Commentary: Iconoclastic Reflections on the ‘Safety’ of Polyunsaturated Fatty Acid-Rich Culinary Frying Oils: Some Cautions regarding the Laboratory Analysis and Dietary Ingestion of Lipid Oxidation Product Toxins
Abstract
1. Introduction
2. Materials and Methods
2.1. Culinary Frying Oils and Fats
2.2. Laboratory-Simulated Shallow Frying Episodes
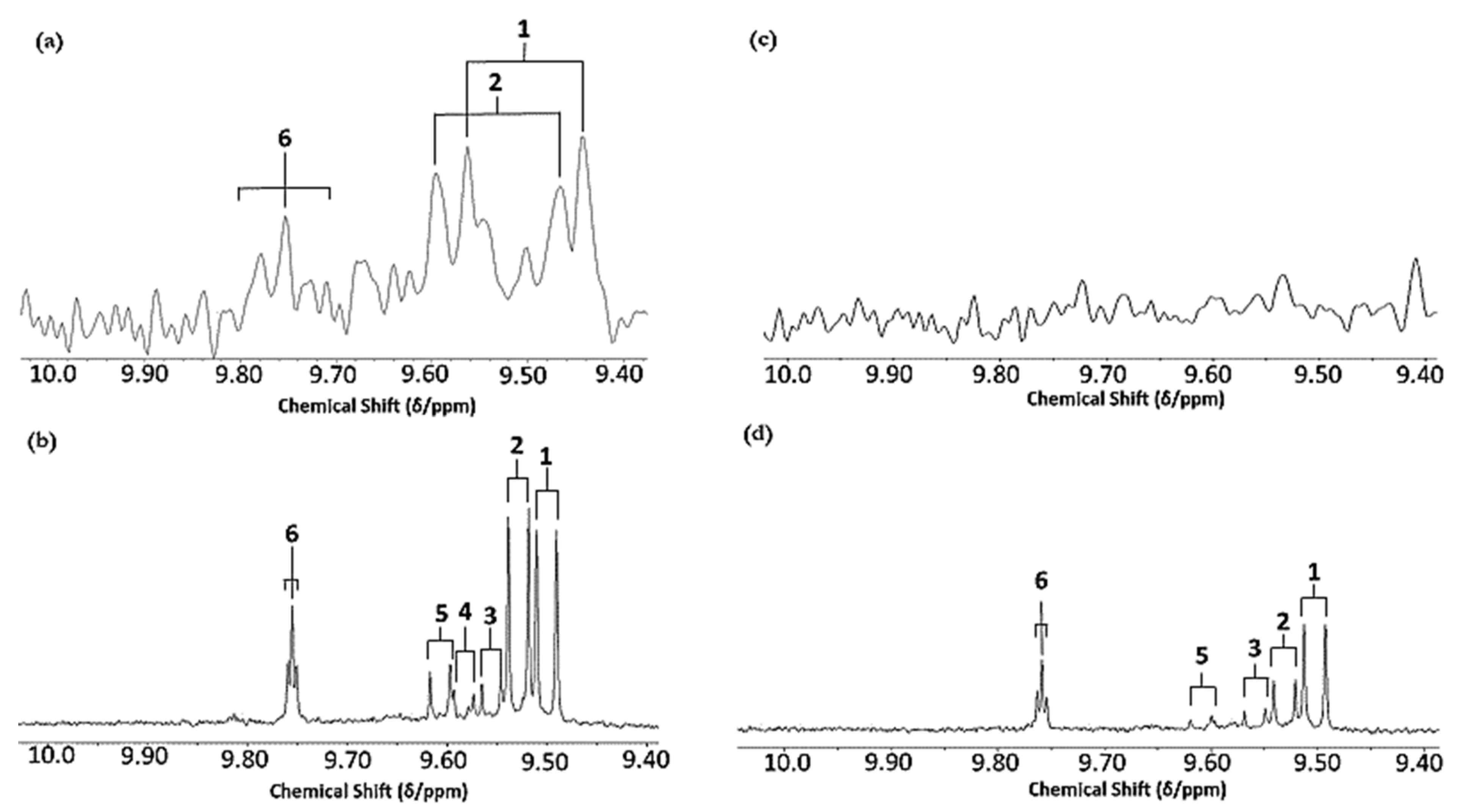
2.3. 1H NMR Analysis
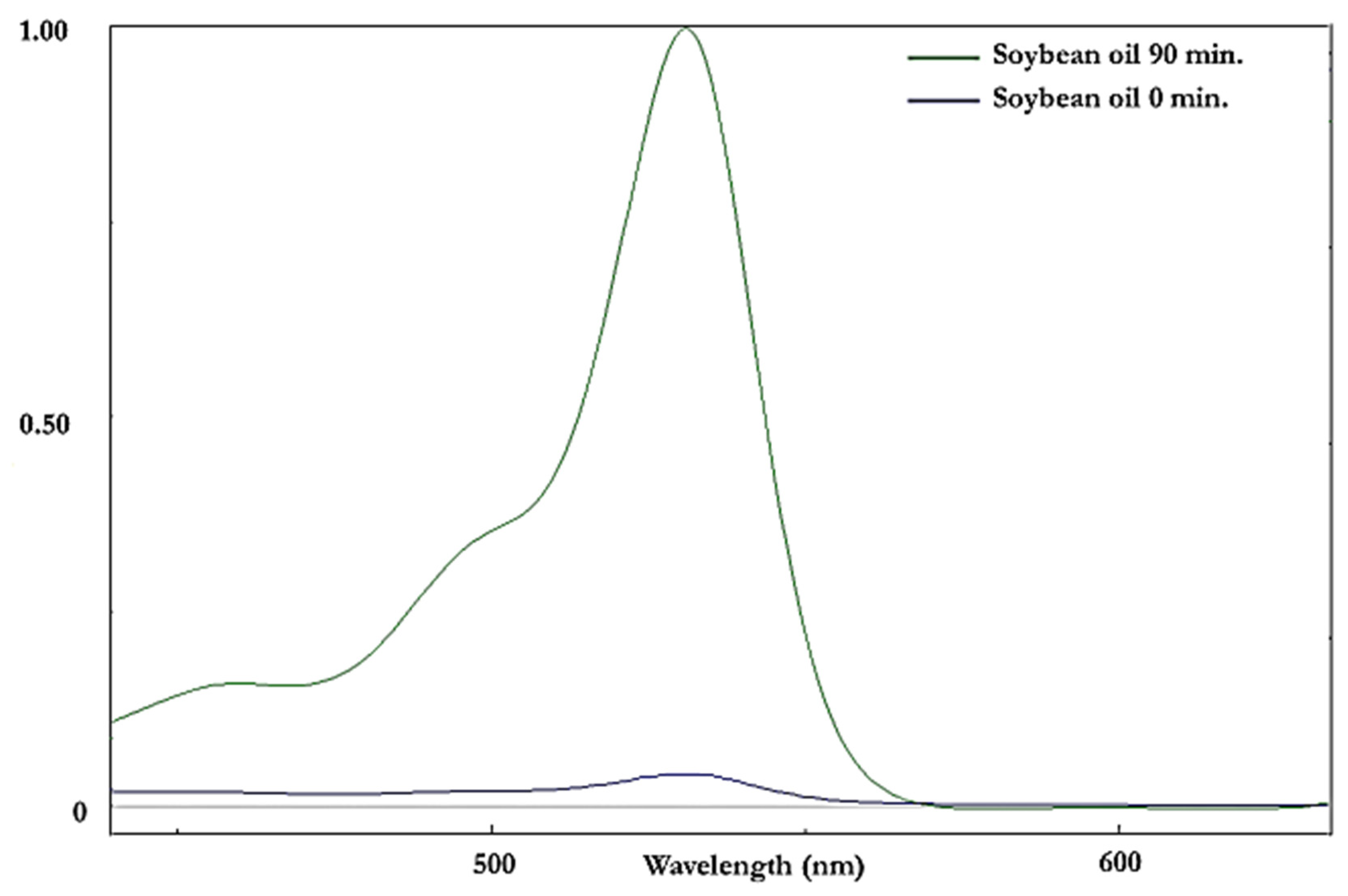
2.4. Specific Spectrophotometric Method for the Analysis of Malondialdehyde
2.5. Statistical Analysis of Patterns of Aldehydic LOPs Generated during LSSFEs
3. Results and Discussion
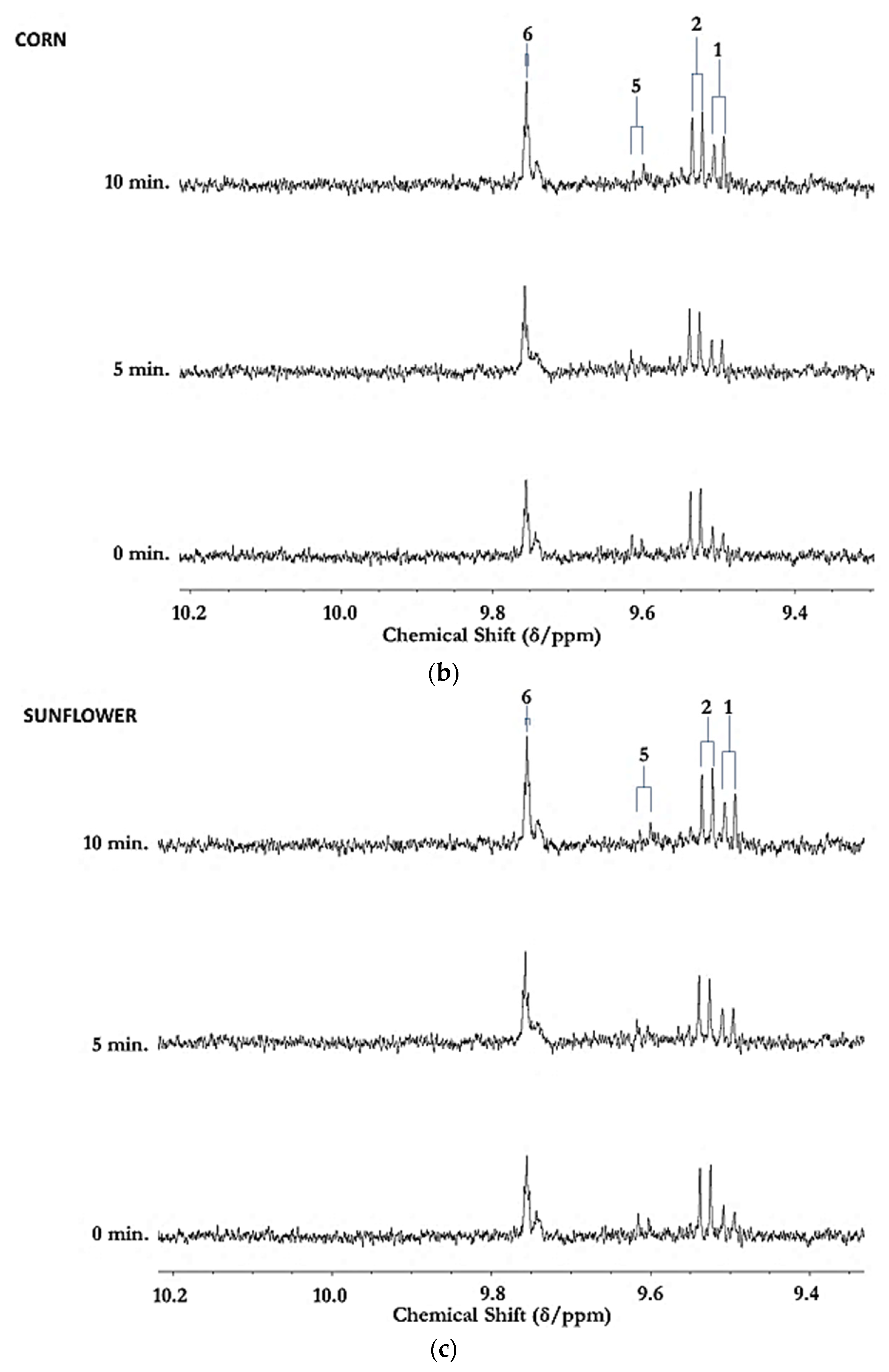
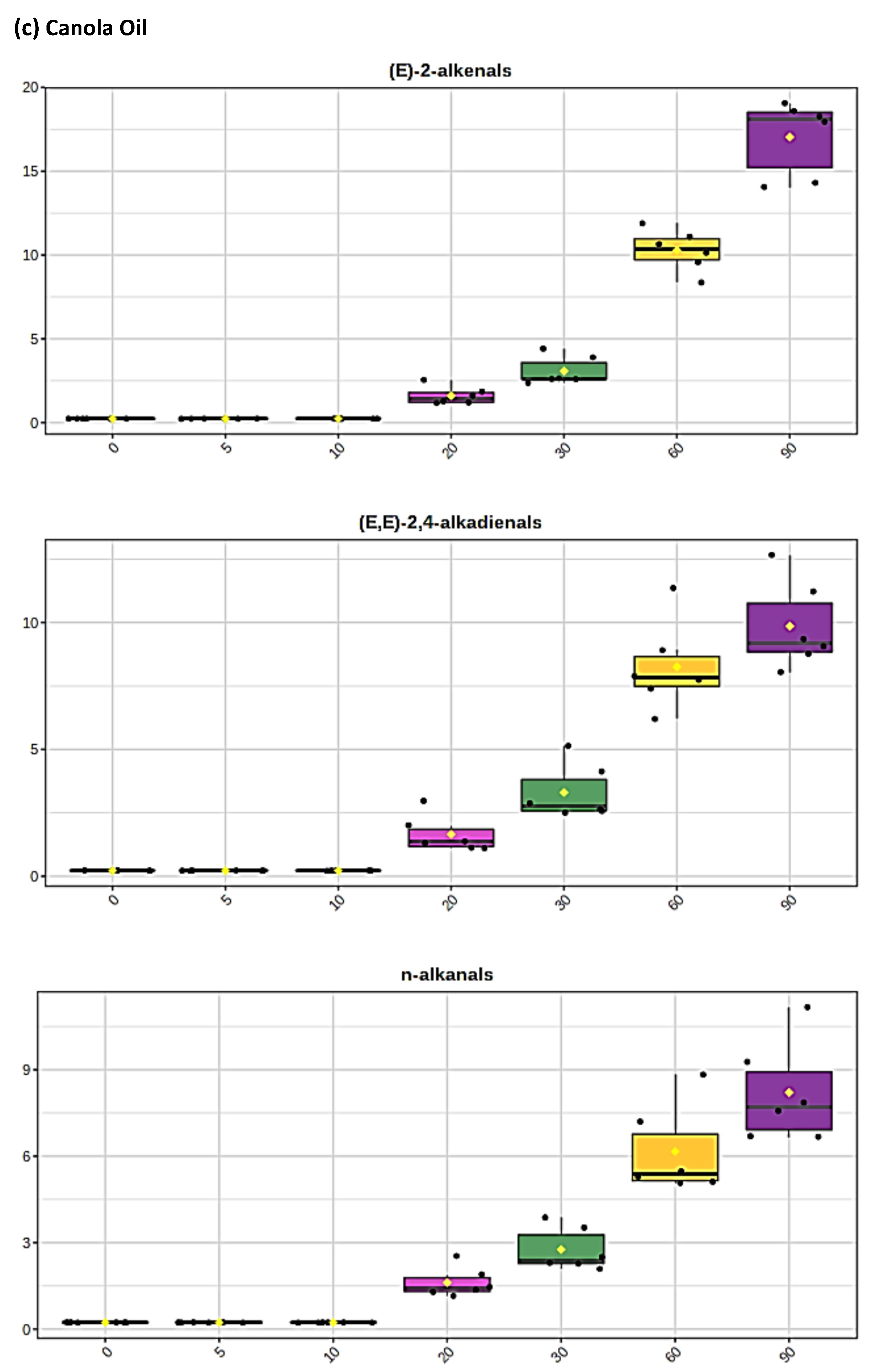
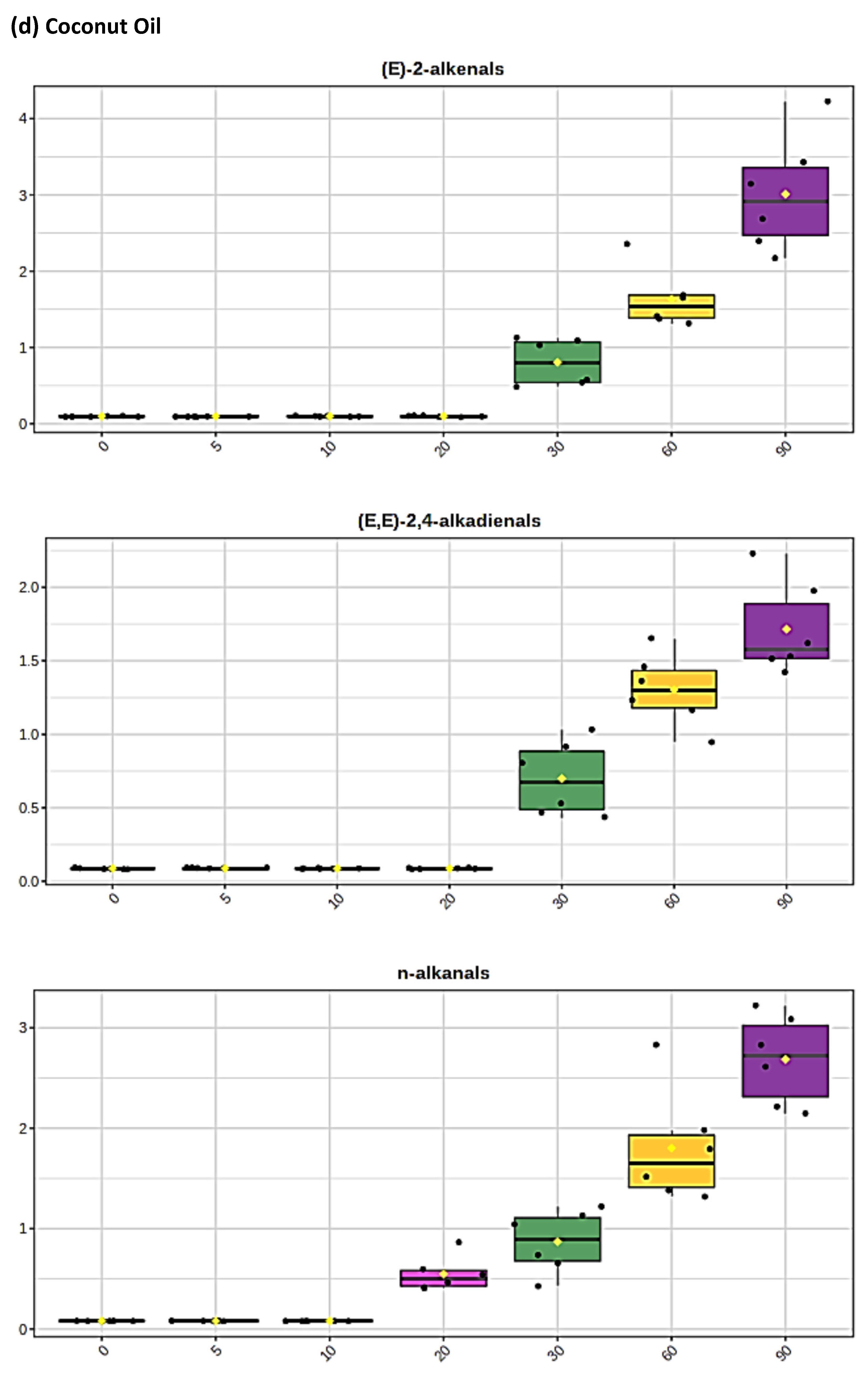
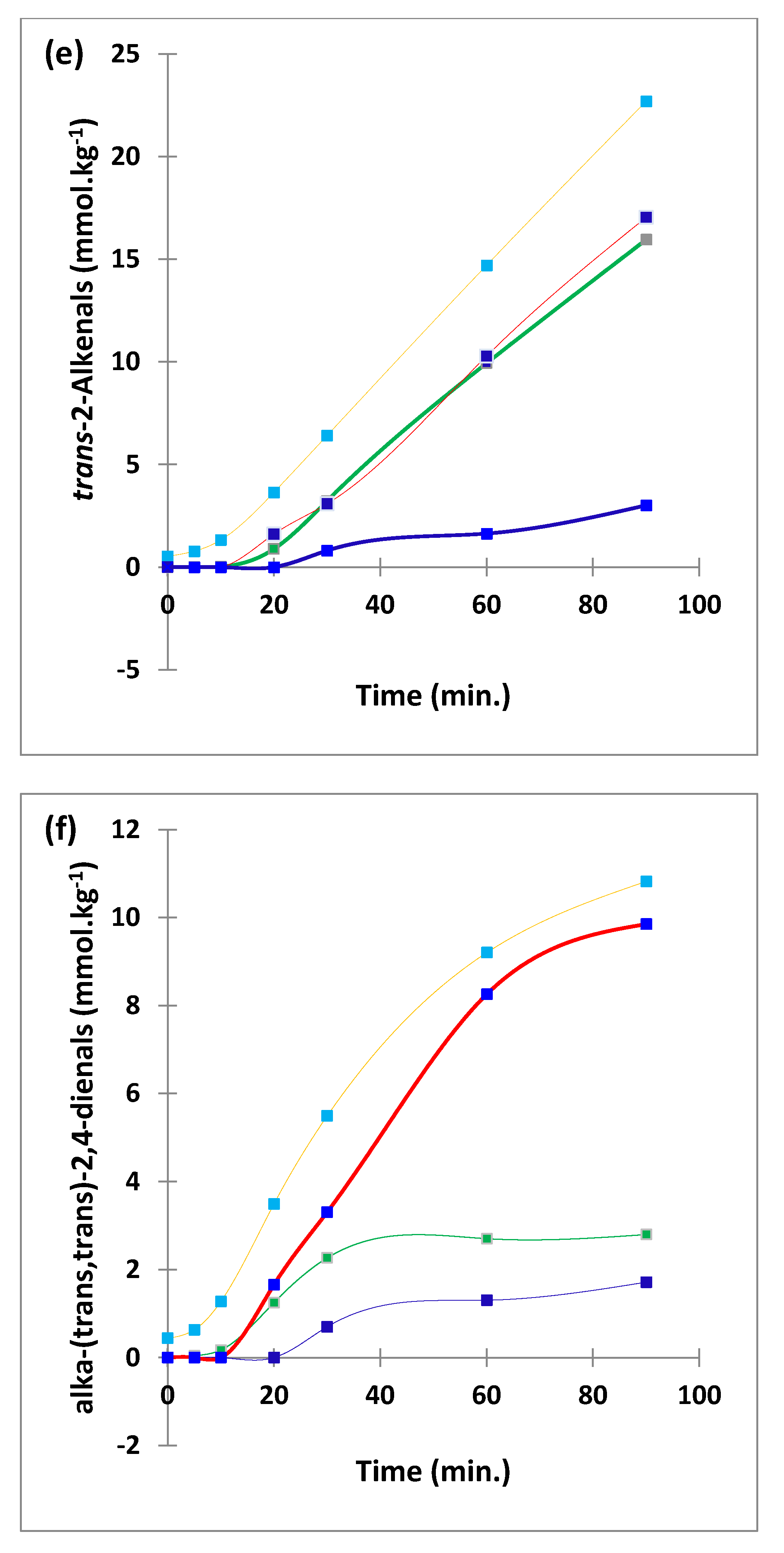
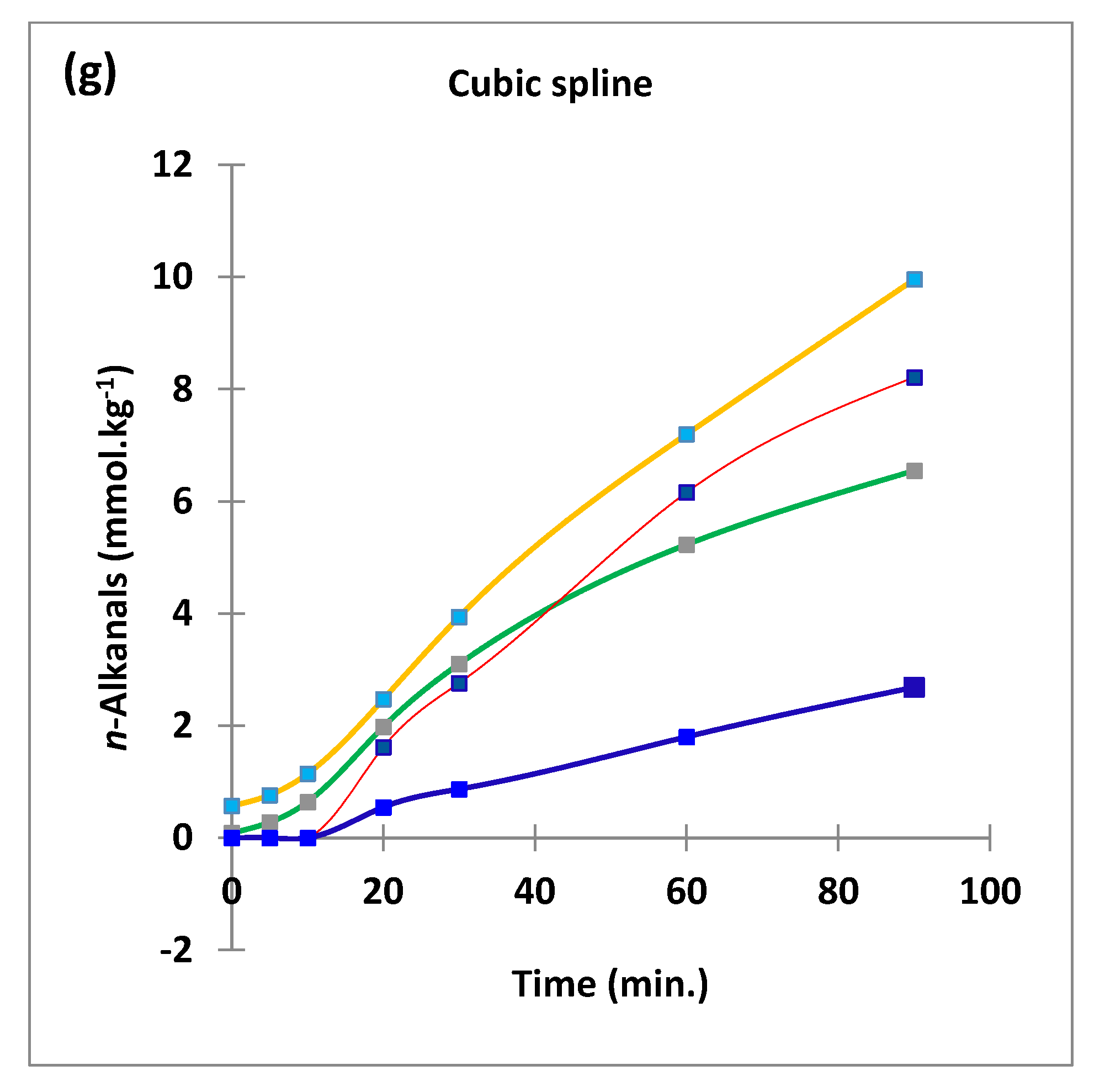
3.1. HF 1H NMR Case Study: Time-Dependent Tracking of Aldehydic LOPs in CFOs Exposed to LSSFEs
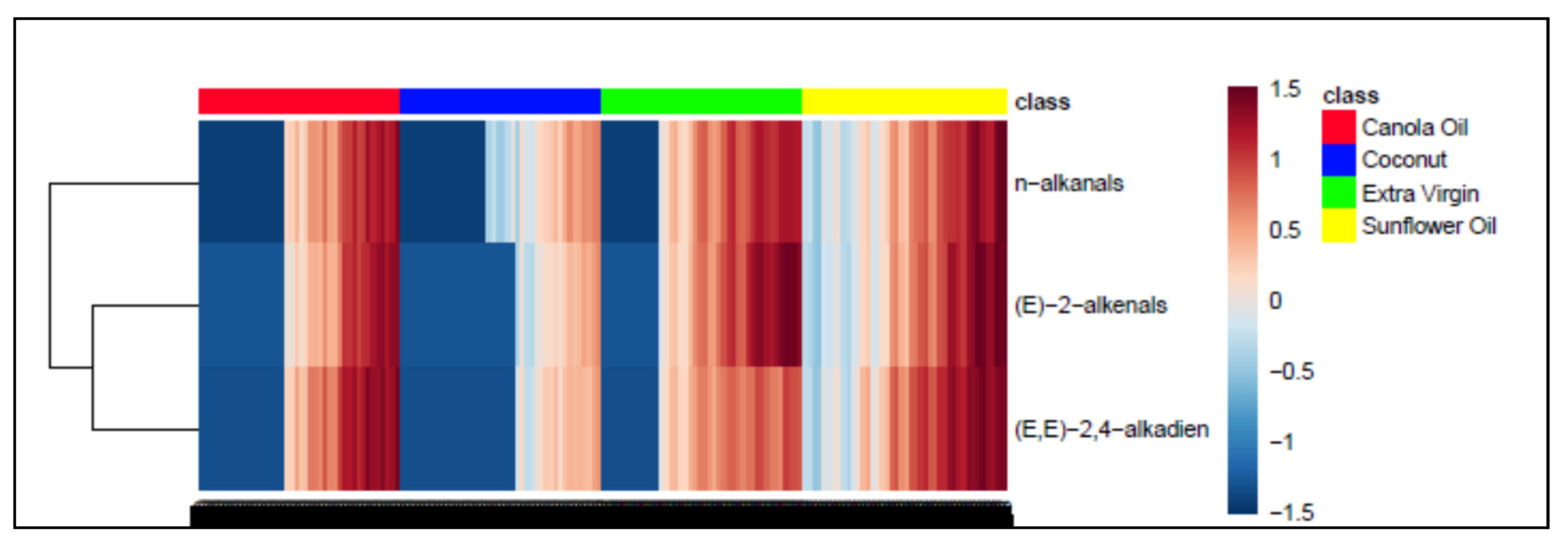
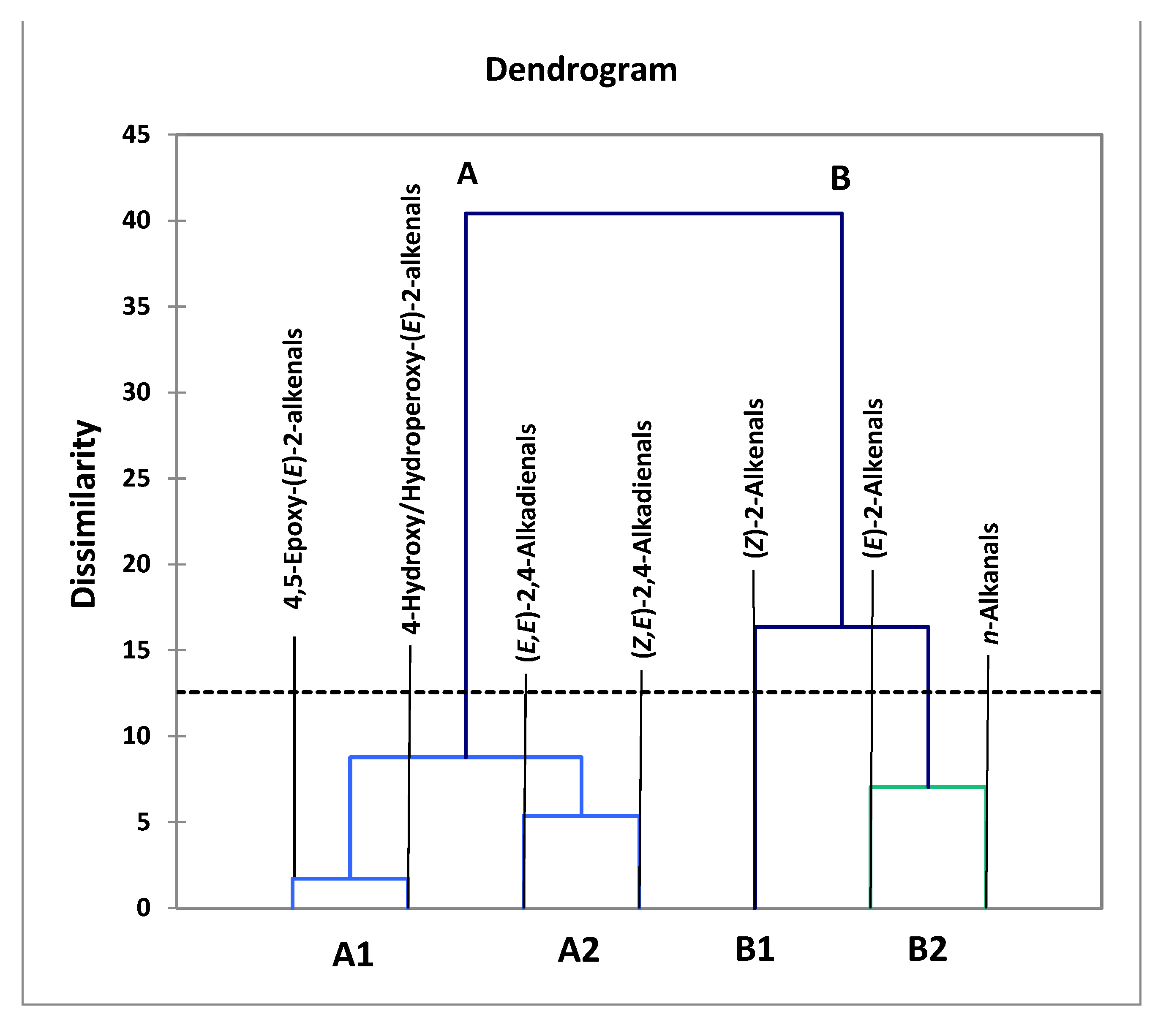
3.2. AHC Analysis of Aldehydic LOP Variables
3.3. Considerations of Possible Evaporative Loss of Culinary Frying Oils during LSSFEs
3.4. LF Benchtop (60 MHz) NMR Analysis of LOPs in Thermally-Stressed CFOs
3.5. Case Study Review: Analytical Advantages Offered by 1H NMR Analysis of LOPs in CFOs
3.6. Critical Review of the Reliabilities and Selectivities of Commonly Employed Non-NMR Methods for LOP Determinations
3.6.1. Spectrophotometric Conjugated Dienes Assay
3.6.2. Spectrophotometric TBARS Test
3.7. Considerations of General Health Risks Posed by Human Exposure to Aldehydic LOPs
3.7.1. Overview of Health Risks
3.7.2. Estimated Daily Dietary Intakes of Aldehydic LOPs: Accordance with Governmental Regulatory Limits?
3.7.3. Carcinogenicities of Selected α,β-Unsaturated Aldehydes
3.7.4. Toxicological Significance of Dietary LOPs in CLOs and CLO-Loaded Fried Foods, and Its Frying Process Type-Dependence
3.7.5. Potential Suppression of LOP Generation during High-Temperature Frying Practices: Do Dietary Antioxidants Work?
3.7.6. Comparative Evaluations of Health Risks Presented by Dietary LOPs and Trans-FAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- FAO/WHO. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO/WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39 (Suppl. 1), 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Choose right carbohydrates and right fats (RCRF)—Keys to optimal health. Hepatobil. Surg. Nutr. 2017, 6, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Brown, L. Should the pharmacological actions of dietary fatty acids in cardiometabolic disorders be classified based on biological or chemical function? Prog. Lipid Res. 2015, 59, 172–200. