Behaviour of Metal(loid)s at the Sediment-Water Interface in an Aquaculture Lagoon Environment (Grado Lagoon, Northern Adriatic Sea, Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Solid Phase Analysis
2.4. Dissolved Phase Analysis
2.5. Diffusive and Benthic Flux Calculation Dissolved Phase Analysis
2.6. Exploratory Multivariate Data Analysis
3. Results and Discussion
3.1. Solid Phase
3.1.1. Sediment Geochemistry and Enrichment Factor (EF) of Metal(loid)s
3.1.2. Labile Fraction of Metal(loid)s in Sediments
3.2. Dissolved Phase
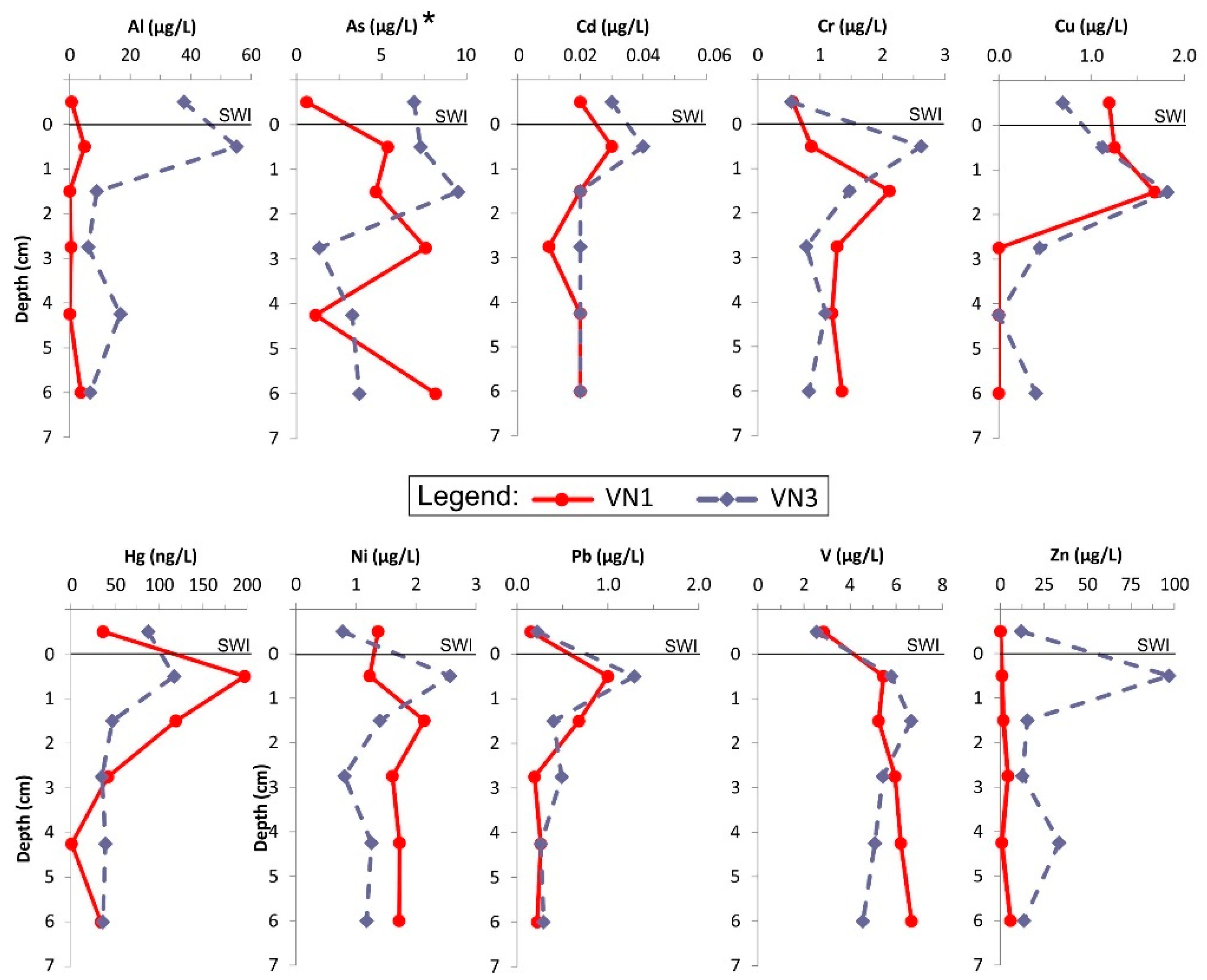
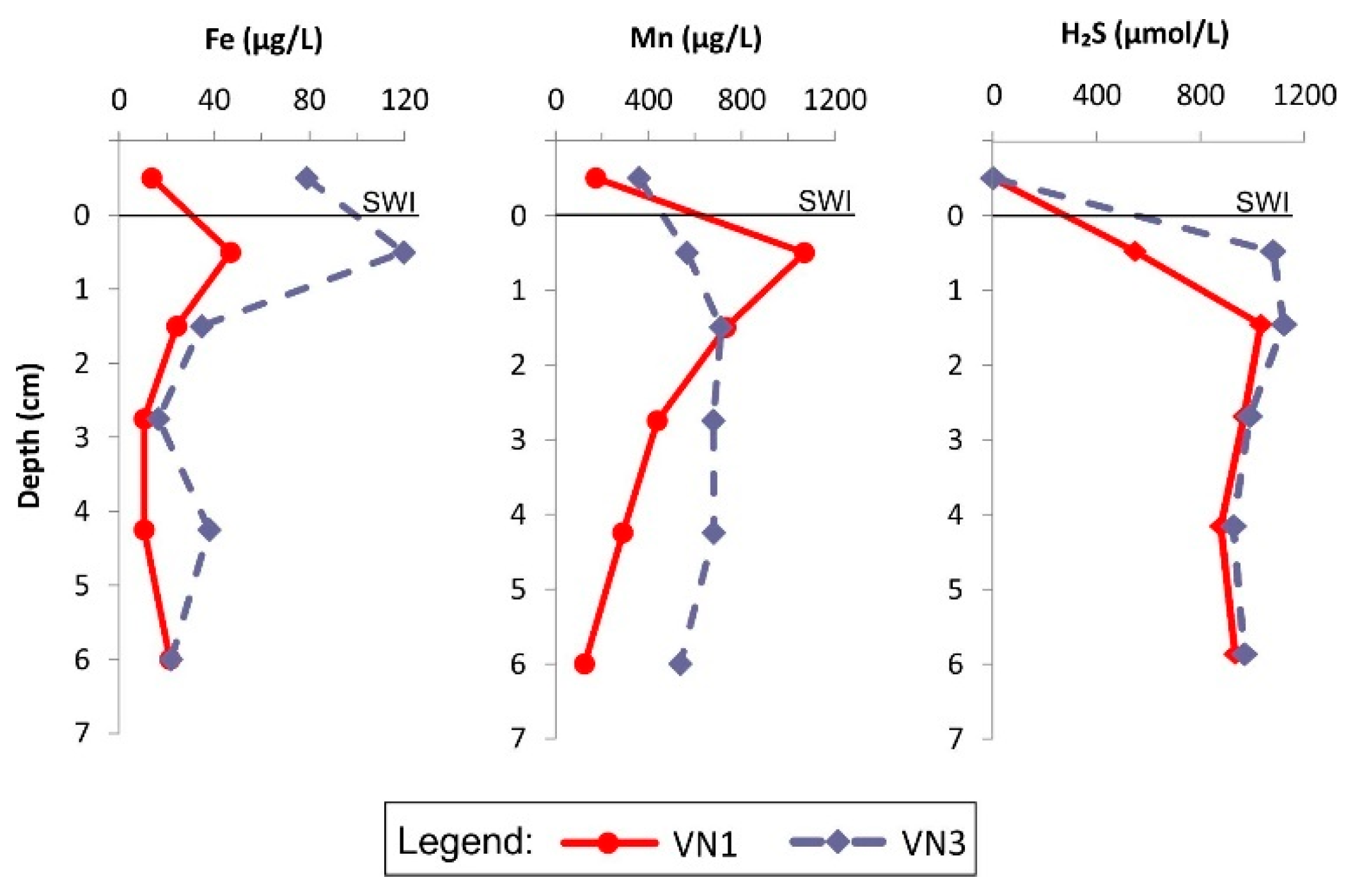
3.2.1. Metal(loid) Concentration Profiles in Porewaters and Distribution Coefficient (KD)
3.2.2. Metal(loid)s in the Benthic Chamber
3.2.3. Diffusive and Benthic Fluxes at the SWI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, R.P. Trace Metals in Aquatic Systems; Wiley-Blackwell: Oxford, UK, 2013; ISBN 978-1-4051-6048-3. [Google Scholar]
- Bastami, K.D.; Neyestani, M.R.; Molamohyedin, N.; Shafeian, E.; Haghparast, S.; Shirzadi, I.A.; Baniamam, M. Bioavailability, Mobility, and Origination of Metals in Sediments from Anzali Wetland, Caspian Sea. Mar. Pollut. Bull. 2018, 136, 22–32. [Google Scholar] [CrossRef]
- Roberts, D.A. Causes and Ecological Effects of Resuspended Contaminated Sediments (RCS) in Marine Environments. Environ. Int. 2012, 40, 230–243. [Google Scholar] [CrossRef]
- Kouassi, N.L.B.; Yao, K.M.; Sangare, N.; Trokourey, A.; Metongo, B.S. The Mobility of the Trace Metals Copper, Zinc, Lead, Cobalt, and Nickel in Tropical Estuarine Sediments, Ebrie Lagoon, Côte d’Ivoire. J. Soils Sediments 2019, 19, 929–944. [Google Scholar] [CrossRef]
