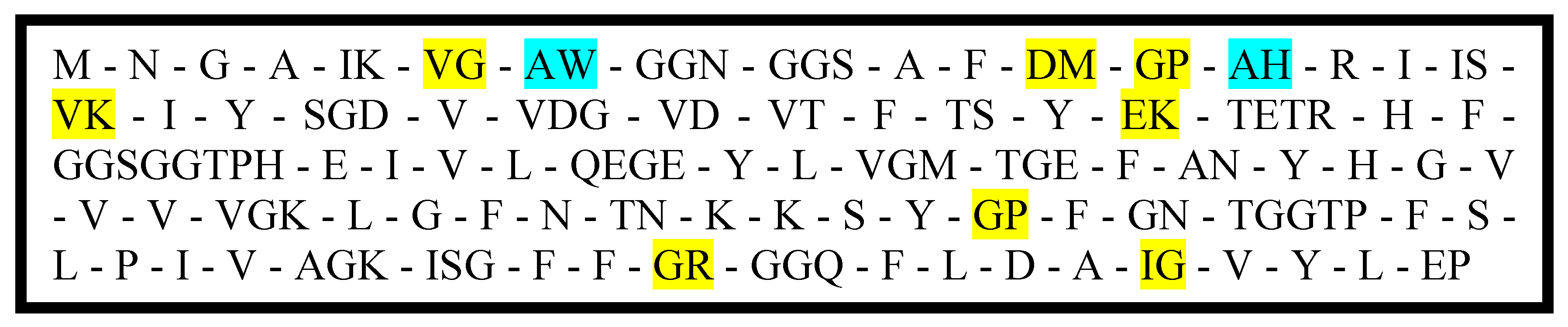1. Introduction
Banana is an important food crop worldwide, together with rice, wheat, and corn [
1]. It contains high amounts of vitamin B
6, carbohydrates and potassium along with moderate amounts of vitamin C, manganese, and dietary fiber [
2]. Given the high consumption of the fruit worldwide, various studies associated to banana consumption have been initiated to prove its health benefits, such as its potent antioxidant, anti-diabetic, hypocholesterolemic and antihypertensive activities. Most of these are found to be associated in its phenolic compounds, carotenoids, flavonoids, biogenic amines, and phytosterols while only few being protein related [
3].
Protein studies require samples with high protein content [
4]. Despite the high availability of bananas [
1], its low protein content (approx. 1% of the fruit pulp) and the presence of interfering compounds in higher amounts (e.g., 20% carbohydrates) makes it a difficult subject for protein studies, thereby, limiting the nature and number of available studies that can be cited up to date [
5]. Moreover, most of these studies focus on optimization and comparison of different protein extraction procedures rather than the identification of isolated proteins.
Extracted crude proteins can be subjected to purification, characterization, and other protein studies in which bioactive peptide researches are included. Bioactive peptides are contained in their precursor proteins and can be released through proteolytic processes. Once released, they can act as potential metabolic regulatory compounds with hormone-like activity. Activities related to bioactive peptides include antimicrobial, blood pressure lowering, antioxidant, and atherosclerotic activities [
6,
7,
8].
Antihypertensive activity is one of the most studied activities associated with bioactive peptides. According to World Health Organization [
9], hypertension causes 7 M deaths yearly and affects 1.5 billion people around the world that suffering from its complications. In the Philippines, 21% of Filipino adults are hypertensive which is expected to increase by the coming years [
10]. Studies on selected commercially available antihypertensive drugs like enalapril showed that they have negative side effects such as kidney damage, cough, diarrhea, and skin rashes [
11]. Angiotensin-converting enzyme (EC 3.4.15.1) (ACE) catalyzes conversion of angiotensin I to angiotensin II, a vasoconstrictor, making it a target of inhibition of antihypertensive compounds. Since commercially available drugs exhibit negative side effects, there are now studies pursuing antihypertensive compounds obtained from natural resources that will not exhibit negative side effects.