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Jarmila, A.V.; Vicha, R.; MLMLcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; HHS Publication #: HHS-ODPHP-2015-2020-01-DGA-A USDA Publication #: Home and Garden Bulletin No. 232; 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines (accessed on 4 June 2019).
- Estévez, M.; Li, Z.; Soladoye, O.P.; Van-Hecke, T. Health Risks of Food Oxidation. Adv. Food Nutr. Res. 2017, 82, 45–81. [Google Scholar] [CrossRef]
- Grootveld, M.; Silwood, C.J.L.; Addis, P.; Claxson, A.W.D.; Bonet Serra, B.; Viana, M. Health effects of oxidised heated oils. Foodserv. Res. Int. 2001, 13, 39–53. [Google Scholar] [CrossRef]
- Grootveld, M.; Ruiz-Rodado, V.; Silwood, C.J.L. Detection, monitoring and deleterious health effects of lipid oxidation products generated in culinary oils during thermal stressing episodes. Inform AOCS 2014, 25, 614–624. [Google Scholar]
- Haywood, R.M.; Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Coumbarides, G.; Hawkes, J.; Lynch, E.J.; Grootveld, M.C. Detection of aldehydes and their conjugated hydroperoxydiene precursors in thermally-stressed culinary oils and fats: Investigations using high resolution proton NMR spectroscopy. Free Radic. Res. 1995, 22, 441–482. [Google Scholar] [CrossRef]
- Moumtaz, S.; Percival, B.C.; Parmar, D.; Grootveld, K.L.; Jansson, P.; Grootveld, M. Toxic aldehyde generation in and food uptake from culinary oils during frying practices: Peroxidative resistance of a monounsaturate-rich algae oil. Sci. Rep. 2019, 9, 4125. [Google Scholar] [CrossRef]
- Frankel, E.N. Volatile lipid oxidation-products. Prog. Lipid Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Perez-Camino, M.C. Fatty acid composition: A useful tool for the determination of alteration level in heated fats. Rev. Fr. Corps Gras 1988, 35, 67–70. [Google Scholar]
- Frankel, E.N.; Selke, E.; Nef, W.E.; Miyashita, K. Autoxidation of polyunsaturated triacylglycerols. IV. Volatile decomposition products from triacylglycerols containing linoleate and linolenate. Lipids 1992, 27, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Neff, W.E.; Byrdwell, W.C. Characterization of model triacylglycerol (triolein, trilinolein and trilinolenin) autoxidation products via high-performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 1998, 818, 169–186. [Google Scholar] [CrossRef]
- Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Haywood, R.M.; Chander, C.L.; Atherton, M.; Lynch, E.J.; Grootveld, M.C. Generation of lipid peroxidation products in culinary oils and fats during episodes of thermal stressing: A high field 1H NMR study. FEBS Lett. 1994, 355, 81–90. [Google Scholar] [CrossRef]
- Silwood, C.J.L.; Grootveld, M. Application of high-resolution two-dimensional 1H and 13C nuclear magnetic resonance techniques to the characterization of lipid oxidation products in autoxidized linoleoyl/linolenoyglycerols. Lipids 1999, 34, 741–756. [Google Scholar] [CrossRef]
- Sacchi, R.; Falcigno, L.; Paduano, A.; Ambrosino, M.L.; Savarese, M.; De Giulio, B.; Addeo, F.; Paolillo, L. Quantitative evaluation of the aldehydes formed in heated vegetable oils using high resolution proton-NMR spectroscopy. Riv. Ital. Sostanze Grasse 2006, 83, 257–263. [Google Scholar]
- Martınez-Yusta, A.; Goicoechea, E.; Guillen, M.D. A Review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Grootveld, M.; Atherton, M.D.; Sheerin, A.N.; Hawkes, J.; Blake, D.R.; Richens, T.E.; Silwood, C.J.L.; Lynch, E.; Claxson, A.W.D. In vivo absorption, metabolism, and urinary excretion of α,β-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally-stressed polyunsaturate-rich culinary oils. J. Clin. Investig. 1998, 101, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Polensek, L.; Hadley, M.; McGirr, L.G. Urinary malondialdehyde as an indicator of lipid peroxidation in the diet and in the tissues. Lipids 1984, 19, 836–843. [Google Scholar] [CrossRef]
- Draper, H.H.; Csallany, A.S.; Hadley, M. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic. Biol. Med. 2000, 29, 1071–1077. [Google Scholar] [CrossRef]
- Pignitter, M.; Somoza, V. Impact of dietary oxidized lipids on energy metabolism. Adv. Food Technol. Nutr. Sci. 2015, 1, 76–81. [Google Scholar] [CrossRef]
- Penumetcha, M.; Khan, N.; Parthasarathy, S. Dietary oxidized fatty acids: An atherogenic risk? J. Lipid Res. 2000, 41, 1473–1480. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A. Cholesterol vehicle in experimental atherosclerosis. 9. Comparison of heated corn oil and heated olive oil. J. Atheroscler. Res. 1967, 7, 647–651. [Google Scholar] [CrossRef]
- Staprãns, I.; Rapp, J.H.; Pan, X.-M.; Hardman, D.A.; Feingold, K.R.; Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 533–538. [Google Scholar] [CrossRef]
- Marnett, L.J.; Hurd, H.K.; Hollstein, M.C.; Levin, D.E.; Esterbauer, H.; Ames, B.N. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA 104. Mutat. Res. 1985, 748, 25–34. [Google Scholar] [CrossRef]
