Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
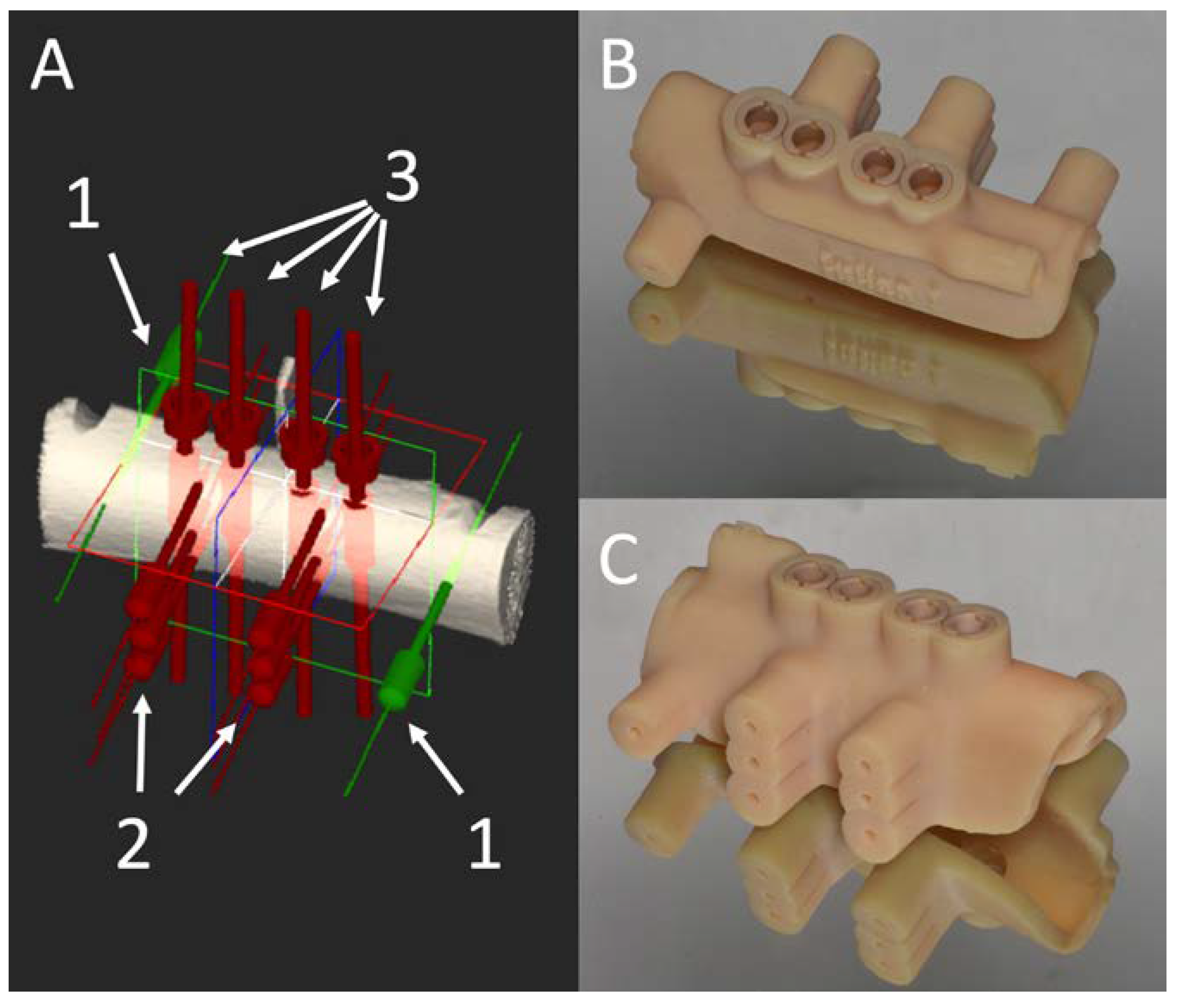
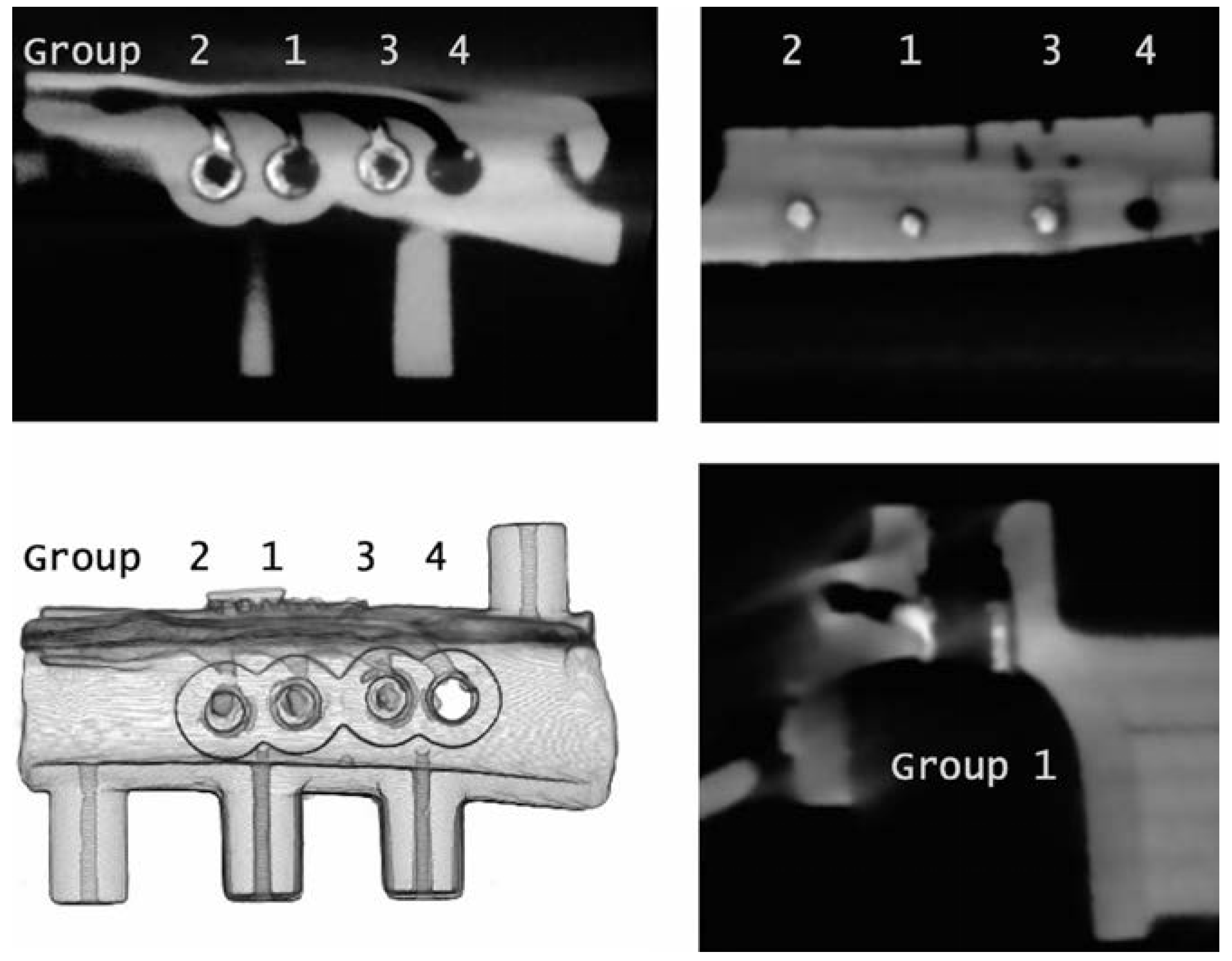
2.2. Surgical Guide Production
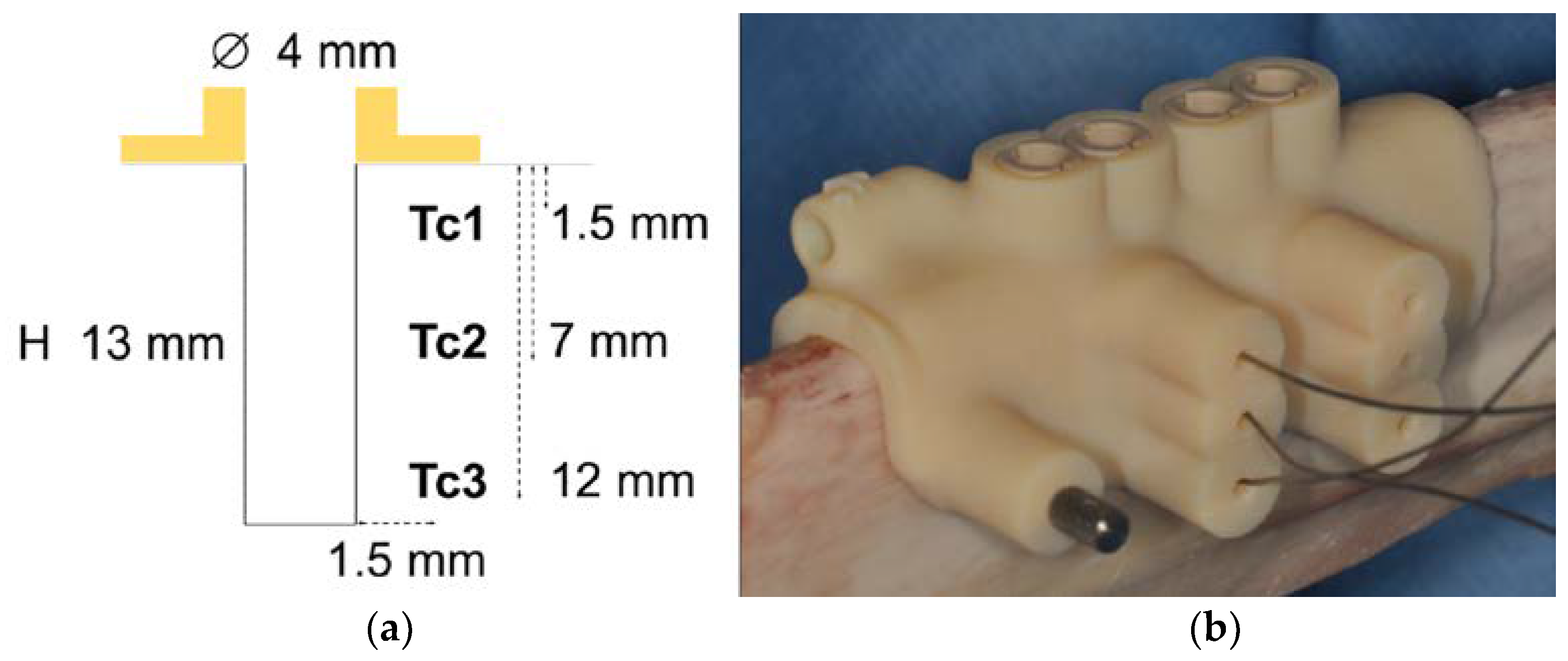
2.3. Temperature Measurement
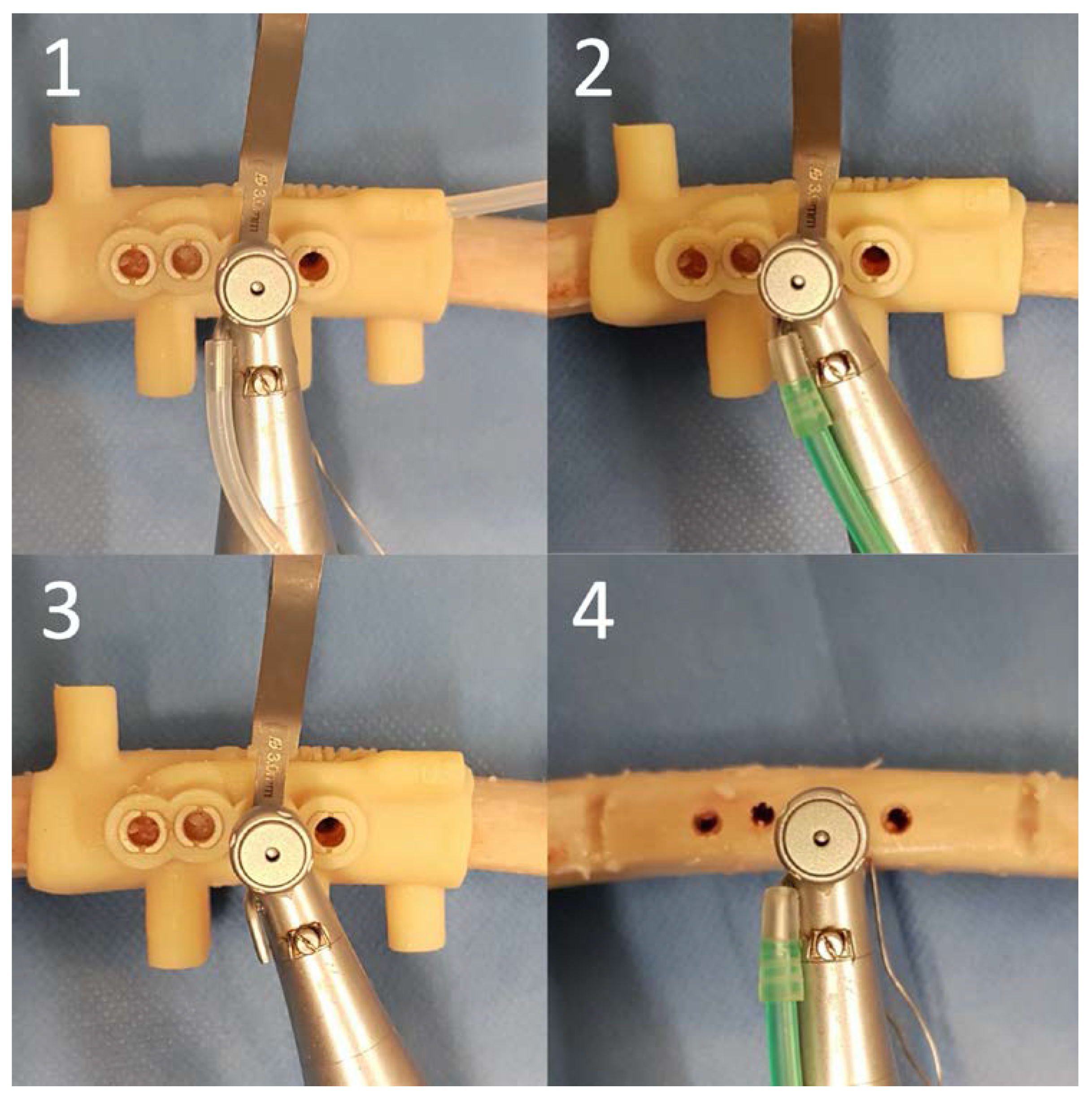
2.4. Surgical Protocol
2.5. Outcome Measures
2.6. Sample Size
2.7. Statistical Analysis
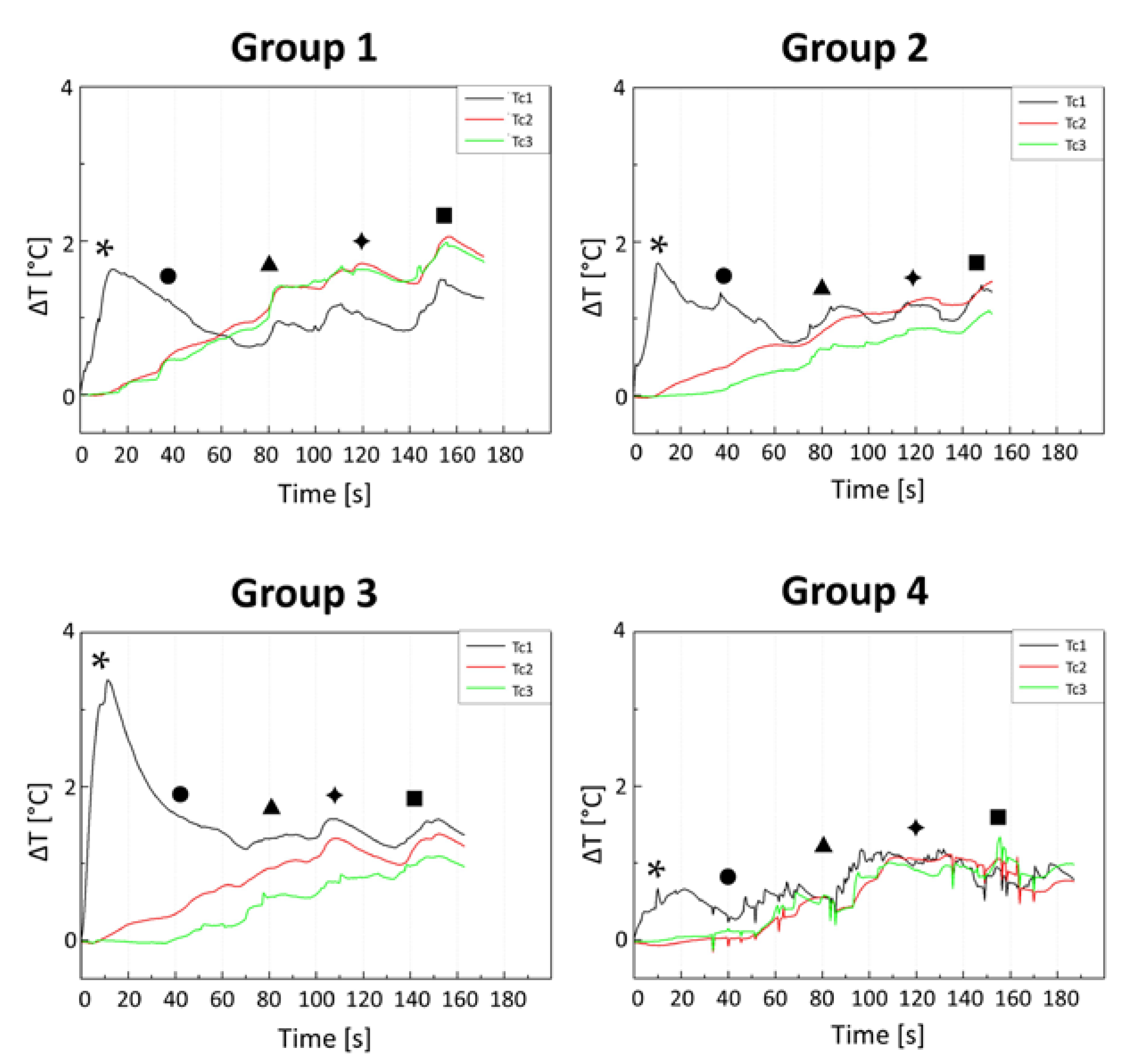
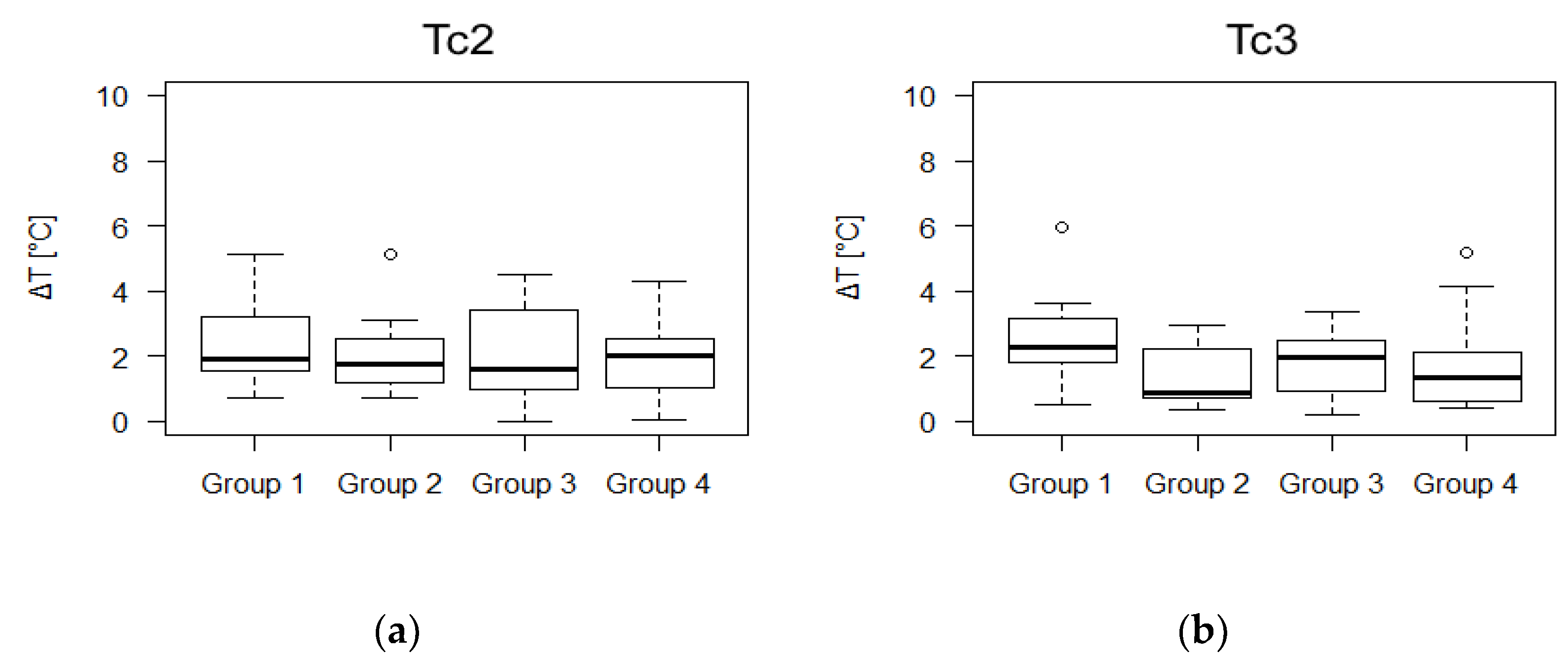
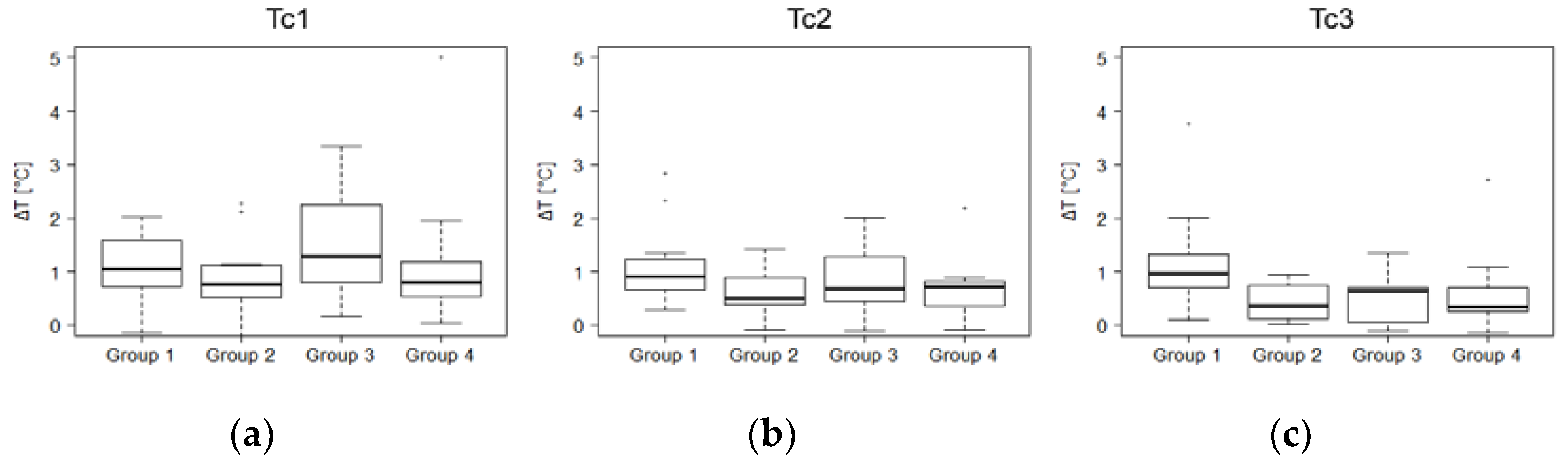
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated Titanium Implants. Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological Factors Contributing to Failures of Osseointegrated Oral Implants. (I). Success Criteria and Epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Wagenberg, B.; Froum, S.J. A Retrospective Study of 1925 Consecutively Placed Immediate Implants from 1988 to 2004. Int. J. Oral Maxillofac. Implant. 2006, 21. [Google Scholar] [CrossRef]
- Sharawy, M.; Misch, C.E.; Weller, N.; Tehemar, S. Heat Generation During Implant Drilling: The Significance of Motor Speed. J. Oral Maxillofac. Surg. 2002, 60, 1160–1169. