google Scholar] [CrossRef]
- Sevim, A.M.; Hojiyev, R.; Gül, A.; Çelik, M.S. An investigation of the kinetics and thermodynamics of the adsorption of a cationic cobalt porphyrazine onto sepiolite. Dye. Pigment. 2011, 88, 25–38. [Google Scholar] [CrossRef]
- Alexandre-Franco, M.; Albarrán-Liso, A.; Gómez-Serrano, V. An identification study of vermiculites and micas: Adsorption of metal ions in aqueous solution. Fuel Process. Technol. 2011, 92, 200–205. [Google Scholar] [CrossRef]
- Walling, S.A.; Kinoshita, H.; Bernal, S.A.; Collier, N.C.; Provis, J.L. Structure and properties of binder gels formed in the system Mg(OH)2–SiO2–H2O for immobilisation of Magnox sludge. Dalton Trans. 2015, 44, 8126–8137. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Pochard, I.; Cau-Dit-Coumes, C. Alkali binding by magnesium silicate hydrates. J. Am. Ceram. Soc. 2019, 102, 6322–6336. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, J.; Li, Y.; Jia, Y.; Cheeseman, C.R. Stabilization/solidification of strontium using magnesium silicate hydrate cement. Processes 2020, 8, 163. [Google Scholar] [CrossRef]
- Zhang, T.; Li, T.; Zou, J.; Li, Y.; Zhi, S.; Jia, Y.; Cheeseman, C. Immobilization of radionuclide 133cs by magnesium silicate hydrate cement. Materials 2020, 13, 146. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C. (Eds.) Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Li, Q.; Gao, J.; Zhang, Y.; An, Z.; Guo, Z. Viscosity Measurement and Structure Analysis of Cr2O3-Bearing CaO-SiO2-MgO-Al2O3 Slags. Metall. Mater. Trans. B 2017, 48, 346–356. [Google Scholar] [CrossRef]
- Hou, D.; Ma, H.; Zhu, Y.; Li, Z. Calcium silicate hydrate from dry to saturated state: Structure, dynamics and mechanical properties. Acta Mater. 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzères, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
- de Villiers, J.P.R. Crystal Structures of Aragonite, Strontianite, and Witherite. Am. Mineral. 1971, 56, 758–767. [Google Scholar]
- de Velasco Maldonado, P.S.; Hernández-Montoya, V.; Concheso, A.; Montes-Morán, M.A. Formation of cerussite and hydrocerussite during adsorption of lead from aqueous solution on oxidized carbons by cold oxygen plasma. Appl. Surf. Sci. 2016, 386, 381–388. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K.J.D. Formation of Layered Magnesium Silicate during the Aging of Magnesium Hydroxide–Silica Mixtures. J. Am. Ceram. Soc. 1998, 81, 754–756. [Google Scholar] [CrossRef]
- Lothenbach, B.; Durdzinski, P.; DeWeerdt, K. Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Oxford, UK, 2016; pp. 177–212. [Google Scholar]
- Cuesta, A.; Ichikawa, R.U.; Londono-Zuluaga, D.; de La Torre, A.G.; Santacruz, I.; Turrillas, X.; Aranda, M.A. Aluminum hydroxide gel characterization within a calcium aluminate cement paste by combined Pair Distribution Function and Rietveld analyses. Cem. Concr. Res. 2017, 96, 1–12. [Google Scholar] [CrossRef]
- Chiang, W.-S.; Ferraro, G.; Fratini, E.; Ridi, F.; Yeh, Y.-Q.; Jeng, U.-S.; Chen, S.-H.; Baglioni, P. Multiscale structure of calcium- and magnesium-silicate-hydrate gels. J. Mater. Chem. A 2014, 2, 12991–12998. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Wang, H.; Liang, C.; Cai, W.; Zhang, L. Chemical-Template Synthesis of Micro/Nanoscale Magnesium Silicate Hollow Spheres for Waste-Water Treatment. Chem. A Eur. J. 2010, 16, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Z.; Zhou, H.; Zhang, Y.; Wang, X. Two-dimensional lamellar magnesium silicate with large spacing as an excellent adsorbent for uranium immobilization. Environ. Sci. Nano 2018, 5, 2406–2414. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Cau-Dit-Coumes, C.; Pochard, I.; Rentsch, D. Aluminum incorporation into magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2020, 128, 105931. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.; Parker, V.B. The solubility of strontianite (SrCO3) in CO2-H2O solutions between 2 and 91 °C, the association constants of SrHCO+3(aq) and SrCO03(aq) between 5 and 80 °C, and an evaluation of the thermodynamic properties of Sr2+(aq) and SrCO3(cr) at 25 °C and 1 atm total pressure. Geochim. Cosmochim. Acta 1984, 48, 2021–2035. [Google Scholar]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural characterization of magnesium silicate hydrate: Towards the design of eco-sustainable cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D. Influence of sodium nitrate on the phases formed in the MgO-Al2O3-SiO2-H2O system. Mater. Des. 2021, 198, 109391. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsiske, M.R.; Debus, C.; Di Lorenzo, F.; Bernard, E.; Churakov, S.V.; Ruiz-Agudo, C. Immobilization of (Aqueous) Cations in Low pH M-S-H Cement. Appl. Sci. 2021, 11, 2968. https://doi.org/10.3390/app11072968
Marsiske MR, Debus C, Di Lorenzo F, Bernard E, Churakov SV, Ruiz-Agudo C. Immobilization of (Aqueous) Cations in Low pH M-S-H Cement. Applied Sciences. 2021; 11(7):2968. https://doi.org/10.3390/app11072968
Chicago/Turabian StyleMarsiske, Maximilian R., Christian Debus, Fulvio Di Lorenzo, Ellina Bernard, Sergey V. Churakov, and Cristina Ruiz-Agudo. 2021. "Immobilization of (Aqueous) Cations in Low pH M-S-H Cement" Applied Sciences 11, no. 7: 2968. https://doi.org/10.3390/app11072968
APA StyleMarsiske, M. R., Debus, C., Di Lorenzo, F., Bernard, E., Churakov, S. V., & Ruiz-Agudo, C. (2021). Immobilization of (Aqueous) Cations in Low pH M-S-H Cement. Applied Sciences, 11(7), 2968. https://doi.org/10.3390/app11072968