- Zhong, L.; Goldberg, M.S.; Parent, M.E.; Hanley, J.A. Risk of developing lung cancer in relation to exposure to fumes from Chinese-style cooking. Scand. J. Work Environ. Health 1999, 25, 309–316. [Google Scholar] [CrossRef]
- Shields, P.G.; Xu, G.X.; Blot, W.J.; Fraumeni, J.F., Jr.; Trivers, G.E.; Pellizzari, E.D.; Qu, Y.H.; Gao, Y.T.; Harris, C.C. Mutagens from heated Chinese and U.S. cooking oils. J. Natl. Cancer Inst. 1995, 87, 836–841. [Google Scholar] [CrossRef]
- Wu, S.C.; Yen, G.C. Effects of cooking oil fumes on the genotoxicity and oxidative stress in human lung carcinoma (A-549) cells. Toxicol. In Vitro 2004, 18, 571–580. [Google Scholar] [CrossRef]
- Young, S.C.; Chang, L.W.; Lee, H.L.; Tsai, L.H.; Liu, Y.C.; Lin, P. DNA damage induced by trans,trans-2,4-decadienal (tt-DD), a component of cooking oil fume, in human bronchial epithelial cells. Environ. Mol. Mutagenes. 2010, 51, 315–321. [Google Scholar]
- Lee, T.; Gany, F. Cooking oil fumes and lung cancer: A review of the literature in the context of the U.S. population. J. Immigr. Minor. Health 2013, 15, 646–652. [Google Scholar] [CrossRef]
- Indart, A.; Viana, M.; Grootveld, M.C.; Silwood, C.J.L.; Sanchez-Vera, I.; Bonet, B. Teratogenic actions of thermally-stressed culinary oils in rats. Free Radic. Res. 2002, 36, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Ferrali, A.; Casini, A.F.; Peiri, S.; Comporti, M. Foot edema induced by carbonyl compounds originating from the peroxidation of microsomal lipids. Biochem. Pharmacol. 1980, 29, 121–124. [Google Scholar] [CrossRef]
- Jayaraj, A.P.; Rees, K.R.; Tovey, F.E.I.; White, J.S. A molecular basis of peptic ulceration due to diet. Br. J. Exp. Pathol. 1986, 67, 149–155. [Google Scholar] [PubMed]
- Long, E.K.; Murphy, T.C.; Leiphon, L.J.; Watt, J.; Morrow, J.D.; Milne, G.L.; Howard, J.R.H.; Picklo, M.J., Sr. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 2008, 105, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.-F.; Mustafa, M.R.; Das, S.; Jaarin, K. Association of elevated blood pressure and impaired vasorelaxation in experimental Sprague-Dawley rats fed with heated vegetable oil. Lipids Health Dis. 2010, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Emerit, I.; Khan, S.H.; Esterbauer, H. Hydroxynonenal, a component of clastogenic factors? Free Radic. Biol. Med. 1991, 10, 371–377. [Google Scholar] [CrossRef]
- Emerit, I. Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogenic factors in carcinogenesis. Free Radic. Biol. Med. 1994, 36, 99–109. [Google Scholar] [CrossRef]