- Schroeder, H.; Fabricius, A.-L.; Ecker, D.; Ternes, T.A.; Duester, L. Impact of Mechanical Disturbance and Acidification on the Metal(Loid) and C, P, S Mobility at the Sediment Water Interface Examined Using a Fractionation Meso Profiling ICP-QQQ-MS Approach. Sci. Total Environ. 2019, 651, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Canuto, F.A.B.; Garcia, C.A.B.; Alves, J.P.H.; Passos, E.A. Mobility and Ecological Risk Assessment of Trace Metals in Polluted Estuarine Sediments Using a Sequential Extraction Scheme. Environ. Monit. Assess. 2013, 185, 6173–6185. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.-W. Bioaccumulation of Heavy Metals Both in Wild and Mariculture Food Chains in Daya Bay, South China. Estuar. Coast. Shelf Sci. 2015, 163, 7–14. [Google Scholar] [CrossRef]
- Acquavita, A.; Covelli, S.; Emili, A.; Berto, D.; Faganeli, J.; Giani, M.; Horvat, M.; Koron, N.; Rampazzo, F. Mercury in the sediments of the Marano and Grado lagoon (northern Adriatic Sea): Sources, distribution and speciation. Estuar. Coast. Shelf Sci. 2012, 13, 20–31. [Google Scholar] [CrossRef]
- Brambati, A. Metalli Pesanti Nelle Lagune di Marano e Grado. Piano di Studi Finalizzato All’accertamento Della Presenza di Eventuali Sostanze Persistenti Nelle Lagune di Grado e Marano e al Loro Risanamento; Regione Autonoma Friuli-Venezia Giulia: Trieste, Italy, 1997. [Google Scholar]
- Piani, R.; Covelli, S. Contributo antropico di metalli pesanti e 137Cs nei sedimenti del bacino di Buso (Laguna di Marano e Grado, Italia settentrionale). Studi Trentini Sci. Nat. Acta Geol. 2001, 77, 169–177. [Google Scholar]
- Ramieri, E.; Barbanti, A.; Picone, M.; Menchini, G.; Bressan, E.; Forno, E.D. Integrated Plan for the Sustainable Management of the Lagoon of Marano and Grado. Littoral 2011, 11. [Google Scholar] [CrossRef]
- Petranich, E.; Covelli, S.; Acquavita, A.; Faganeli, J.; Horvat, M.; Contin, M. Evaluation of Mercury Biogeochemical Cycling at the Sediment–Water Interface in Anthropogenically Modified Lagoon Environments. J. Environ. Sci. 2018, 68, 5–23. [Google Scholar] [CrossRef]
