Multidimensional Analytical Characterization of Water-Soluble Organic Aerosols: Challenges and New Perspectives
Abstract
:1. Introduction
2. Coping with Water-Soluble OA Complexity
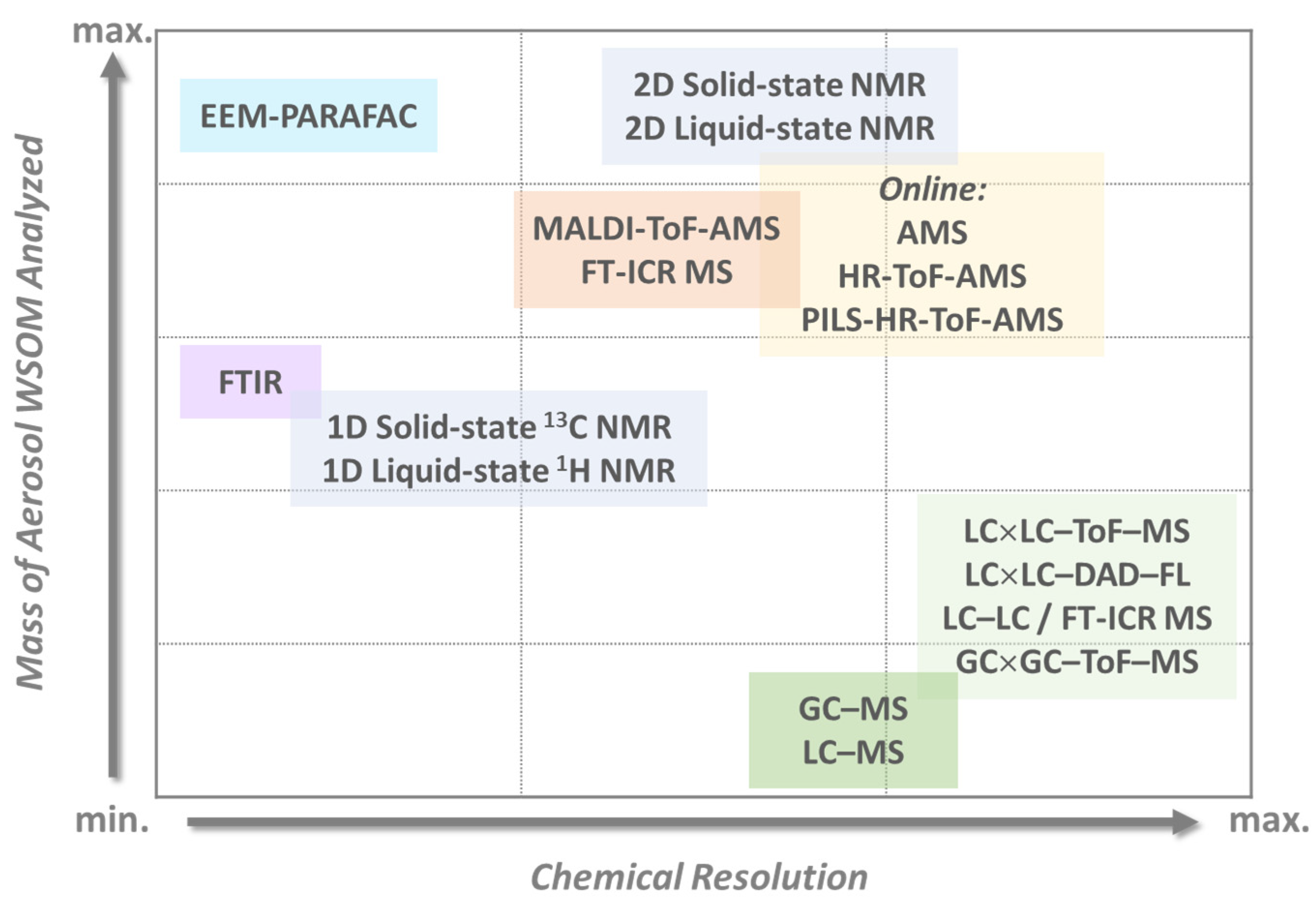
3. Advanced Multidimensional Techniques for Aerosol WSOM Analysis
3.1. Online vs. Offline Techniques: Where Does Multidimensionality Stand?
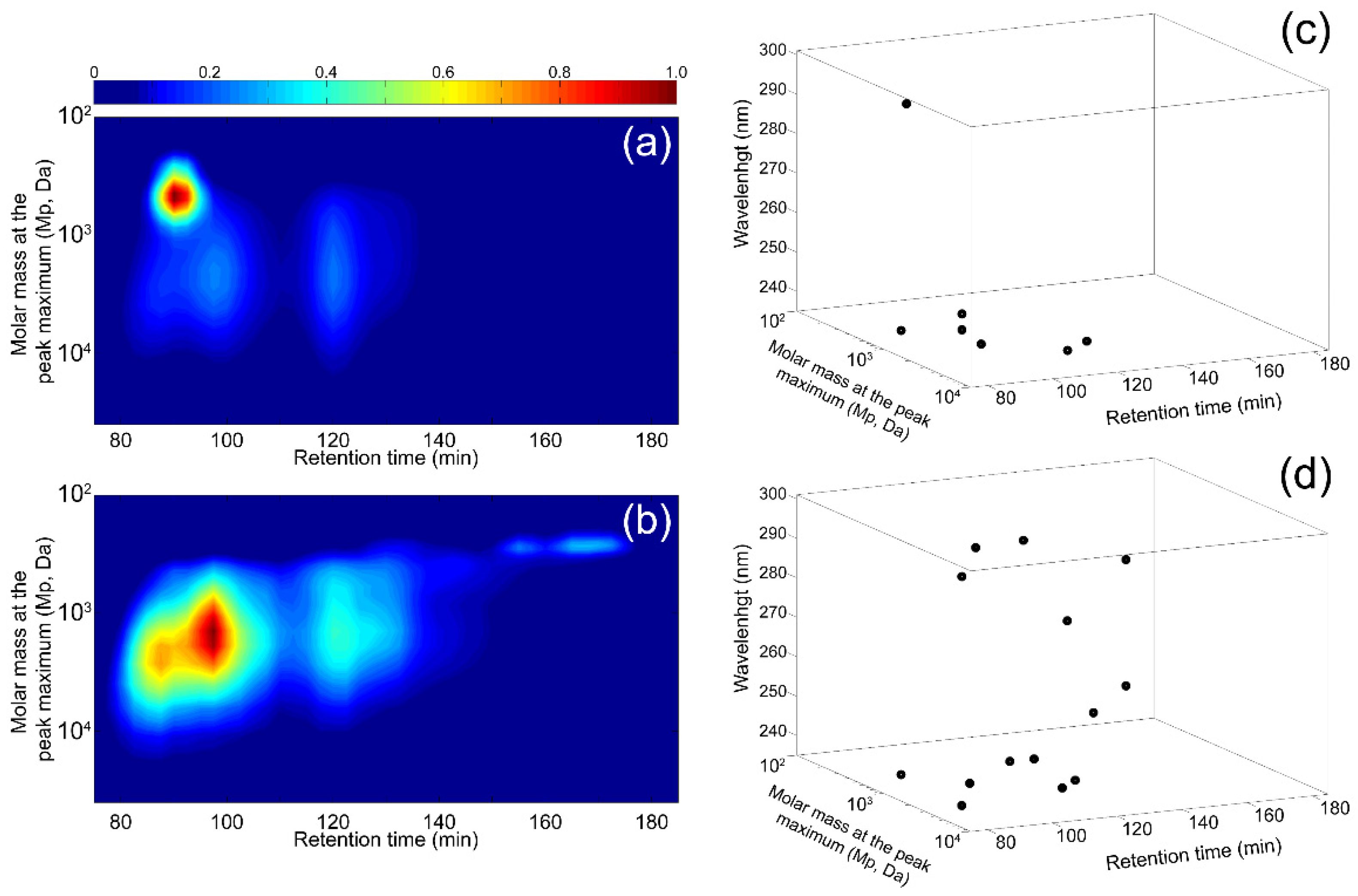
3.2. Application of Off-Line Multidimensional MS Methodologies for Water-Soluble OA Analysis
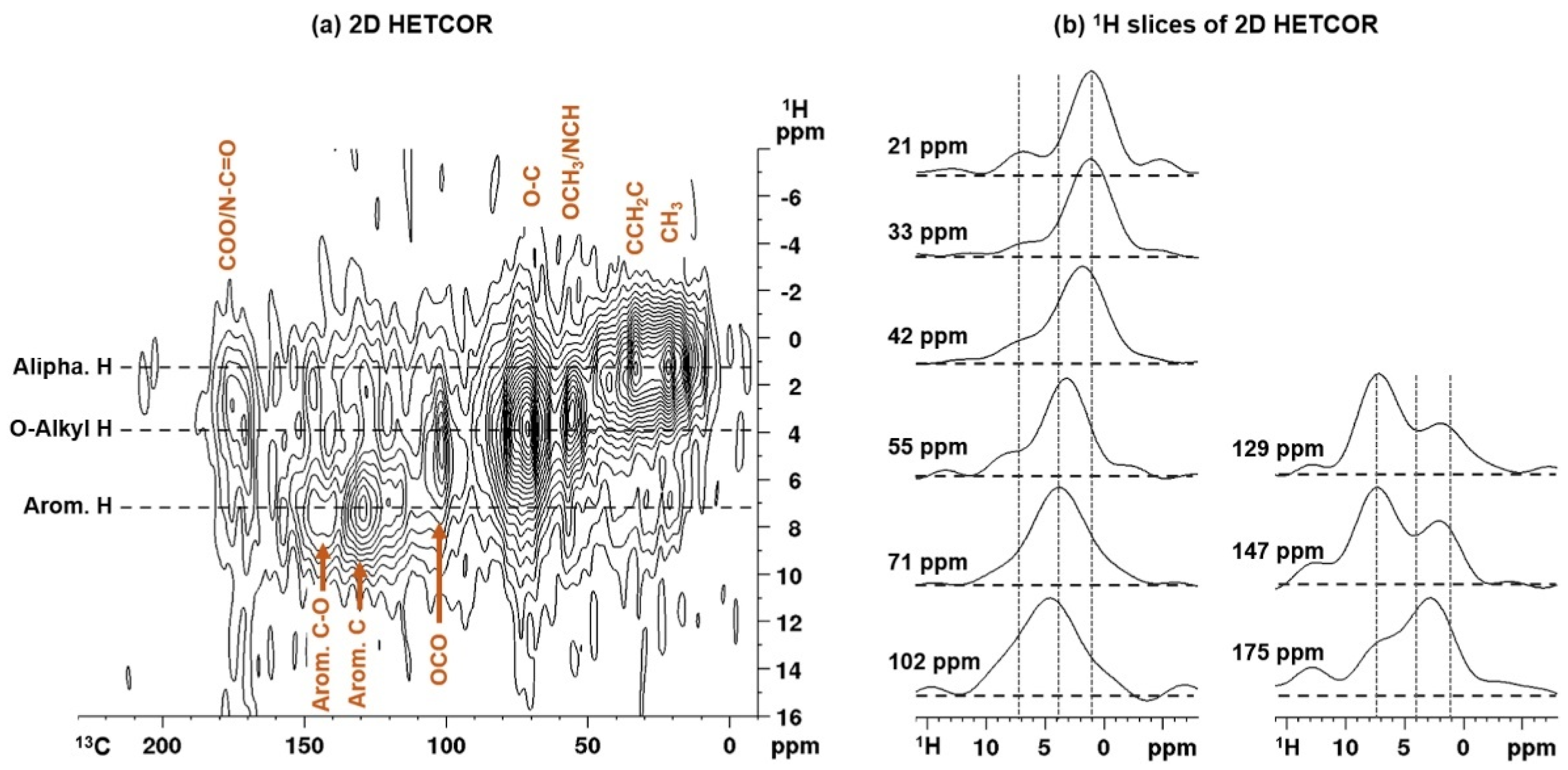
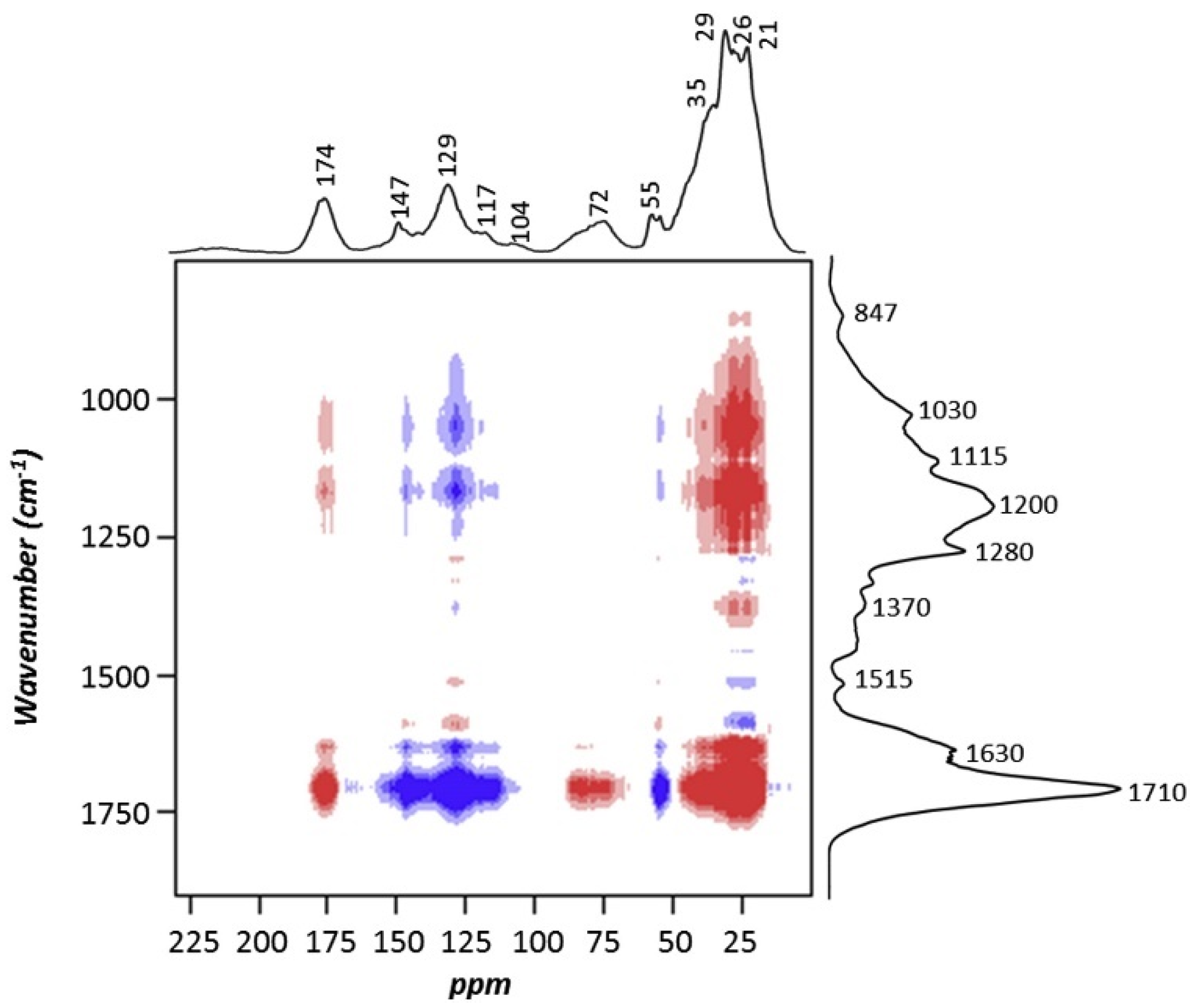
3.3. Application of Off-Line Multidimensional NMR Spectroscopy for Water-Soluble OA Analysis
3.4. Excitation–Emission Matrix (EEM) Fluorescence Spectroscopy for Water-Soluble OA Analysis
4. Multivariate Analysis for Source Apportionment of Water-Soluble OA
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef] [PubMed]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.; Ferreira, R.M.P.; Silva, A.M.S.; Duarte, A.C.; Neves, B.M.; Duarte, R.M.B.O. Structural Features and Pro-Inflammatory Effects of Water-Soluble Organic Matter in Inhalable Fine Urban Air Particles. Environ. Sci. Technol. 2020, 54, 1082–1091. [Google Scholar] [CrossRef]
- Lopes, S.P.; Matos, J.T.V.; Silva, A.M.S.; Duarte, A.C.; Duarte, R.M.B.O. 1 H NMR studies of water- and alkaline-soluble organic matter from fine urban atmospheric aerosols. Atmos. Environ. 2015, 119, 374–380. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Freire, S.M.S.C.; Duarte, A.C. Investigating the water-soluble organic functionality of urban aerosols using two-dimensional correlation of solid-state 13C NMR and FTIR spectral data. Atmos. Environ. 2015, 116, 245–252. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Piñeiro-Iglesias, M.; López-Mahía, P.; Muniategui-Lorenzo, S.; Moreda-Piñeiro, J.; Silva, A.M.S.; Duarte, A.C. Comparative study of atmospheric water-soluble organic aerosols composition in contrasting suburban environments in the Iberian Peninsula Coast. Sci. Total Environ. 2019, 648, 430–441. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Matos, J.T.V.; Paula, A.S.; Lopes, S.P.; Pereira, G.; Vasconcellos, P.; Gioda, A.; Carreira, R.; Silva, A.M.S.; Duarte, A.C.; et al. Structural signatures of water-soluble organic aerosols in contrasting environments in South America and Western Europe. Environ. Pollut. 2017, 227, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Padró, L.T.; Tkacik, D.; Lathem, T.; Hennigan, C.J.; Sullivan, A.P.; Weber, R.J.; Huey, L.G.; Nenes, A. Investigation of cloud condensation nuclei properties and droplet growth kinetics of the water-soluble aerosol fraction in Mexico City. J. Geophys. Res. 2010, 115, D09204. [Google Scholar] [CrossRef]