- Carletto, C.; Nicolay, J.F.; Courbebaisse, Y. Oxidative stress and cutaneous ageing: The ‘toxic second messengers’ concept and an interesting family of products, ‘pseudodipeptides’. Int. J. Cosmet. Sci. 2000, 22, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Opperman, M.; Benadé, A.; Abrecht, C.; Matsheka, L. South African seed oils are safe for consumption. S. Afr. J. Clin. Nutr. 2016, 29, 7–11. [Google Scholar] [CrossRef][Green Version]
- Karimi, S.; Wawire, M.; Mathooko, F.M. Impact of frying practices and frying conditions on the quality and safety of frying oils used by street vendors and restaurants in Nairobi, Kenya. J. Food Compos. Anal. 2017, 62, 239–244. [Google Scholar] [CrossRef]
- Friere, P.C.M.; Lobo, L.C.B.; da Silva Freites, G.; de Castro Ferreria, T.A.P. The quality of deep-frying oils and fats used in street-fairs in Goiânia. Braz. Food Sci. Technol. 2013, 33, 569–576. [Google Scholar] [CrossRef][Green Version]
- Percival, B.C.; Savel, E.; Ampem, G.; Gibson, M.; Edgar, M.; Jafari, F.; Frederick, K.; Wilson, P.; Grootveld, M. Molecular composition of and potential health benefits offered by natural East African virgin sunflower oil products: A 400 MHz 1H NMR analysis study. Int. J. Nutr. 2019, 3, 22–43. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Estimation of free fatty acid content in oils, fats, and biodiesel by 1H NMR spectroscopy. Energy Fuels 2009, 23, 2273–2277. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α,β-unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Moumtaz, S.; Grootveld, K.L. A 1H NMR-linked PCR modelling strategy for tracking the fatty acid sources of aldehydic lipid oxidation products in culinary oils exposed to simulated shallow-frying episodes. Int. J. Chem. Mol. Eng. 2019, 13, 251–263. [Google Scholar]
- Parker, T.; Limer, E.; Watson, A.D.; Defernez, M.; Williamson, D.; Kemsley, E.K. 60 MHz 1H-NMR spectroscopy for the analysis of edible oils. Trends Anal. Chem. 2014, 57, 147–158. [Google Scholar] [CrossRef]
- Siddiqui, N.; Sim, J.; Silwood, C.J.L.; Toms, H.; Iles, R.A.; Grootveld, M. Multicomponent analysis of encapsulated marine oil supplements using high resolution 1H and 13C NMR nuclear magnetic resonance (NMR) techniques. J. Lipid Res. 2003, 44, 2406–2427. [Google Scholar] [CrossRef]
- Cortinas, L.; Galobart, J.; Barroeta, A.C.; Baucells, M.D.; Grashorn, M.A. Change in α-tocopherol contents, lipid oxidation and fatty acid profile in eggs enriched with linolenic acid or very long-chain w-3 polyunsaturated fatty acids after different processing methods. J. Sci. Food Agric. 2008, 83, 820–829. [Google Scholar] [CrossRef]