- Petranich, E.; Covelli, S.; Acquavita, A.; De Vittor, C.; Faganeli, J.; Contin, M. Benthic Nutrient Cycling at the Sediment-Water Interface in a Lagoon Fish Farming System (Northern Adriatic Sea, Italy). Sci. Total Environ. 2018, 644, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Covelli, S.; Faganeli, J.; De Vittor, C.; Predonzani, S.; Acquavita, A.; Horvat, M. Benthic Fluxes of Mercury Species in a Lagoon Environment (Grado Lagoon, Northern Adriatic Sea, Italy). Appl. Geochem. 2008, 23, 529–546. [Google Scholar] [CrossRef]
- Acquavita, A.; Aleffi, I.F.; Benci, C.; Bettoso, N.; Crevatin, E.; Milani, L.; Tamberlich, F.; Toniatti, L.; Barbieri, P.; Licen, S.; et al. Annual Characterization of the Nutrients and Trophic State in a Mediterranean Coastal Lagoon: The Marano and Grado Lagoon (Northern Adriatic Sea). Reg. Stud. Mar. Sci. 2015, 2, 132–144. [Google Scholar] [CrossRef]
- Emili, A.; Acquavita, A.; Koron, N.; Covelli, S.; Faganeli, J.; Horvat, M.; Žižek, S.; Fajon, V. Benthic Flux Measurements of Hg Species in a Northern Adriatic Lagoon Environment (Marano and Grado Lagoon, Italy). Estuar. Coast. Shelf Sci. 2012, 113, 71–84. [Google Scholar] [CrossRef]
- De Vittor, C.; Faganeli, J.; Emili, A.; Covelli, S.; Predonzani, S.; Acquavita, A. Benthic Fluxes of Oxygen, Carbon and Nutrients in the Marano and Grado Lagoon (Northern Adriatic Sea, Italy). Estuar. Coast. Shelf Sci. 2012, 113, 57–70. [Google Scholar] [CrossRef]
- Hedges, J.L.; Stern, J.H. Carbon and Nitrogen Determinations of Carbonate Containing Solids. Limnol. Oceanogr. 1984, 29, 657–663. [Google Scholar] [CrossRef]
- EPA. Method 3052 Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; EPA: Washington, DC, USA, 1996.
- EPA. Method 7473 (SW-846) Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry; EPA: Washington, DC, USA, 1998.
- Adami, G.; Barbieri, P.; Reisenhofer, E. A comparison on five sediment decomposition procedures for determining anthropogenic trace metal pollution. Int. J. Environ. Anal. Chem. 1999, 75, 251–260. [Google Scholar] [CrossRef]
- EPA. Method 1631, Revison e. Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry; EPA: Washington, DC, USA, 2002.