- Müller, A.; Miyazaki, Y.; Tachibana, E.; Kawamura, K.; Hiura, T. Evidence of a reduction in cloud condensation nuclei activity of water-soluble aerosols caused by biogenic emissions in a coolerate forest. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Chemistry of Atmospheric Brown Carbon. Chem. Rev. 2015, 115, 4335–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moise, T.; Flores, J.M.; Rudich, Y. Optical Properties of Secondary Organic Aerosols and Their Changes by Chemical Processes. Chem. Rev. 2015, 115, 4400–4439. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Ammann, M.; D’Anna, B.; Donaldson, D.J.; Nizkorodov, S.A. Heterogeneous photochemistry in the atmosphere. Chem. Rev. 2015, 115, 4218–4258. [Google Scholar] [CrossRef] [Green Version]
- Iavorivska, L.; Boyer, E.W.; De Walle, D.R. Atmospheric deposition of organic carbon via precipitation. Atmos. Environ. 2016, 146, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Witkowska, A.; Lewandowska, A.; Falkowska, L.M. Parallel measurements of organic and elemental carbon dry (PM1, PM2.5) and wet (rain, snow, mixed) deposition into the Baltic Sea. Mar. Pollut. Bull. 2016, 104, 303–312. [Google Scholar] [CrossRef]
- Bao, H.; Niggemann, J.; Luo, L.; Dittmar, T.; Kao, S.J. Aerosols as a source of dissolved black carbon to the ocean. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5. Environ. Sci. Technol. 2015, 49, 4646–4656. [Google Scholar] [CrossRef] [PubMed]
- Samara, C. On the redox activity of urban aerosol particles: Implications for size distribution and relationships with organic aerosol components. Atmosphere 2017, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Duan, P.; Mao, J.; Chu, W.; Duarte, A.C.; Schmidt-Rohr, K. Exploring water-soluble organic aerosols structures in urban atmosphere using advanced solid-state 13C NMR spectroscopy. Atmos. Environ. 2020, 230, 117503. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Duarte, A.C. NMR Studies of Organic Aerosols. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Academic Press: Oxford, UK, 2017; Volume 92, pp. 83–135. ISBN 978-0-12-812084-2. [Google Scholar]
- Maksymiuk, C.S.; Gayahtri, C.; Gil, R.R.; Donahue, N.M. Secondary organic aerosol formation from multiphase oxidation of limonene by ozone: Mechanistic constraints via two-dimensional heteronuclear NMR spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 7810–7818. [Google Scholar] [CrossRef]
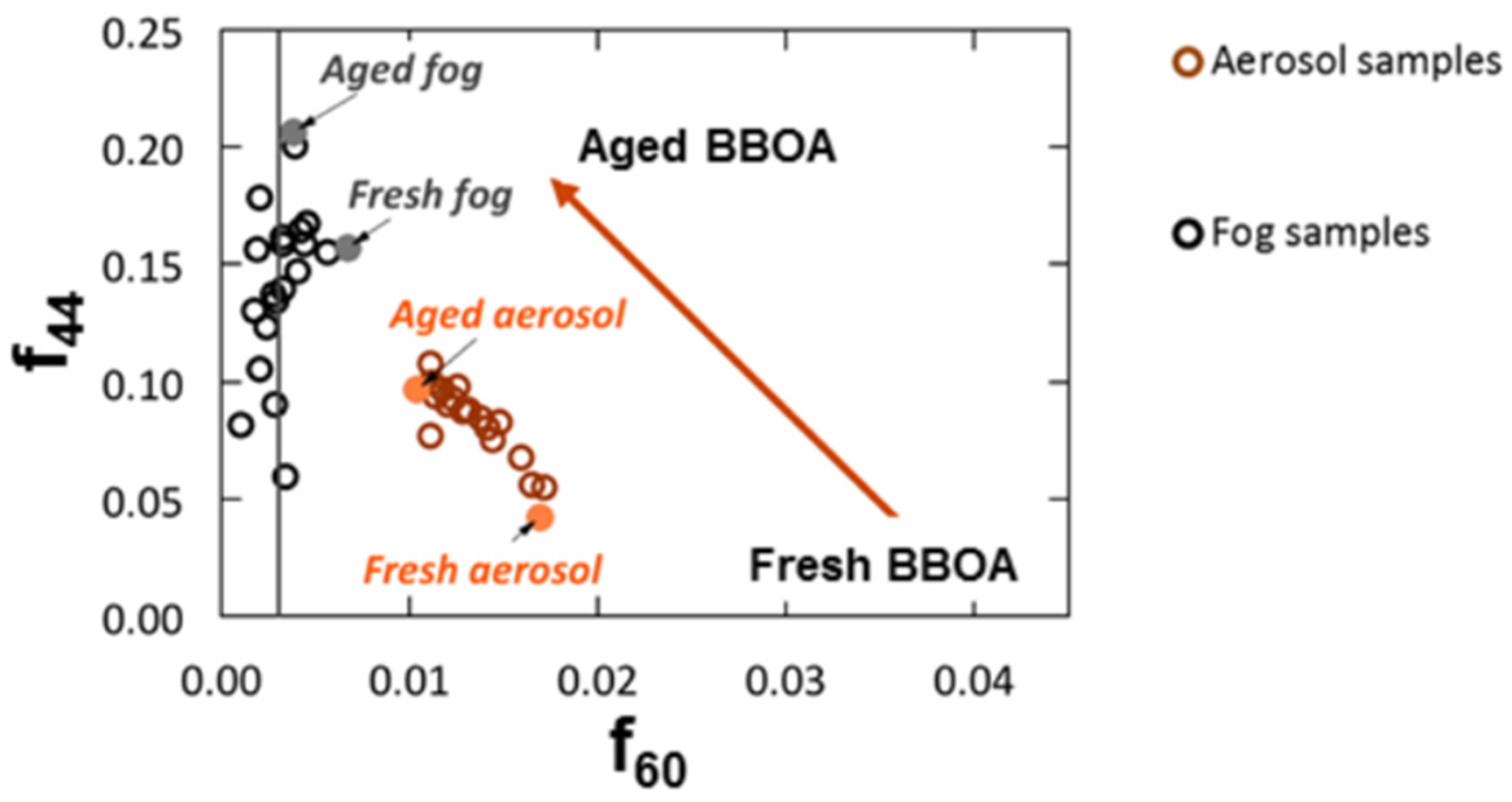
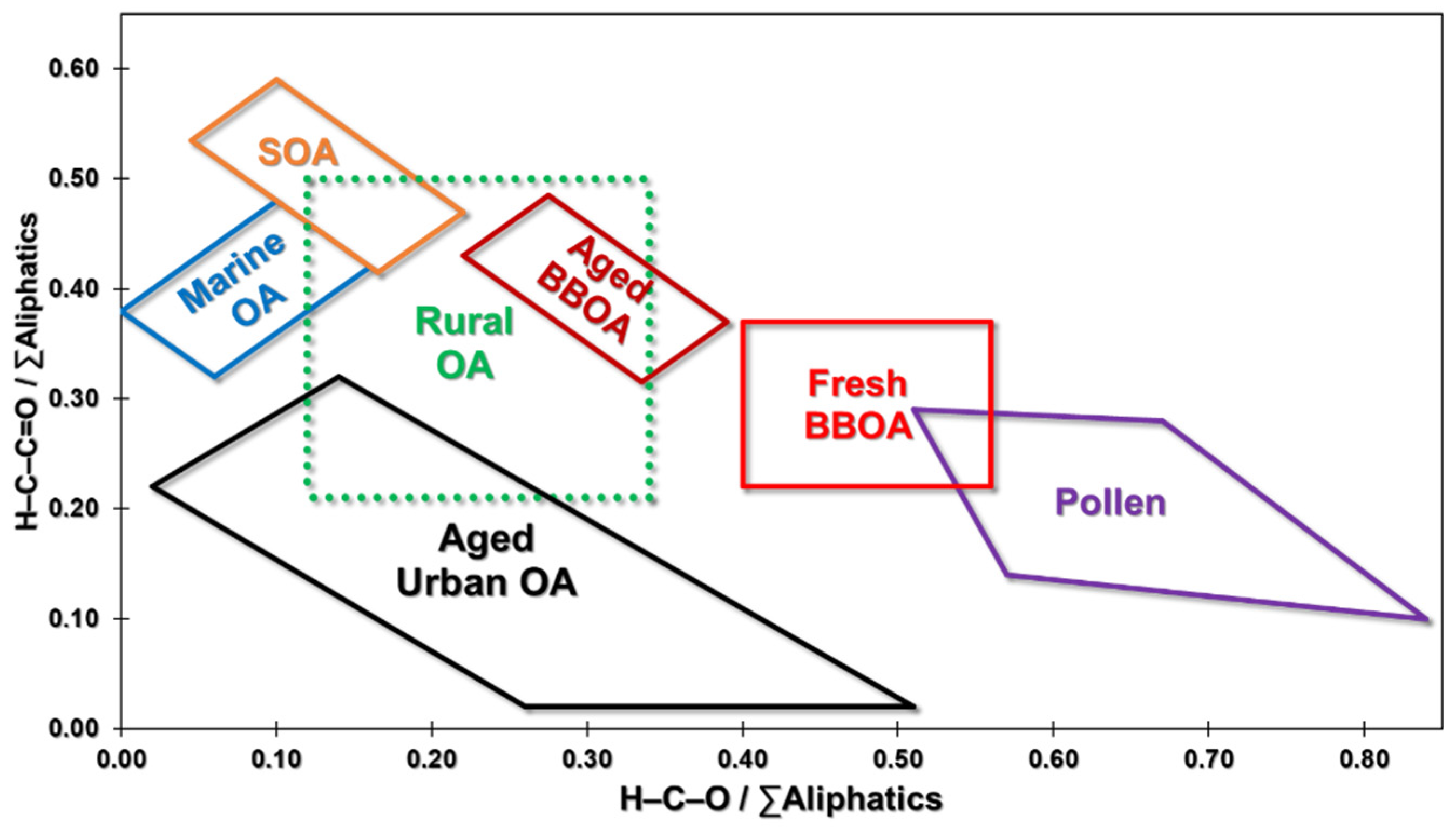
- Brege, M.; Paglione, M.; Gilardoni, S.; Decesari, S.; Cristina Facchini, M.; Mazzoleni, L.R. Molecular insights on aging and aqueous-phase processing from ambient biomass burning emissions-influenced Po Valley fog and aerosol. Atmos. Chem. Phys. 2018, 18, 13197–13214. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.T.V.; Freire, S.M.S.C.; Duarte, R.M.B.O.; Duarte, A.C. Profiling water-soluble organic matter from urban aerosols using comprehensive two-dimensional liquid chromatography. Aerosol Sci. Technol. 2015, 49. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.T.V.; Duarte, R.M.B.O.; Lopes, S.P.; Silva, A.M.S.; Duarte, A.C. Persistence of urban organic aerosols composition: Decoding their structural complexity and seasonal variability. Environ. Pollut. 2017, 231, 281–290. [Google Scholar] [CrossRef]