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Delgado-Peña, J.; Maté-Sánchez, J.E.; Mareque Bueno, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Novel Hybrid Drilling Protocol: Evaluation for the Implant Healing–Thermal Changes, Crestal Bone Loss, and Bone-to-Implant Contact. Clin. Oral Implant. Res. 2015, 26, 753–760. [Google Scholar] [CrossRef]
- Stocchero, M.; Jinno, Y.; Toia, M.; Ahmad, M.; Papia, E.; Yamaguchi, S.; Becktor, J.P. Intraosseous Temperature Change during Installation of Dental Implants with Two Different Surfaces and Different Drilling Protocols: An in Vivo Study in Sheep. J. Clin. Med. 2019, 8, 1198. [Google Scholar] [CrossRef]
- Eriksson, A.; Albrektsson, T.; Grane, B.; McQueen, D. Thermal Injury to Bone: A Vital-Microscopic Description of Heat Effects. Int. J. Oral Surg. 1982, 11, 115–121. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Albrektsson, T. The Effect of Heat on Bone Regeneration: An Experimental Study in the Rabbit Using the Bone Growth Chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Augustin, G.; Zigman, T.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Cortical Bone Drilling and Thermal Osteonecrosis. Clin. Biomech. 2012, 27, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Katsumi, U.; Kimimitsu, O.; Takeyasu, M. Influence of Heat Stress to Matrix on Bone Formation. Clin. Oral Implant. Res. 2009, 20, 782–790. [Google Scholar] [CrossRef]