- Emili, A.; Acquavita, A.; Covelli, S.; Spada, L.; Di Leo, A.; Giandomenico, S.; Cardellicchio, N. Mobility of Heavy Metals from Polluted Sediments of a Semi-Enclosed Basin: In Situ Benthic Chamber Experiments in Taranto’s Mar Piccolo (Ionian Sea, Southern Italy). Environ. Sci. Pollut. Res. 2016, 23, 12582–12595. [Google Scholar] [CrossRef] [PubMed]
- Petranich, E.; Croce, S.; Crosera, M.; Pavoni, E.; Faganeli, J.; Adami, G.; Covelli, S. Mobility of Metal(loid)s at the Sediment-Water Interface in Two Tourist Port Areas of the Gulf of Trieste (Northern Adriatic Sea). Environ. Sci. Pollut. Res. 2018, 25, 26887–26902. [Google Scholar] [CrossRef]
- Boudreau, B.P. Metals and Models: Diagenetic Modelling in Freshwater Lacustrine Sediments. Met. Models Diagenetic Model. Freshw. Lacustrine Sediments 1999, 22, 227–251. [Google Scholar]
- Li, Y.H.; Gregory, S. Diffusion of Ions in Sea Water and in Deep-Sea Sediments. Geochim. Cosmochima Acta 1974, 38, 703–714. [Google Scholar]
- Gobeil, C.; Cossa, D. Mercury in Sediments and Sediment Pore Water in the Laurentian Trough. Can. J. Fish. Aquat. 1993, 50, 1794–1800. [Google Scholar] [CrossRef]
- Zago, C.; Capodaglio, G.; Ceradini, S.; Ciceri, G.; Abelmoschi, L.; Soggia, F.; Cescon, P.; Scarponi, G. Benthic Fluxes of Cadmium, Lead, Copper and Nitrogen Species in the Northern Adriatic Sea in Front of the River Po Outflow, Italy. Sci. Total Environ. 2000, 246, 121–137. [Google Scholar] [CrossRef]
- Oliveri, P.; Malegori, C.; Casale, M. Chemometrics: Multivariate Analysis of Chemical Data. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128132661. [Google Scholar]
- Oliveri, P.; Malegori, C.; Simonetti, R.; Casale, M. The impact of signal pre-processing on the final interpretation of analytical outcomes–A tutorial. Anal. Chim. Acta 2019, 1058, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://gruppochemiometria.it/index.php/software (accessed on 3 September 2019).