- Willoughby, A.S.; Wozniak, A.S.; Hatcher, P.G. Detailed source-specific molecular composition of ambient aerosol organic matter using ultrahigh resolution mass spectrometry and 1H NMR. Atmosphere 2016, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Duarte, R.M.B.O.; Santos, E.B.H.; Pio, C.A.; Duarte, A.C. Comparison of structural features of water-soluble organic matter from atmospheric aerosols with those of aquatic humic substances. Atmos. Environ. 2007, 41, 8100–8113. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Sullivan, A.P.; Weber, R.J.; Ingall, E.D. Characterization of water-soluble organic carbon in urban atmospheric aerosols using solid-state C-13 NMR spectroscopy. Environ. Sci. Technol. 2006, 40, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Decesari, S.; Mircea, M.; Cavalli, F.; Fuzzi, S.; Moretti, F.; Tagliavini, E.; Facchini, M.C. Source Attribution of Water-Soluble Organic Aerosol by Nuclear Magnetic Resonance Spectroscopy. Environ. Sci. Technol. 2007, 41, 2479–2484. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Silva, A.M.S.; Duarte, A.C. Two-Dimensional NMR Studies of Water-Soluble Organic Matter in Atmospheric Aerosols. Environ. Sci. Technol. 2008, 42, 8224–8230. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.T.V.; Freire, S.M.S.C.; Duarte, R.M.B.O.; Duarte, A.C. Natural organic matter in urban aerosols: Comparison between water and alkaline soluble components using excitation–emission matrix fluorescence spectroscopy and multiway data analysis. Atmos. Environ. 2015, 102, 1–10. [Google Scholar] [CrossRef]
- Paglione, M.; Saarikoski, S.; Carbone, S.; Hillamo, R.; Facchini, M.C.; Finessi, E.; Giulianelli, L.; Carbone, C.; Fuzzi, S.; Moretti, F.; et al. Primary and secondary biomass burning aerosols determined by proton nuclear magnetic resonance (1H-NMR) spectroscopy during the 2008 EUCAARI campaign in the Po Valley (Italy). Atmos. Chem. Phys. 2014, 14, 5089–5110. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, A.S.; Willoughby, A.S.; Gurganus, S.C.; Hatcher, P.G. Distinguishing molecular characteristics of aerosol water soluble organic matter from the 2011 trans-North Atlantic US GEOTRACES cruise. Atmos. Chem. Phys. 2014, 14, 8419–8434. [Google Scholar] [CrossRef] [Green Version]
- Ng, N.L.; Canagaratna, M.R.; Zhang, Q.; Jimenez, J.L.; Tian, J.; Ulbrich, I.M.; Kroll, J.H.; Docherty, K.S.; Chhabra, P.S.; Bahreini, R.; et al. Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry. Atmos. Chem. Phys. 2010, 10, 4625–4641. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, M.J.; Ziemba, L.D.; Griffin, R.J.; Dibb, J.E.; Anderson, C.H.; Lefer, B.; Rappenglück, B. Characterization of urban aerosol using aerosol mass spectrometry and proton nuclear magnetic resonance spectroscopy. Atmos. Environ. 2012, 54, 511–518. [Google Scholar] [CrossRef]