Another health benefit associated with bioactive peptides is their antioxidant activity. Oxidative stress refers to an imbalance between the free radical production and the body’s ability to combat its harmful effects [
12]. Free radicals and reactive oxygen species (ROS) generated by different metabolic processes and environmental stress can damage biomolecules and modify their functions leading to cellular dysfunction and cell death. These are manifested in the form of health problems such as cancer and accelerated cell aging [
13]. Human tissues are protected against these reactive compounds by endogenous and exogenous antioxidants from a natural or synthetic origin [
14]. One mechanism in which these delay or inhibit oxidation processes is through interfering with the nonbeneficial chain reactions caused by free radicals and ROS. Studies on natural antioxidants showed that their activity are comparable to the activity of the synthetic ones with the advantage of being cheap and readily available in the diet [
15]. Natural antioxidants were also being studied for their prophylactic and therapeutic properties which can serve as a possible countermeasure for radiation, combating cancer and other age-related diseases [
16].
Given that there are only few studies which can be cited on banana proteomics and bioactive peptides, this study aims to provide preliminary data on ‘Señorita’ banana protein extraction, purification, and characterization methods, which may serve as a foundation for further banana protein studies. Second, it aims to determine the antihypertensive and antioxidant activities of its protein extracts to prove health beneficial effects associated to banana consumption.
2. Materials and Methods
2.1. Sample Preparation and Protein Extraction
The ‘Señorita’ banana sample obtained from the local market was peeled and the pulp was chopped into tiny pieces, flash frozen in liquid nitrogen, and powdered using mortar and pestle. The crude proteins from the powdered pulp were extracted according to procedure by Esteve et al. [
17] with few modifications. Forty grams of the powdered pulp was mixed with 80.00 mL of cold extraction buffer (0.125 M tris-HCl [pH 7.4] with 50 mM NaCl) and was stirred overnight, with the container submerged in an ice bath. The resulting mixture was centrifuged in a refrigerated centrifuge (Z 326K centrifuge, Hermle, Wehingen, Germany) for 30 min at 4 °C, 56,448×
g. Upon separation of the layers, the supernatant (fraction containing the crude protein extract) was collected and stored in the freezer with the temperature at approximately −20 °C while the residue was discarded.
2.2. Purification of the Protein Isolate
2.2.1. Ammonium Sulfate Precipitation/Fractionation
The percent ammonium sulfate saturation of the solution in which most of the proteins will precipitate was determined first. Percent saturations used were 0–20%, 20–40%, 40–60%, 60–80% and 80–95%. Then, the protein extract was saturated up to the optimized percent saturation, 40% to 60%, was gently stirred for 30 min in an ice bath, and was centrifuged at 10,000× g rpm for 30 min at 4 °C. After centrifugation, the precipitate was collected while the supernatant was discarded.
2.2.2. Dialysis
The precipitate obtained from ammonium sulfate precipitation was dissolved in minimum amount of extraction buffer and placed in a dialyzing bag with a molecular weight cut-off of 10 kDa. The dialysis was done for 12 h against distilled water with stirring in a beaker submerged in an ice bath. The distilled water used was changed every 3 h.
2.2.3. Gel-Filtration Chromatography
A column containing Sephacryl® S-100 as resin was pre-equilibrated and eluted with extraction prior to loading of the dialyzed samples. The samples were eluted at a flow rate of 2.00 mL/min. The resulting sixty 1.5 mL fractions were collected and the absorbance at 280 nm of each fractions were determined.
2.3. Protein Quantification Using Colorimetric Bradford Assay
The protein content of the protein extracts was calculated from a standard protein curve of bovine serum albumin (BSA) using a colorimetric Bradford assay [
18]. Five μL of sample protein extract was added to 250 μL of 1× Bradford reagent in every well of a 300 μL capacity microtiter plate. The resulting solutions were incubated for 5 min with interval shaking in a UV-Vis microtiter plate holder, before the absorbance was determined at 595 nm.
2.4. Enzymatic Protein Digestion
The crude, partially purified and purified protein extracts were hydrolyzed while being submerged in a water bath with shaker at 37 °C using two sets of freshly prepared enzymes: Set A consists of pepsin while set B consists of trypsin, chymotrypsin and thermolysin. For the first part of protein digestion, pepsin was added to the sample and the digestion was allowed to proceed for two hours at pH 2.0. Then, Set B of enzymes were added, and the digestion was allowed to proceed for additional 1, 2, 10 and 22 h at pH 7.0. At every specified time, a 2.0 mL aliquot was obtained from the extracts followed by boiling for 5 min in a boiling water bath to terminate the digestion process. After boiling, the aliquot was stored to freezer prior to activity testing.