- Shepard, F.P. Nomenclature Based on Sand–Silt–Clay Ratios. J. Sediment. Petrol. 1954, 24, 151–158. [Google Scholar]
- Goñi, M.A.; Teixeira, M.J.; Perkey, D.W. Sources and Distribution of Organic Matter in a River-Dominated Estuary (Winyah Bay, SC, USA). Estuar. Coast. Shelf Sci. 2003, 57, 1023–1048. [Google Scholar] [CrossRef]
- Ogrinc, N.; Fontolan, G.; Faganeli, J.; Covelli, S. Carbon and Nitrogen Isotope Compositions of Organic Matter in Coastal Marine Sediments (the Gulf of Trieste, N Adriatic Sea): Indicators of Sources and Preservation. Mar. Chem. 2005, 95, 163–181. [Google Scholar] [CrossRef]
- Covelli, S.; Fontolan, G.; Faganeli, J.; Ogrinc, N. Anthropogenic Markers in the Holocene Stratigraphic Sequence of the Gulf of Trieste (Northern Adriatic Sea). Mar. Geol. 2006, 230, 29–51. [Google Scholar] [CrossRef]
- Covelli, S.; Langone, L.; Acquavita, A.; Piani, R.; Emili, A. Historical Flux of Mercury Associated with Mining and Industrial Sources in the Marano and Grado Lagoon (Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 2012, 113, 7–19. [Google Scholar] [CrossRef]
- Sutherland, R.A. Bed Sediment-Associated Trace Metals in an Urban Stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury Contamination of Coastal Sediments as the Result of Long-Term Cinnabar Mining Activity (Gulf of Trieste, Northern Adriatic Sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Covelli, S.; Piani, R.; Acquavita, A.; Predonzani, S.; Faganeli, J. Transport and Dispersion of Particulate Hg Associated with a River Plume in Coastal Northern Adriatic Environments. Mar. Pollut. Bull. 2007, 55, 436–450. [Google Scholar] [CrossRef]
- Bastami, K.D.; Neyestani, M.R.; Esmaeilzadeh, M.; Haghparast, S.; Alavi, C.; Fathi, S.; Nourbakhsh, S.; Shirzadi, E.A.; Parhizgar, R. Geochemical Speciation, Bioavailability and Source Identification of Selected Metals in Surface Sediments of the Southern Caspian Sea. Mar. Pollut. Bull. 2017, 114, 1014–1023. [Google Scholar] [CrossRef]
- Warnken, K.W.; Gill, G.A.; Griffin, L.L.; Santschi, P.H. Sediment-Water Exchange of Mn, Fe, Ni and Zn in Galveston Bay, Texas. Mar. Chem. 2001, 73, 215–231. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Morse, J.W. Pyritization of Trace Metals in Anoxic Marine Sediments. Geochim. Cosmochim. Acta 1992, 56, 2681–2702. [Google Scholar] [CrossRef]
- Rigaud, S.; Radakovitch, O.; Couture, R.-M.; Deflandre, B.; Cossa, D.; Garnier, C.; Garnier, J.-M. Mobility and Fluxes of Trace Elements and Nutrients at the Sediment–Water Interface of a Lagoon under Contrasting Water Column Oxygenation Conditions. Appl. Geochem. 2013, 31, 35–51. [Google Scholar] [CrossRef]