- Guillod, T. Interpolations, courbes de bézier et B-splines. Bull. Soc. Enseign. Neuchâtel. Sci. 2008, 34, 1–50. [Google Scholar]
- Matthews, R.F.; Scanlan, R.A.; Libbey, L.M. Autoxidation products of 2,4-decadienal. J. Am. Oil Chem. Soc. 1971, 48, 745–747. [Google Scholar] [CrossRef]
- Frankel, E.N. Frying fats. In Lipid Oxidation; Frankel, E.N., Ed.; The Oily Press Ltd.: Dundee, UK, 2005; pp. 227–248. [Google Scholar]
- Hrncirik, K.; Zeelenberg, M. Stability of essential fatty Acids and Formation of Nutritionally Undesirable Compounds in Baking and Shallow Frying. J. Am. Oil Chem. Soc. 2014, 91, 591–598. [Google Scholar] [CrossRef]
- Mateos, R.; Uceda, M.; Aguilera, M.P.; Escuderos, M.E.; Maza, G.B. Relationship of Rancimat method values at varying temperatures for virgin olive oils. Eur. Food Res. Technol. 2006, 223, 246–252. [Google Scholar] [CrossRef]
- Fanelli, S.; Zimmermann, T.E.G.; Salgado, H.R.N. FTIR Spectrophotometry as a green tool for quantitative analysis of drugs: Practical application to amoxicillin. J. Chem. 2018, 2018, 3920810. [Google Scholar] [CrossRef]
- Giese, E.; Rohn, S.; Fritsche, J. Chemometric tools for the authentication of cod liver oil based on nuclear magnetic resonance and infrared spectroscopy data. Anal. Bioanal. Chem. 2019, 411, 6931–6942. [Google Scholar] [CrossRef]
- Xu, L.; Fei, T.; Yu, X.; Li, Q.; Liu, L. Qualitative analysis of edible oil oxidation by FTIR spectroscopy using mesh “cell”. Anal. Methods 2015, 7, 4328–4333. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Gibson, M.; Osman, Y.; Edgar, M.; Molinari, M.; Mather, M.L.; Casanova, F.; Wilson, P.B. Progress in low-field benchtop NMR spectroscopy in chemical and biochemical Analysis—A review. Anal. Chim. Acta 2019, 1067, 11–30. [Google Scholar] [CrossRef]
- Saguy, I.S.; Shani, A.; Weinberg, P.; Garti, N. Utilization of jojoba oil for deep-fat frying of foods. Lebensm. Wiss. Technol. 1996, 29, 573–577. [Google Scholar] [CrossRef]
- Sochr, J.; Cinkova, K.; Svorc, L. Degradation markers in nutritional products: A review. Aust. J. Anal. Pharm. Chem. 2014, 1, 1005. [Google Scholar]
- Shahidi, F.; Wanasundara, U.N. Methods for measuring oxidative rancidity in fats and oils. In Food Lipids: Chemistry, Nutrition and Biotechnology; Akah, C.C., Min, D.B., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 465–487. [Google Scholar]