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed using the method developed by Laemmli [
19]. The run was carried out in a 15% discontinuous denaturing stacking and resolving gels using BIORAD tetracell electrophoresis apparatus. The electrophoretic run was completed for 60 min at 110 V. The gel was stained using staining solution 0.10% (
w/
v) Coomassie Brilliant Blue R-250, 50% (
v/
v) methanol, 10% (
v/
v) acetic acid) for 1 h with shaking and was destained using destaining solution (50% (
v/
v) methanol, 10% (
v/
v) acetic acid). The molecular weights of the subunits were estimated using a Benchmark Protein Ladder with a molecular weight (MW) range of 10–250 kDa.
2.6. Densitometric Analysis
Molecular weights of the proteins present in the crude, partially purified, purified extracts, and enzyme digests were estimated from the SDS-PAGE profiles using the TotalLab software. The same software was used to determine the extent of protein hydrolysis in the digested protein extracts. Peak areas and band volume of the protein bands were also determined.
2.7. In Silico Analysis of the ‘Señorita’ Banana Major Protein
The amino acid sequence of the putative major protein lectin (E9NX13) extracted from ‘Señorita’ banana was obtained from
http://www.uniprot.org/ (retrieval date: February 2019) [
20]. The peptides released when the major protein was subjected to simulated digestion with Pepsin (EC 3.4.23.1), chymotrypsin (EC 3.4.21.1), Trypsin (EC 3.4.21.4) and thermolysin (EC 3.4.24.27) was determined using the protein sequence analyzer in
http://www.uwm.edu.pl/biochemia/index.php/en/biopep (retrieval date: February 2019) [
21]. The same site was used for the determination of the bioactive peptides exhibiting antioxidant and antihypertensive activities.
2.8. Determination of the Percent (%) Angiotensin Converting Enzyme (ACE) Inhibition
The ACE activity is determined using the method described by Cushman et al. [
22] with few modifications. Initially, 100 μL of HHL buffer (5 mM hippuryl-L-histidine-L-leucine [HHL] in 0.1 M phosphate buffer [pH 8.2] with 0.3 M NaCl) was placed in different 2.0 mL Eppendorf tubes. For the sample replicates (undigested and digested crude, partially purified, and purified protein extracts), 25 μL of the sample was added to the HHL buffer in the Eppendorf tubes. For the method blank, 25 μL of enzyme blank (mixture containing only the enzymes used for digestion) was added. For the positive control, 25 μL of 50 μg/mL captopril solution was added. For the main control (full reaction control where ACE was not inhibited), only 25 μL of the extraction buffer was added. The resulting mixtures were incubated in a water bath at 37 °C for 4 min with occasional stirring in an incubation chamber. Then, 25 μL of purified ACE was added to the sample tubes except for the method blank. For the method blank, 125 μL of 1.0 M HCl was added. The resulting mixtures were incubated again in a water bath at 37 °C for 30 min. After incubation, 125 μL of 1.0 M HCl was added to the mixtures (sample replicates, positive control and main control) to terminate the reaction, while for the method blank, 25 μL of ACE was added. Afterwards, 750 μL of ethyl acetate was added to all the sample tubes and the mixture was stirred using vortex mixer. The resulting mixtures were allowed to stand under room temperature condition until there was a visible separation of layers. The top layer of the mixture was obtained and placed in another set of empty Eppendorf tubes and was evaporated to dryness in a steam bath until there were no more traces of ethyl acetate detected. These replicates were then reconstituted with 500 μL of 1.0 M NaCl and the absorbance of the resulting solution was read at 228 nm using a UV-Vis spectrophotometer. The percent (%) ACE inhibition of the samples was calculated using the formula:
where
C is the absorbance of the main control,
A is the absorbance of the sample or the positive control, and
B is the absorbance of the method blank.