- Misir, A. Ferhat, Mahmut Sumer, Murat Yenisey, and Erol Ergioglu. Effect of Surgical Drill Guide on Heat Generated from Implant Drilling. J. Oral Maxillofac. Surg. 2009, 67, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Wikesjö, U.M.; Kang, H.S.; Ku, Y.; Eom, T.G.; Koo, K.T. Effect of Implant Drill Characteristics on Heat Generation in Osteotomy Sites: A Pilot Study. Clin. Oral Implant. Res. 2011, 22, 722–726. [Google Scholar] [CrossRef]
- Yacker, M.J.; Klein, M. The Effect of Irrigation on Osteotomy Depth and Bur Diameter. Int. J. Oral Maxillofac. Implant. 1996, 11, 634–638. [Google Scholar]
- Ercoli, C.; Funkenbusch, P.D.; Lee, H.J.; Moss, M.E.; Graser, G.N. The Influence of Drill Wear on Cutting Efficiency and Heat Production During Osteotomy Preparation for Dental Implants: A Study of Drill Durability. Int. J. Oral Maxillofac. Implant. 2004, 19, 335–349. [Google Scholar]
- Trisi, P.; Marco, B.; Antonello, F.; Michele, P.V.; Giorgio, P. Insufficient Irrigation Induces Peri-Implant Bone Resorption: An in Vivo Histologic Analysis in Sheep. Clin. Oral Implant. Res. 2014, 25, 696–701. [Google Scholar] [CrossRef]