- Shakya, K.M.; Place, P.F.; Griffin, R.J.; Talbot, R.W. Carbonaceous content and water-soluble organic functionality of atmospheric aerosols at a semi-rural New England location. J. Geophys. Res. Atmos. 2012, 117, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Timonen, H.; Carbone, S.; Aurela, M.; Saarnio, K.; Saarikoski, S.; Ng, N.L.; Canagaratna, M.R.; Kulmala, M.; Kerminen, V.-M.; Worsnop, D.R.; et al. Characteristics, sources and water-solubility of ambient submicron organic aerosol in springtime in Helsinki, Finland. J. Aerosol Sci. 2013, 56, 61–77. [Google Scholar] [CrossRef]
- Chalbot, M.-C.G.; Brown, J.; Chitranshi, P.; Gamboa da Costa, G.; Pollock, E.D.; Kavouras, I.G. Functional characterization of the water-soluble organic carbon of size-fractionated aerosol in the southern Mississippi Valley. Atmos. Chem. Phys. 2014, 14, 6075–6088. [Google Scholar] [CrossRef] [Green Version]
- Willoughby, A.S.; Wozniak, A.S.; Hatcher, P.G. A molecular-level approach for characterizing water-insoluble components of ambient organic aerosol particulates using ultrahigh-resolution mass spectrometry. Atmos. Chem. Phys. 2014, 14, 10299–10314. [Google Scholar] [CrossRef] [Green Version]
- Duarte, R.M.B.O.; Duarte, A.C. A critical review of advanced analytical techniques for water-soluble organic matter from atmospheric aerosols. TrAC Trends Anal. Chem. 2011, 30, 1659–1671. [Google Scholar] [CrossRef]
- Pratt, K.A.; Prather, K.A. Mass spectrometry of atmospheric aerosols-Recent developments and applications. Part II: On-line mass spectrometry techniques. Mass Spectrom. Rev. 2012, 31, 17–48. [Google Scholar] [CrossRef]
- Pratt, K.A.; Prather, K.A. Mass spectrometry of atmospheric aerosols-Recent developments and applications. Part I: Off-line mass spectrometry techniques. Mass Spectrom. Rev. 2012, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Paglione, M.; Kiendler-Scharr, A.; Mensah, A.A.; Finessi, E.; Giulianelli, L.; Sandrini, S.; Facchini, M.C.; Fuzzi, S.; Schlag, P.; Piazzalunga, A.; et al. Identification of humic-like substances (HULIS) in oxygenated organic aerosols using NMR and AMS factor analyses and liquid chromatographic techniques. Atmos. Chem. Phys. 2014, 14, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef] [Green Version]
- Cubison, M.J.; Ortega, A.M.; Hayes, P.L.; Farmer, D.K.; Day, D.; Lechner, M.J.; Brune, W.H.; Apel, E.; Diskin, G.S.; Fisher, J.A.; et al. Effects of aging on organic aerosol from open biomass burning smoke in aircraft and laboratory studies. Atmos. Chem. Phys. 2011, 11, 12049–12064. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Guo, H.; Weber, R.J.; Ng, N.L. Chemical Characterization of Water-Soluble Organic Aerosol in Contrasting Rural and Urban Environments in the Southeastern United States. Environ. Sci. Technol. 2017, 51, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Paglione, M.; Decesari, S.; Harrison, R.M.; Beddows, D.C.S.; Ovadnevaite, J.; Ceburnis, D.; O’Dowd, C.D.; Simó, R.; Dall’Osto, M. Contribution of Water-Soluble Organic Matter from Multiple Marine Geographic Eco-Regions to Aerosols around Antarctica. Environ. Sci. Technol. 2020, 54, 7807–7817. [Google Scholar] [CrossRef]