2.9. Determination of the Radical Scavenging Activity by 2.2-Diphenyl-1-picrylhydrazil (DPPH) Assay
A 2.2-diphenyl-1-picrylhydrazyl (DPPH) assay was carried out according to the method of Pownall et al. [
23] with few modifications. For the sample replicates, 150 μL of sample (undigested and digested crude, partially purified and purified protein extract) was placed in each well of 300 μL capacity microtiter plate, followed by the addition of 50 μL of 50 μg/mL DPPH in methanol. For the positive control, 150 μL of 50 μg/mL ascorbic acid solution in extraction buffer was added instead of samples, while for the main control, 150 μL methanol was added. The resulting replicate mixtures were incubated for 30 min in the dark. After incubation, the absorbance of the replicates was read at 517 nm using a UV-Vis spectrophotometer. The percent (%) scavenging activity of the samples was calculated using the formula:
where
Acontrol corresponds to the absorbance of the main control while
Asample corresponds to the absorbance of the sample.
2.10. Statistical Analysis
The activities were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). The activities of the samples were compared to the activity of the corresponding standard used for each assay using one-way ANOVA and Tukey’s multiple comparisons test to determine whether these activities are significantly different with respect to the activity of the standard.
3. Results and Discussion
3.1. Extraction of Proteins from ‘Señorita’ Banana Pulp
This study extracted proteins from ripe ‘Señorita’ banana pulp to determine protein related health beneficial effects associated to consumption of the fruit, although other plant parts such as root [
24] and peel [
25] are also known to contain extractable proteins. The SDS-PAGE profile (
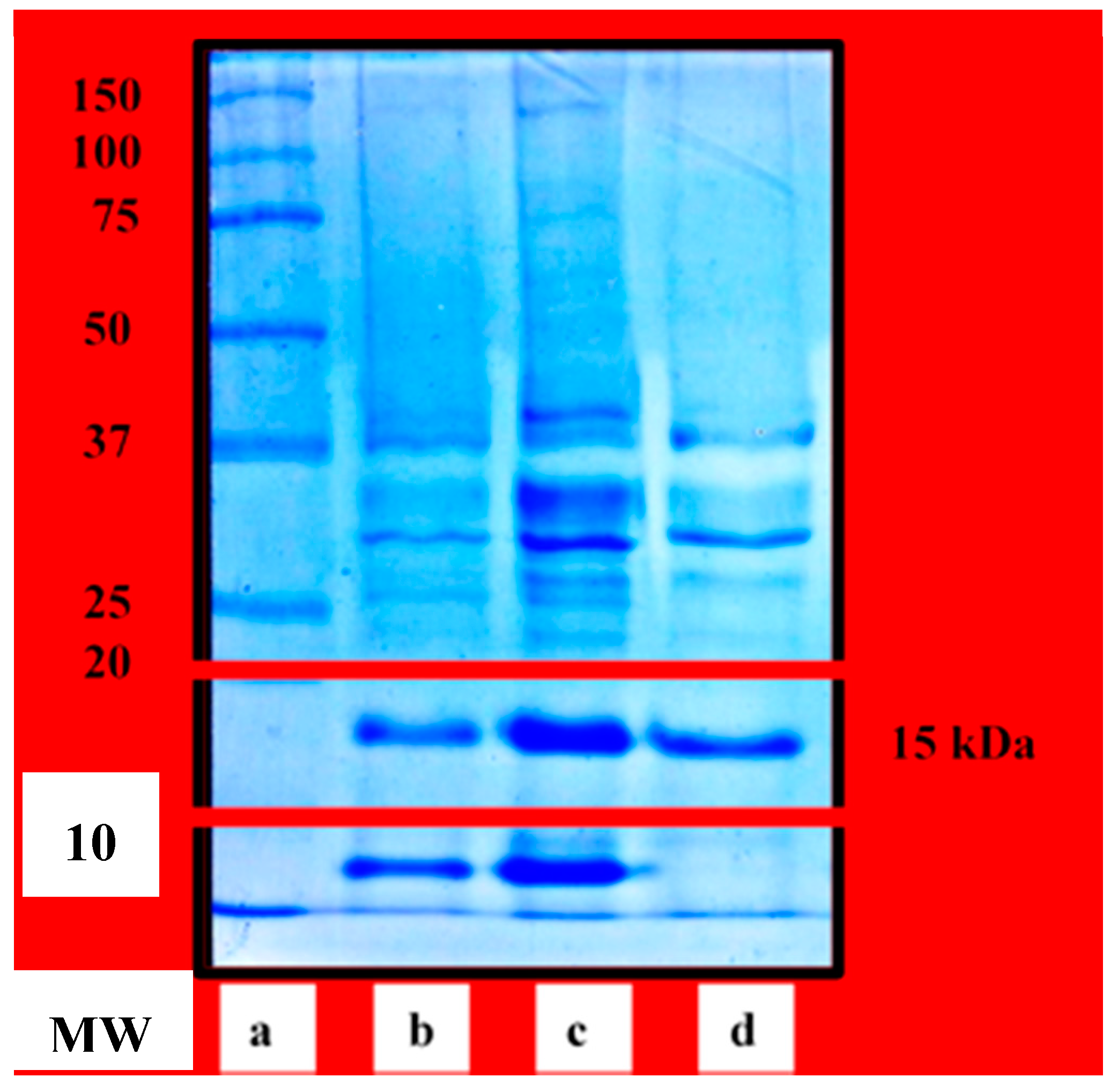
Figure 1, lane b) of the crude protein extract is similar to the profile obtained by Surabhi et al. [
26] with both profiles showing major proteins with MW of approximately 15, 30 and 40 kDa. This proves that proteins were successfully extracted from the powdered pulp.