- Dos Santos, P.L.; Pereira Queiroz, T.; Margonar, R.; de Souza Carvalho, A.C.G.; Betoni, W., Jr.; Rodrigues Rezende, R.R.; Garcia, R., Jr. Evaluation of Bone Heating, Drill Deformation, and Drill Roughness after Implant Osteotomy: Guided Surgery and Classic Drilling Procedure. Int. J. Oral Maxillofac. Implant. 2014, 29. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Aramburú Júnior, J.S.; Pérez-Albacete Martínez, C.; Ramirez Fernandez, M.P.; Maté Sánchez de Val, J.E.; Calvo-Guirado, J.L. The Influence of Drill Length and Irrigation System on Heat Production During Osteotomy Preparation for Dental Implants: An Ex Vivo Study. Clin. Oral Implant. Res. 2018, 29, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Tehemar, S.H. Factors Affecting Heat Generation During Implant Site Preparation: A Review of Biologic Observations and Future Considerations. Int. J. Oral Maxillofac. Implant. 1999, 14, 127–136. [Google Scholar] [PubMed]
- Tatakis, D.N.; Chien, H.H.; Parashis, A.O. Guided Implant Surgery Risks and Their Prevention. Periodontol. 2000 2019, 81, 194–208. [Google Scholar] [CrossRef]