- Prather, K.A.; Hatch, C.D.; Grassian, V.H. Analysis of atmospheric aerosols. Annu. Rev. Anal. Chem. 2008, 1, 485–514. [Google Scholar] [CrossRef]
- May, J.C.; McLean, J.A. Advanced Multidimensional Separations in Mass Spectrometry: Navigating the Big Data Deluge. Annu. Rev. Anal. Chem. 2016, 9, 387–409. [Google Scholar] [CrossRef] [Green Version]
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Mass spectrometric approaches for chemical characterisation of atmospheric aerosols: Critical review of the most recent advances. Environ. Chem. 2012, 9, 163–189. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.T.V.; Duarte, R.M.B.O.; Duarte, A.C. Challenges in the identification and characterization of free amino acids and proteinaceous compounds in atmospheric aerosols: A critical review. TrAC Trends Anal. Chem. 2016, 75. [Google Scholar] [CrossRef]
- Cai, J.; Zeng, X.; Zhi, G.; Gligorovski, S.; Sheng, G.; Yu, Z.; Wang, X.; Peng, P. Molecular composition and photochemical evolution of water-soluble organic carbon (WSOC) extracted from field biomass burning aerosols using high-resolution mass spectrometry. Atmos. Chem. Phys. 2020, 20, 6115–6128. [Google Scholar] [CrossRef]
- Alam, M.S.; Harrison, R.M. Recent advances in the application of 2-dimensional gas chromatography with soft and hard ionisation time-of-flight mass spectrometry in environmental analysis. Chem. Sci. 2016, 7, 3968–3977. [Google Scholar] [CrossRef] [Green Version]
- Pól, J.; Hohnová, B.; Jussila, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography-time-of-flight mass spectrometry in the analysis of acidic compounds in atmospheric aerosols. J. Chromatogr. A 2006, 1130, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.S.; Matos, J.T.V.; Duarte, R.M.B.O.; Duarte, A.C. Two chemically distinct light-absorbing pools of urban organic aerosols: A comprehensive multidimensional analysis of trends. Chemosphere 2016, 145. [Google Scholar] [CrossRef] [PubMed]
- Spranger, T.; Van Pinxteren, D.; Herrmann, H. Two-Dimensional Offline Chromatographic Fractionation for the Characterization of Humic-Like Substances in Atmospheric Aerosol Particles. Environ. Sci. Technol. 2017, 51, 5061–5070. [Google Scholar] [CrossRef]
- Spranger, T.; Pinxteren, D.; Van Reemtsma, T.; Lechtenfeld, O.J.; Herrmann, H. 2D Liquid Chromatographic Fractionation with Ultra-high Resolution MS Analysis Resolves a Vast Molecular Diversity of Tropospheric Particle Organics. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pirok, B.W.J.; Stoll, D.R.; Schoenmakers, P.J. Recent Developments in Two-Dimensional Liquid Chromatography: Fundamental Improvements for Practical Applications. Anal. Chem. 2019, 91, 240–263. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-K.; Jackson, S.N.; Murray, K.K. Matrix-assisted laser desorption-ionization mass spectrometry of collected bioaerosol particles. Rapid Commun. Mass Spectrom. 2005, 19, 1725–1729. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Su, T.; Han, Y.; Mo, Y.; Jiang, H.; Cui, M.; Jiang, B.; Chen, Y.; Tang, J.; et al. Molecular compositions and optical properties of dissolved brown carbon in biomass burning, coal combustion, and vehicle emission aerosols illuminated by excitation—Emission matrix spectroscopy and Fourier transform ion cyclotron resonance mass spectr. Atmos. Chem. Phys. 2020, 20, 2513–2532. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Cao, T.; Thompson, J.E. The chemical evolution & physical properties of organic aerosol: A molecular structure based approach. Atmos. Environ. 2012, 62, 199–207. [Google Scholar] [CrossRef]
- Chalbot, M.-C.G.; Chitranshi, P.; Gamboa da Costa, G.; Pollock, E.; Kavouras, I.G. Characterization of water-soluble organic matter in urban aerosol by 1H-NMR spectroscopy. Atmos. Environ. 2016, 128, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbalakshmi, Y.; Patti, A.F.; Lee, G.S.H.; Hooper, M.A. Structural characterisation of macromolecular organic material in air particulate matter using Py-GC-MS and solid state C-13-NMR. J. Environ. Monit. 2000, 2, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.M.B.O.; Pio, C.A.; Duarte, A.C. Spectroscopic study of the water-soluble organic matter isolated from atmospheric aerosols collected under different atmospheric conditions. Anal. Chim. Acta 2005, 530, 7–14. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Duarte, A.C. Unraveling the structural features of organic aerosols by NMR spectroscopy: A review. Magn. Reson. Chem. 2015, 53, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.M.B.O.; Duarte, A.C. Atmospheric Organic Matter. In NMR Spectroscopy: A Versatile Tool for Environmental Research; Simpson, M.J., Simpson, A.J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 193–206. ISBN 9780470034590. [Google Scholar]
- Schmitt-Kopplin, P.; Gelencsér, A.; Dabek-Zlotorzynska, E.; Kiss, G.; Hertkorn, N.; Harir, M.; Hong, Y.; Gebefügi, I. Analysis of the Unresolved Organic Fraction in Atmospheric Aerosols with Ultrahigh-Resolution Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy: Organosulfates As Photochemical Smog Constituents. Anal. Chem. 2010, 82, 8017–8026. [Google Scholar] [CrossRef] [PubMed]
- Chalbot, M.C.G.; Gamboa da Costa, G.; Kavouras, I.G. NMR Analysis of the Water-Soluble Fraction of Airborne Pollen Particles. Appl. Magn. Reson. 2013, 44, 1347–1358. [Google Scholar] [CrossRef]
- Decesari, S.; Finessi, E.; Rinaldi, M.; Paglione, M.; Fuzzi, S.; Stephanou, E.G.; Tziaras, T.; Spyros, A.; Ceburnis, D.; O’Dowd, C.; et al. Primary and secondary marine organic aerosols over the North Atlantic Ocean during the MAP experiment. J. Geophys. Res. Atmos. 2011, 116, 1–21. [Google Scholar] [CrossRef]