- Chapter 19, Section 3: Spectroscopy of Aldehydes and Ketones. pp. 895–903. Available online: http://www.saplinglearning.com/media/loudon/loudon5ech19sec03.pdf (accessed on 2 March 2020).
- Kalaliana, C.; Samira, B.; Rotha, E.; Chakira, A. UV absorption spectra of trans-2-pentenal, trans-2-hexenal and 2-methyl-2-pentenal. Chem. Phys. Lett. 2019, 718, 22–26. [Google Scholar] [CrossRef]
- Cao, J.; Li, H.; Xia, X.; Zou, X.-G.; Li, J.; Zhu, X.-M.; Deng, Z.-Y. Effect of fatty acid and tocopherol on oxidative stability of vegetable oils with limited air. Int. J. Food Prop. 2015, 184, 808–820. [Google Scholar] [CrossRef]
- Zhanyuan, D.; BramLmLage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Hodges, D.; DeLong, J.; Forney, C.; Prange, R. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Hazard Symbols and Hazard Pictograms—Chemical Classification—HSE. Available online: http://www.hse.gov.uk (accessed on 11 March 2020).
- Mehta, U.; Swinburn, B. A review of factors affecting fat absorption in hot chips. Crit. Rev. Food Sci. Nutr. 2001, 41, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.G.; Castell-Perez, M.E.; Barrufet, M.A. Deep-Fat Frying Fundamentals and Applications; Aspen Publishers Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Mellema, M. Mechanism and reduction of fat uptake in deep-fat fried foods. Trends Food Sci. Technol. 2000, 14, 364–373. [Google Scholar] [CrossRef]
- Alireza, S.; Tan, C.P.; Hamed, M.; Che Man, Y.B. Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Int. Food Res. J. 2010, 17, 295–302. [Google Scholar]
- Naseri, M.; Abedi, E.; Mohammadzadeh, B.; Afsharnaderi, A. Effect of frying in different culinary fats on the fatty acid composition of silver carp. Food Sci. Nutr. 2013, 1, 292–297. [Google Scholar] [CrossRef]
- Csallany, A.S.; Han, I.; Shoeman, D.W.; Chen, C.; Yuan, J. 4-Hydroxynonenal (HNE), a toxic aldehyde in french fries from fast food restaurants. JAOCS 2015, 92, 1413–1419. [Google Scholar] [CrossRef]
- Powers, S.J.; Mottram, D.S.; Curtis, A.; Halford, N.G. Acrylamide levels in potato crisps in Europe from 2002 to 2016. Food Add. Contam. Part A 2017, 34, 2085–2100. [Google Scholar] [CrossRef]
- Krishnakumar, T.; Visvanathan, R. Acrylamide in food products: A review. J. Food Process. Technol. 2014, 5, 7. [Google Scholar]
- Ehling, S.; Hengel, M.; Shibamoto, T. Formation of acrylamide from lipids. Adv. Exp. Med. Biol. 2005, 56, 223–233. [Google Scholar]
- Acceptable Daily Intakes for Agricultural and Veterinary Chemicals: Current as of 31 March 2016; The Office of Chemical Safety, Department of Health: Sydney, Australia, 2016.