- Migliorati, M.; Amorfini, L.; Signori, A.; Barberis, F.; Benedicenti, S. Internal Bone Temperature Change During Guided Surgery Preparations for Dental Implants: An in Vitro Study. Int. J. Oral Maxillofac. Implant. 2013, 28, 1464–1469. [Google Scholar] [CrossRef]
- Azari, A.; Nikzad, S. Flapless Implant Surgery: Review of the Literature and Report of 2 Cases with Computer-Guided Surgical Approach. J. Oral Maxillofac. Surg. 2008, 66, 1015–1021. [Google Scholar] [CrossRef]
- Kivovics, M.; Pénzes, D.; Németh, O.; Mijiritsky, E. The Influence of Surgical Experience and Bone Density on the Accuracy of Static Computer-Assisted Implant Surgery in Edentulous Jaws Using a Mucosa-Supported Surgical Template with a Half-Guided Implant Placement Protocol—A Randomized Clinical Study. Materials 2020, 13, 5759. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, M.; Laleman, I.; Jacobs, R.; Quirynen, M. Computer-Supported Implant Planning and Guided Surgery: A Narrative Review. Clin. Oral Implant. Res. 2015, 26, 69–76. [Google Scholar] [CrossRef]
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. 2020, 29, 534–541. [Google Scholar] [CrossRef]
- Deeb, G.R.; Allen, R.K.; Hall, V.P.; Whitley, D., III; Laskin, D.M.; Bencharit, S. How Accurate Are Implant Surgical Guides Produced with Desktop Stereolithographic 3-Dimentional Printers? J. Oral Maxillofac. Surg. 2017, 75, 2559.e1–2559.e8. [Google Scholar] [CrossRef] [PubMed]