- Mehta, N.; Kocar, B.D. Geochemical conditions conductive for retention of trace elements and radionuclides during shale-fluid interactions. Environ. Sci. Proc. Imp. 2019, 21, 1764–1776. [Google Scholar]
- Froelich, P.N.; Klinkhammer, G.P.; Bender, M.L.; Luedtke, N.A.; Heath, G.R.; Cullen, D.; Dauphin, P.; Hammond, D.; Hartman, B.; Maynard, V. Early Oxidation of Organic Matter in Pelagic Sediments of the Eastern Equatorial Atlantic: Suboxic Diagenesis. Geochim. Cosmochim. Acta 1979, 43, 1075–1090. [Google Scholar] [CrossRef]
- Lee, P.-K.; Baillif, P.; Touray, J.-C. Geochemical Behaviour and Relative Mobility of Metals (Mn, Cd, Zn and Pb) in Recent Sediments of a Retention Pond along the A-71 Motorway in Sologne, France. Environ. Geol. 1997, 32, 142–152. [Google Scholar] [CrossRef]
- Hammerschmidt, C.R.; Fitzgerald, W.F. Geochemical Controls on the Production and Distribution of Methylmercury in Near-Shore Marine Sediments. Environ. Sci. Technol. 2004, 38, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Gavriil, A.M.; Angelidis, M.O. Metal Diagenesis in a Shallow Semi-Enclosed Marine System in the Aegean Sea, Greece. Estuar. Coast. Shelf Sci. 2006, 70, 487–498. [Google Scholar] [CrossRef]
- De Vittor, C.; Relitti, F.; Kralj, M.; Covelli, S.; Emili, A. Oxygen, Carbon, and Nutrient Exchanges at the Sediment–Water Interface in the Mar Piccolo of Taranto (Ionian Sea, Southern Italy). Environ. Sci. Pollut. Res. 2016, 23, 12566–12581. [Google Scholar] [CrossRef] [PubMed]
- Bettoso, N. Macrozoobenthos Monitoring in Relation to Dredged Sediment Disposal: The Case of the Marano and Grado Lagoon (Northern Adriatic Sea, Italy). Reg. Stud. Mar. Sci. 2020, 33, 100916. [Google Scholar] [CrossRef]
- Point, D.; Monperrus, M.; Tessier, E.; Amouroux, D.; Chauvaud, L.; Thouzeau, G.; Jean, F.; Amice, E.; Grall, J.; Leynaert, A.; et al. Biological Control of Trace Metal and Organometal Benthic Fluxes in a Eutrophic Lagoon (Thau Lagoon, Mediterranean Sea, France). Estuar. Coast. Shelf Sci. 2007, 72, 457–471. [Google Scholar] [CrossRef]
- Ehrlich, H.L.; Newman, D.K.; Kappler, A. Ehrlich’s Geomicrobiology; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-0-367-65872-4. [Google Scholar]
- Westerlund, S.F.G.; Anderson, L.G.; Hall, P.O.J.; Iverfeldt, Å.; Van Der Loeff, M.M.R.; Sundby, B. Benthic Fluxes of Cadmium, Copper, Nickel, Zinc and Lead in the Coastal Environment. Geochim. Cosmochim. Acta 1986, 50, 1289–1296. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Porewater Distribution and Benthic Flux Measurements of Mercury and Methylmercury in the Gulf of Trieste (Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 1999, 48, 415–428. [Google Scholar] [CrossRef]