- World Health Organization; Gomes, R.; Meek, M.E. Concise International Chemical Assessment Document 43 ACROLEIN; United Nations Environment Programme, the International Labour Organization, and the World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Grootveld, M.; Percival, B.C.; Grootveld, K.L. Chronic non-communicable disease risks presented by lipid oxidation products in fried foods. Hepatobil. Surg. Nutr. 2018, 7, 305–312. [Google Scholar] [CrossRef]
- van Andel, I.; Sleijffers, A.; Schenk, E.; Rambali, B.; Wolterink, G.; van de Werken, G.; van Aerts, L.A.G.J.M.; Vleeming, W.; van Amsterdam, J.G.C. Adverse Health Effects of Cigarette Smoke: Aldehydes Crotonaldehyde, Butyraldehyde, Hexanal, and Malonaldehyde; RIVM Report 340630002/2006; RIVM: Bilthoven, The Netherlands, 2006. [Google Scholar]
- National Institute of Occupational Safety and Health’s (NIOSH’s). Immediately Dangerous to Life or Health (IDLH) Concentrations; CAS Number 107-02-08; Centers for Disease Control and Prevention, NIOSH Publications and Products: Atlanta, GA, USA, 1994. [Google Scholar]
- Henderson, Y.; Haggard, H.W. Noxious Gases, 2nd ed.; Reinhold Publishing Corporation: New York, NY, USA, 1943; p. 138. [Google Scholar]
- NRC. Emergency and Continuous Exposure Limits for Selected Airborne Contaminants; National Academy Press, Committee on Toxicology, Board on Toxicology and Environmental Health Hazards, Commission on Life Sciences, National Research Council: Washington, DC, USA, 1984; Volume 1, pp. 27–34. [Google Scholar]
- Darley, E.F.; Middleton, J.T.; Garber, M.J. Plant damage and eye irritation from ozone-hydrocarbon reactions. J. Agric. Food Chem. 1960, 8, 483–485. [Google Scholar] [CrossRef]
- Sim, V.M.; Pattle, R.E. Effect of possible smog irritants on human subjects. J. Am. Med. Assoc. 1957, 165, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Japan Bioassay Research Center. Summary of Inhalation Carcinogenicity Study of Acrolein in B6D2F1 Mice; Japan Organization of Health and Safety: Kanagawa, Japan, 2016. [Google Scholar]
- Japan Bioassay Research Center. Summary of Inhalation Carcinogenicity Study of Acrolein in F344 Rats; Japan Organization of Health and Safety: Kanagawa, Japan, 2016. [Google Scholar]
- Nath, R.G.; Ocando, J.E.; Guttenplan, J.B.; Chung, F.L. 1,N2-propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998, 58, 581–584. [Google Scholar] [PubMed]
- Chen, H.J.; Lin, W.P. Quantitative analysis of multiple exocyclic DNA adducts in human salivary DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Anal. Chem. 2011, 83, 8543–8551. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hu, W.; Hu, Y.; Tang, M.S. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. USA 2006, 103, 15404–15409. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.L.; Tanaka, T.; Hecht, S.S. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986, 46, 1285–1289. [Google Scholar] [PubMed]
- Dittberner, U.; Eisenbrand, G.; Zankl, H. Genotoxic effects of the alpha,beta-unsaturated aldehydes 2-trans-butenal, 2-trans-hexenal and 2-trans, 6-cis-nonadienal. Mutat. Res. 1995, 335, 259–265. [Google Scholar] [CrossRef]
- IARC Monograph Working Group. Carcinogenicity of acrolein, crotonaldehyde, and arecoline. Lancet Oncol. 2020. [Google Scholar] [CrossRef]
- Pamies, D.; Vilanova, E.A.; Wexler, P. Encyclopedia of Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 63–68. ISBN 9780123864550. [Google Scholar] [CrossRef]
- Esterbauer, H.; Ertl, A.; Scholz, N. The reaction of cysteine with α,β-unsaturated aldehydes. Tetrahedron 1976, 32, 285–289. [Google Scholar] [CrossRef]
- Allam, S.S.M.; Mohamed, H.M.A. Thermal stability of some commercial natural and synthetic antioxidants and their mixtures. J. Food Lipids 2002, 9, 277–293. [Google Scholar] [CrossRef]
- Battino, M.; Quiles, J.L.; Huertas, J.R.; Ramirez-Tortosa, M.C.; Cassinello, M.; Mañas, M.; Lopez-Frias, M.; Mataix, J. Feeding fried oil changes antioxidant and fatty acid pattern of rat and affects rat liver mitochondrial respiratory chain components. J. Bioenerget. Biomem. 2002, 34, 127–134. [Google Scholar] [CrossRef]
- Quiles, J.L.; Huertas, J.R.; Battino, M.; Ramirez-Tortosa, M.C.; Cassinello, M.; Mataix, J.; Lopez-Frias, M.; Mañas, M. The intake of fried virgin olive or sunflower oils differentially induces oxidative stress in rat liver microsomes. Br. J. Nutr. 2002, 88, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, N.K.; Dedoussis, G.V.Z.; Falirea, A.; Kalogeropoulos, N.; Hatzinikola, H.S. Deterioration of natural antioxidant species of vegetable edible oils during the domestic deep-frying and pan-frying of potatoes. Int. J. Food Sci. Nutr. 2002, 53, 351–363. [Google Scholar] [CrossRef]
- Percival, B.C.; Zbasnik, R.; Schlegel, V.; Edgar, M.; Zhang, J.; Grootveld, M. Determinations of the peroxidative susceptibilities of cod liver oils by a newly-developed 1H NMR-based method: Resistance of an antioxidant-fortified product isolated from pre-fermented sources. Biomed. Cent. Res. Notes 2020, 13, 73. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Jung, J.; Lee, C.; Gim, S.Y.; Ka, H.; Yi, B.R.; Kim, M.-J.; Kim, C.-I.; Lee, J.H. Analysis of trans fat in edible oils with cooking process. Toxicol. Res. 2015, 31, 307–312. [Google Scholar] [CrossRef]
- Countdown to 2023: WHO Report on Global Trans-Fat Elimination 2020; World Health Organization: Geneva, Switzerland, 2020. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/334170/9789240010178-eng.pdf (accessed on 9 December 2020).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grootveld, M.; Percival, B.C.; Moumtaz, S.; Gibson, M.; Woodason, K.; Akhtar, A.; Wawire, M.; Edgar, M.; Grootveld, K.L. Commentary: Iconoclastic Reflections on the ‘Safety’ of Polyunsaturated Fatty Acid-Rich Culinary Frying Oils: Some Cautions regarding the Laboratory Analysis and Dietary Ingestion of Lipid Oxidation Product Toxins. Appl. Sci. 2021, 11, 2351. https://doi.org/10.3390/app11052351
Grootveld M, Percival BC, Moumtaz S, Gibson M, Woodason K, Akhtar A, Wawire M, Edgar M, Grootveld KL. Commentary: Iconoclastic Reflections on the ‘Safety’ of Polyunsaturated Fatty Acid-Rich Culinary Frying Oils: Some Cautions regarding the Laboratory Analysis and Dietary Ingestion of Lipid Oxidation Product Toxins. Applied Sciences. 2021; 11(5):2351. https://doi.org/10.3390/app11052351
Chicago/Turabian StyleGrootveld, Martin, Benita C. Percival, Sarah Moumtaz, Miles Gibson, Katy Woodason, Azeem Akhtar, Michael Wawire, Mark Edgar, and Kerry L. Grootveld. 2021. "Commentary: Iconoclastic Reflections on the ‘Safety’ of Polyunsaturated Fatty Acid-Rich Culinary Frying Oils: Some Cautions regarding the Laboratory Analysis and Dietary Ingestion of Lipid Oxidation Product Toxins" Applied Sciences 11, no. 5: 2351. https://doi.org/10.3390/app11052351
APA StyleGrootveld, M., Percival, B. C., Moumtaz, S., Gibson, M., Woodason, K., Akhtar, A., Wawire, M., Edgar, M., & Grootveld, K. L. (2021). Commentary: Iconoclastic Reflections on the ‘Safety’ of Polyunsaturated Fatty Acid-Rich Culinary Frying Oils: Some Cautions regarding the Laboratory Analysis and Dietary Ingestion of Lipid Oxidation Product Toxins. Applied Sciences, 11(5), 2351. https://doi.org/10.3390/app11052351