- Team R Core. R: A Language and Environment for Statistical Computing; Team R Core: Vienna, Austria, 2013. [Google Scholar] [CrossRef]
- Alevizakos, V.; Mitov, G.; von See, C. Guided Implant Placement Using an Internally Cooling Surgical Template–a Technical Note. J. Oral Implantol. 2020, 46, 533–535. [Google Scholar] [CrossRef]
- Bulloch, S.E.; Olsen, R.G.; Bulloch, B. Comparison of Heat Generation between Internally Guided (Cannulated) Single Drill and Traditional Sequential Drilling with and without a Drill Guide for Dental Implants. Int. J. Oral Maxillofac. Implant. 2012, 27, 1456–1460. [Google Scholar]
- Calttenburg, R.; Cohen, J.; Conner, S.; Cook, N. Thermal Properties of Cancellous Bone. J. Biomed Mater. Res. 1975, 9, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Strbac, G.D.; Unger, E.; Donner, R.; Bijak, M.; Watzek, G.; Zechner, W. Thermal Effects of a Combined Irrigation Method during Implant Site Drilling. A Standardized in Vitro Study Using a Bovine Rib Model. Clin. Oral Implant. Res. 2014, 25, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Aghvami, M.; Brunski, J.B.; Serdar Tulu, U.; Chen, C.H.; Helms, J.A. A Thermal and Biological Analysis of Bone Drilling. J. Biomech. Eng. 2018, 140. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wu, J.L.; Zhang, J.X.; Peng, W.; Liao, W.Q. Numerical and Experimental Analyses on the Temperature Distribution in the Dental Implant Preparation Area When Using a Surgical Guide. J. Prosthodont. 2018, 27, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Barrak, I.; Joób-Fancsaly, Á.; Braunitzer, G.; Varga, E., Jr.; Boa, K.; Piffkó, J. Intraosseous Heat Generation During Osteotomy Performed Freehand and through Template with an Integrated Metal Guide Sleeve: An in Vitro Study. Implant Dent. 2018, 27, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.R.; James, D.F. Measurement of Thermal Conductivity of Bovine Cortical Bone. Med Eng. Phys. 2000, 22, 741–747. [Google Scholar] [CrossRef]
- Di Fiore, A.; Sivolella, S.; Stocco, E.; Favero, V.; Stellini, E. Experimental Analysis of Temperature Differences During Implant Site Preparation: Continuous Drilling Technique Versus Intermittent Drilling Technique. J. Oral Implantol. 2018, 44, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Marković, A.; Lazić, Z.; Mišić, T.; Šćepanović, M.; Todorović, A.; Thakare, K.; Glišić, M. Effect of Surgical Drill Guide and Irrigans Temperature on Thermal Bone Changes During Drilling Implant Sites-Thermographic Analysis on Bovine Ribs. Vojnosanit. Pregl. 2016, 73, 744–750. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocchero, M.; Sivolella, S.; Brunello, G.; Zoppello, A.; Cavallin, F.; Biasetto, L. Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation. Appl. Sci. 2021, 11, 2588. https://doi.org/10.3390/app11062588
Stocchero M, Sivolella S, Brunello G, Zoppello A, Cavallin F, Biasetto L. Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation. Applied Sciences. 2021; 11(6):2588. https://doi.org/10.3390/app11062588
Chicago/Turabian StyleStocchero, Michele, Stefano Sivolella, Giulia Brunello, Arianna Zoppello, Francesco Cavallin, and Lisa Biasetto. 2021. "Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation" Applied Sciences 11, no. 6: 2588. https://doi.org/10.3390/app11062588
APA StyleStocchero, M., Sivolella, S., Brunello, G., Zoppello, A., Cavallin, F., & Biasetto, L. (2021). Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation. Applied Sciences, 11(6), 2588. https://doi.org/10.3390/app11062588