- Muresan, B.; Cossa, D.; Jézéquel, D.; Prévot, F.; Kerbellec, S. The Biogeochemistry of Mercury at the Sediment–Water Interface in the Thau Lagoon. 1. Partition and Speciation. Estuar. Coast. Shelf Sci. 2007, 72, 472–484. [Google Scholar] [CrossRef]



| Site | Level | Al | Fe | As | Cd | Cr | Cu | Hg | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | % | % | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | |
| VN1 | 0–1 | 7.03 | 5.71 | 7.62 | 1.64 | 84.8 | 25.9 | 5.10 | 404 | 55.7 | 32.6 | 78.3 | 85.0 |
| 1–2 | 8.88 | 6.78 | 7.73 | 1.94 | 83.7 | 27.4 | 4.19 | 359 | 57.0 | 37.4 | 81.1 | 87.0 | |
| 2–3.5 | 7.26 | 6.19 | 6.44 | 1.89 | 78.8 | 23.6 | 3.62 | 340 | 55.2 | 36.8 | 77.7 | 100 | |
| 3.5–5 | 8.74 | 6.93 | 5.93 | 2.13 | 90.3 | 30.7 | 3.20 | 313 | 64.9 | 41.2 | 93.4 | 121 | |
| 5–7 | 6.76 | 4.99 | 6.47 | 1.90 | 74.0 | 19.0 | 2.15 | 396 | 47.6 | 32.9 | 67.2 | 72.2 | |
| VN3 | 0–1 | 5.34 | 7.18 | 8.29 | 2.22 | 86.5 | 32.8 | 4.20 | 366 | 60.2 | 43.2 | 87.1 | 95.4 |
| 1–2 | 6.18 | 6.55 | 11.2 | 2.29 | 85.3 | 29.9 | 5.11 | 444 | 58.4 | 43.1 | 86.3 | 93.4 | |
| 2–3.5 | 6.86 | 6.82 | 12.3 | 2.26 | 83.1 | 25.4 | 6.87 | 497 | 54.8 | 40.9 | 80.0 | 85.9 | |
| 3.5–5 | 7.86 | 5.97 | 11.5 | 2.39 | 85.7 | 24.3 | 5.98 | 512 | 54.2 | 39.8 | 78.5 | 80.7 | |
| 5–7 | 6.87 | 6.09 | 10.7 | 2.00 | 84.8 | 24.6 | 5.23 | 529 | 57.4 | 37.1 | 83.5 | 86.4 |
| Site | Level (cm) | Al | As | Cd | Cr | Cu | Fe | Hg | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Labile Fraction (%) | |||||||||||||
| VN1 | 0–1 | 0.09 | 1.59 | 9.96 | u. f. | u. f. | 0.01 | u. f. | 35.2 | 2.95 | 0.18 | 0.05 | 9.30 |
| 1–2 | 0.09 | 0.89 | 8.86 | u. f. | u. f. | 0.00 | u. f. | 34.1 | 3.69 | u. f. | 0.03 | 12.2 | |
| 2–3.5 | < LOD | 0.85 | 9.30 | u. f. | u. f. | 0.04 | u. f. | 36.1 | 3.71 | 0.04 | 0.03 | 11.9 | |
| 3.5–5 | 0.02 | 1.33 | 8.63 | u. f. | u. f. | 0.11 | u. f. | 33.2 | 4.27 | 0.12 | 0.03 | 10.6 | |
| 5–7 | 0.03 | 1.42 | 6.77 | u. f. | u. f. | 0.01 | u. f. | 37.9 | 2.32 | u. f. | 0.19 | 4.55 | |
| VN3 | 0–1 | 0.08 | 1.30 | 7.91 | u. f. | u. f. | 0.37 | u. f. | 28.2 | 3.86 | 0.28 | 0.03 | 13.6 |
| 1–2 | 0.06 | 0.63 | 7.63 | u. f. | u. f. | 0.11 | u. f. | 28.4 | 3.17 | 0.06 | 0.02 | 12.2 | |
| 2–3.5 | 0.10 | 0.51 | 4.45 | u. f. | u. f. | 0.00 | u. f. | 25.4 | 0.34 | u. f. | 0.22 | 0.34 | |
| 3.5–5 | 0.08 | 0.85 | 4.62 | 19.9 | u. f. | 0.08 | u. f. | 29.6 | 14.4 | 0.04 | 0.88 | 1.44 | |
| 5–7 | 0.11 | 0.80 | 4.62 | 0.07 | u. f. | 0.00 | u. f. | 30.1 | 0.43 | u. f. | 1.23 | 0.37 | |
| Site | Diffusive Fluxes (µg/m2 d) | (mmol/m2 d) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | As | Cd | Cr | Cu | Hg | Fe | Mn | Ni | Pb | V | Zn | H2S | |