- Chalbot, M.; Siddiqui, S.; Kavouras, I.G. Molecular Speciation of Size Fractionated Particulate Water-Soluble Organic Carbon by Two-Dimensional Nuclear Magnetic Resonance (NMR) Spectroscopy. Int. J. Environ. Res. Public Health 2021, 18, 1334. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Laskin, A.; Laskin, J.; Nizkorodov, S.A. Brown carbon formation from ketoaldehydes of biogenic monoterpenes. Faraday Discuss. 2013, 165, 473–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finessi, E.; Lidster, R.T.; Whiting, F.; Elliott, T.; Alfarra, M.R.; McFiggans, G.B.; Hamilton, J.F. Improving the quantification of secondary organic aerosol using a microflow reactor coupled to HPLC-MS and NMR to manufacture ad hoc calibration standards. Anal. Chem. 2014, 86, 11238–11245. [Google Scholar] [CrossRef] [PubMed]
- Kitanovski, Z.; Čusak, A.; Grgić, I.; Claeys, M. Chemical characterization of the main products formed through aqueous-phase photonitration of guaiacol. Atmos. Meas. Tech. 2014, 7, 2457–2470. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Fu, P.; Ram, K.; Song, J.; Chen, Q.; Kawamura, K.; Wan, X.; Kang, S.; Wang, X.; Laskin, A.; et al. Fluorescence characteristics of water-soluble organic carbon in atmospheric aerosol. Environ. Pollut. 2021, 268, 115906. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, M.; Kang, S.; Yan, F.; Han, X.; Gautam, S.; Hu, Z.; Zheng, H.; Chen, P.; Gao, S.; et al. Light absorption and fluorescence characteristics of water-soluble organic compounds in carbonaceous particles at a typical remote site in the southeastern Himalayas and Tibetan Plateau. Environ. Pollut. 2021, 272, 116000. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Laskin, A.; Laskin, J.; Nizkorodov, S.A. Excitation-emission spectra and fluorescence quantum yields for fresh and aged biogenic secondary organic aerosols. Environ. Sci. Technol. 2013, 47, 5763–5770. [Google Scholar] [CrossRef]
- Lee, H.J.; Aiona, P.K.; Laskin, A.; Laskin, J.; Nizkorodov, S.A. Effect of Solar Radiation on the Optical Properties and Molecular Composition of Laboratory Proxies of Atmospheric Brown Carbon. Environ. Sci. Technol. 2014, 48, 10217–10226. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Kawamura, K.; Chen, J.; Qin, M.; Ren, L.; Sun, Y.; Wang, Z.; Barrie, L.A.; Tachibana, E.; Ding, A.; et al. Fluorescent water-soluble organic aerosols in the High Arctic atmosphere. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Miyazaki, Y.; Kawamura, K.; Matsumoto, K.; Coburn, S.; Volkamer, R.; Iwamoto, Y.; Kagami, S.; Deng, Y.; Ogawa, S.; et al. Characterization of Chromophoric Water-Soluble Organic Matter in Urban, Forest, and Marine Aerosols by HR-ToF-AMS Analysis and Excitation–Emission Matrix Spectroscopy. Environ. Sci. Technol. 2016, 50, 10351–10360. [Google Scholar] [CrossRef]