| VN1 (this study) | 13.3 | 240 | 0.04 | 0.99 | 0.24 | 0.44 | 132 | 3415 | −0.53 | 4.44 | 8.62 | −23.7 | 28.6 [12] |
| VN3 (this study) | 55.1 | 2.06 | 0.04 | 7.01 | 1.79 | 0.08 | 167 | 803 | 6.90 | 5.74 | 11.0 | 345 | 55.5 [12] |
| SL, Lucija (SLO) [23] | n.d. | 21.0 | 0.02 | 5.47 | 8.05 | 932 | 0.03 | 499 | 6.36 | −52.2 | n.d. | −3.36 | 0.006 |
| SR-1, San Rocco (ITA) [23] | n.d. | 50.4 | 0.00 | 0.21 | −17.7 | 283 | 1.47 | 40.9 | −8.27 | −62.7 | n.d. | −90.7 | n.d. |
| SR-2, San Rocco (ITA) [23] | n.d. | 11.7 | −0.03 | 0.56 | −157 | 92.6 | 0.97 | 6980 | 1.11 | 4.75 | n.d. | 37.2 | 0.001 |
| 1 E, Taranto (ITA) [22] | n.d. | 0.99 | 0.00 | 0.01 | 0.14 | 1.50 | 0.03 | 78.0 | 0.02 | −0.13 | −0.61 | −2.87 | −0.0002 |
| 1 I, Taranto (ITA) [22] | n.d. | 1.31 | 0.03 | 0.02 | −0.08 | 12.1 | 0.00 | 47.9 | 0.04 | 0.20 | −0.92 | −0.38 | 0.0021 |
| Daily Benthic Fluxes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/m2 d | µmol/m2 d | ||||||||||||
| Site | Al | As | Cd | Cr | Cu | Fe | Hg | Mn | Ni | Pb | V | Zn | H2S |
| VN1 (this study) | 784 | 101 | 3.15 | −44 | −60 | 1638 | −1.00 | −1.033 | 139 | 22 | 161 | 954 | 0.00 |
| VN3 (this study) | n.d. | 602 | 0.00 | −6.30 | 126 | 63 | 0.60 | 9009 | 50 | 60 | 50 | 154 | 11,987 |
| SL [24] | n.d. | 5040 | −9.45 | −1654 | 340,200 | −882 | −0.07 | 658 | 1260 | 95 | 315 | 1134 | −135 |
| SR1 [24] | n.d. | 5355 | 9.45 | 630 | −4095 | −1166 | −3.92 | 1093 | 504 | 4199 | 5670 | 3339 | −132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petranich, E.; Crosera, M.; Pavoni, E.; Faganeli, J.; Covelli, S. Behaviour of Metal(loid)s at the Sediment-Water Interface in an Aquaculture Lagoon Environment (Grado Lagoon, Northern Adriatic Sea, Italy). Appl. Sci. 2021, 11, 2350. https://doi.org/10.3390/app11052350
Petranich E, Crosera M, Pavoni E, Faganeli J, Covelli S. Behaviour of Metal(loid)s at the Sediment-Water Interface in an Aquaculture Lagoon Environment (Grado Lagoon, Northern Adriatic Sea, Italy). Applied Sciences. 2021; 11(5):2350. https://doi.org/10.3390/app11052350
Chicago/Turabian StylePetranich, Elisa, Matteo Crosera, Elena Pavoni, Jadran Faganeli, and Stefano Covelli. 2021. "Behaviour of Metal(loid)s at the Sediment-Water Interface in an Aquaculture Lagoon Environment (Grado Lagoon, Northern Adriatic Sea, Italy)" Applied Sciences 11, no. 5: 2350. https://doi.org/10.3390/app11052350
APA StylePetranich, E., Crosera, M., Pavoni, E., Faganeli, J., & Covelli, S. (2021). Behaviour of Metal(loid)s at the Sediment-Water Interface in an Aquaculture Lagoon Environment (Grado Lagoon, Northern Adriatic Sea, Italy). Applied Sciences, 11(5), 2350. https://doi.org/10.3390/app11052350