- Fan, X.; Wei, S.; Zhu, M.; Song, J.; Peng, P. Comprehensive characterization of humic-like substances in smoke PM2.5 emitted from the combustion of biomass materials and fossil fuels. Atmos. Chem. Phys. 2016, 16, 13321–13340. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ikemori, F.; Mochida, M. Light Absorption and Excitation–Emission Fluorescence of Urban Organic Aerosol Components and Their Relationship to Chemical Structure. Environ. Sci. Technol. 2016, 50, 10859–10868. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, L.; Zhou, X.; Duan, J.; Mu, S.; Xiao, K.; Hu, J.; Tan, J. Fluorescence fingerprinting properties for exploring water-soluble organic compounds in PM2.5 in an industrial city of northwest China. Atmos. Environ. 2018, 184, 203–211. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Alier, M.; Van Drooge, B.L.; Dall’Osto, M.; Querol, X.; Grimalt, J.O.; Tauler, R. Source apportionment of submicron organic aerosol at an urban background and a road site in Barcelona (Spain) during SAPUSS. Atmos. Chem. Phys. 2013, 13, 10353–10371. [Google Scholar] [CrossRef] [Green Version]
- Viana, M.; Kuhlbusch, T.A.J.; Querol, X.; Alastuey, A.; Harrison, R.M.; Hopke, P.K.; Winiwarter, W.; Vallius, M.; Szidat, S.; Prévôt, A.S.H.; et al. Source apportionment of particulate matter in Europe: A review of methods and results. J. Aerosol Sci. 2008, 39, 827–849. [Google Scholar] [CrossRef]
- Shrivastava, M.K.; Subramanian, R.; Rogge, W.F.; Robinson, A.L. Sources of organic aerosol: Positive matrix factorization of molecular marker data and comparison of results from different source apportionment models. Atmos. Environ. 2007, 41, 9353–9369. [Google Scholar] [CrossRef]
- Bove, M.C.; Brotto, P.; Cassola, F.; Cuccia, E.; Massabò, D.; Mazzino, A.; Piazzalunga, A.; Prati, P. An integrated PM2.5 source apportionment study: Positive Matrix Factorisation vs. the chemical transport model CAMx. Atmos. Environ. 2014, 94, 274–286. [Google Scholar] [CrossRef]
- Bell, N.G.A.; Michalchuk, A.A.L.; Blackburn, J.W.T.; Graham, M.C.; Uhrín, D. Isotope-Filtered 4D NMR Spectroscopy for Structure Determination of Humic Substances. Angew. Chem. Int. Ed. 2015, 54, 8382–8385. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, R.M.B.O.; Matos, J.T.V.; Duarte, A.C. Multidimensional Analytical Characterization of Water-Soluble Organic Aerosols: Challenges and New Perspectives. Appl. Sci. 2021, 11, 2539. https://doi.org/10.3390/app11062539
Duarte RMBO, Matos JTV, Duarte AC. Multidimensional Analytical Characterization of Water-Soluble Organic Aerosols: Challenges and New Perspectives. Applied Sciences. 2021; 11(6):2539. https://doi.org/10.3390/app11062539
Chicago/Turabian StyleDuarte, Regina M. B. O., João T. V. Matos, and Armando C. Duarte. 2021. "Multidimensional Analytical Characterization of Water-Soluble Organic Aerosols: Challenges and New Perspectives" Applied Sciences 11, no. 6: 2539. https://doi.org/10.3390/app11062539
APA StyleDuarte, R. M. B. O., Matos, J. T. V., & Duarte, A. C. (2021). Multidimensional Analytical Characterization of Water-Soluble Organic Aerosols: Challenges and New Perspectives. Applied Sciences, 11(6), 2539. https://doi.org/10.3390/app11062539