The extracted protein has a concentration of 167.32 μg/mL in terms of extraction buffer used or 334.64 μg/g in terms of fruit pulp used. The low concentration of the protein extracted can be associated to the initially low protein content of the sample, difference in the actual protein content of the sample compared to the representative species reported in the literature, and difference or modification in the implemented extraction procedure. For example, although the extraction buffer used in the study is almost the same with the extraction buffer used in in the reference studies [
24,
25], the implemented extraction procedure was simplified resulting to a decreased amount of protein extracted. Extraction procedures on the reference studies have coupled extraction with different protein precipitation methods and/or have added other components to the buffer to increase extraction efficiency and yield a protein with higher purity.
Throughout the experiment, pH and temperature were carefully observed since proteins are sensitive to changes in these parameters. Drastic changes in these parameters can lead to protein denaturation, affecting negatively the efficiency of the protein extraction procedure and the quality of the protein extracted. Low temperature condition was employed to prevent any endogenous protease activity which may lead to denaturation of the protein of interest [
4].
3.2. Purification of the Crude Protein Extract
Partial purification of the crude extract using ammonium sulfate precipitation removed proteins that did not precipitate at 40–60% salt saturation. This also concentrated the dilute crude extract, shown by more intense protein bands (
Figure 1, lane c), through the dissolution of the precipitated proteins in a smaller buffer volume [
4]. Dialysis, on the other hand, removed most of the ammonium sulfate present in the partially purified sample which could affect the results of the succeeding protein characterization procedures. Lastly, gel filtration chromatography (
Figure 1, lane d) as a purification procedure effectively separated substances with MW range between 10 kDa to 100 kDa which refers to the effective separative capacity of the resin used.
Comparison of the protein profiles in
Figure 1 (lanes b, c and d) showed that the purification scheme was effective. Protein band at 10 kDa and other faint protein bands at 25–37 kDa area of the crude profile were not observed in the purified profile. Moreover, the protein band at 15 kDa became more distinct after purification.
Densitometric analysis allowed estimation of the molecular weights and calculation of the percent band volume of each protein present in the SDS-PAGE profiles of the crude, partially purified, and purified protein extracts. This showed that the major proteins have MW of 15 kDa, since this protein band had the highest percent band volume ranging from 26% to 34% per sample lane.
Data from
Table 1 shows that there was a significant decrease in protein concentration after every purification procedure. This means that non-major proteins were subsequently removed from the crude and partially purified extract leaving only the major proteins in the purified extract. Although the results of the colorimetric Bradford assay in
Table 1 showed a decrease in concentration after every purification step, the SDS-PAGE profile of partially purified (lane c) and purified extract (lane d) in
Figure 1 had more intense bands compared to the crude extract (lane b). This is due to loading of preconcentrated partially purified and purified extract into the gel system. If preconcentration of the said extract were not done, their dilute concentration might result to very faint protein bands. Meanwhile, the crude extract was not pre-concentrated prior to SDS-PAGE since it already gave a workable protein profile band with the concentration as it is.
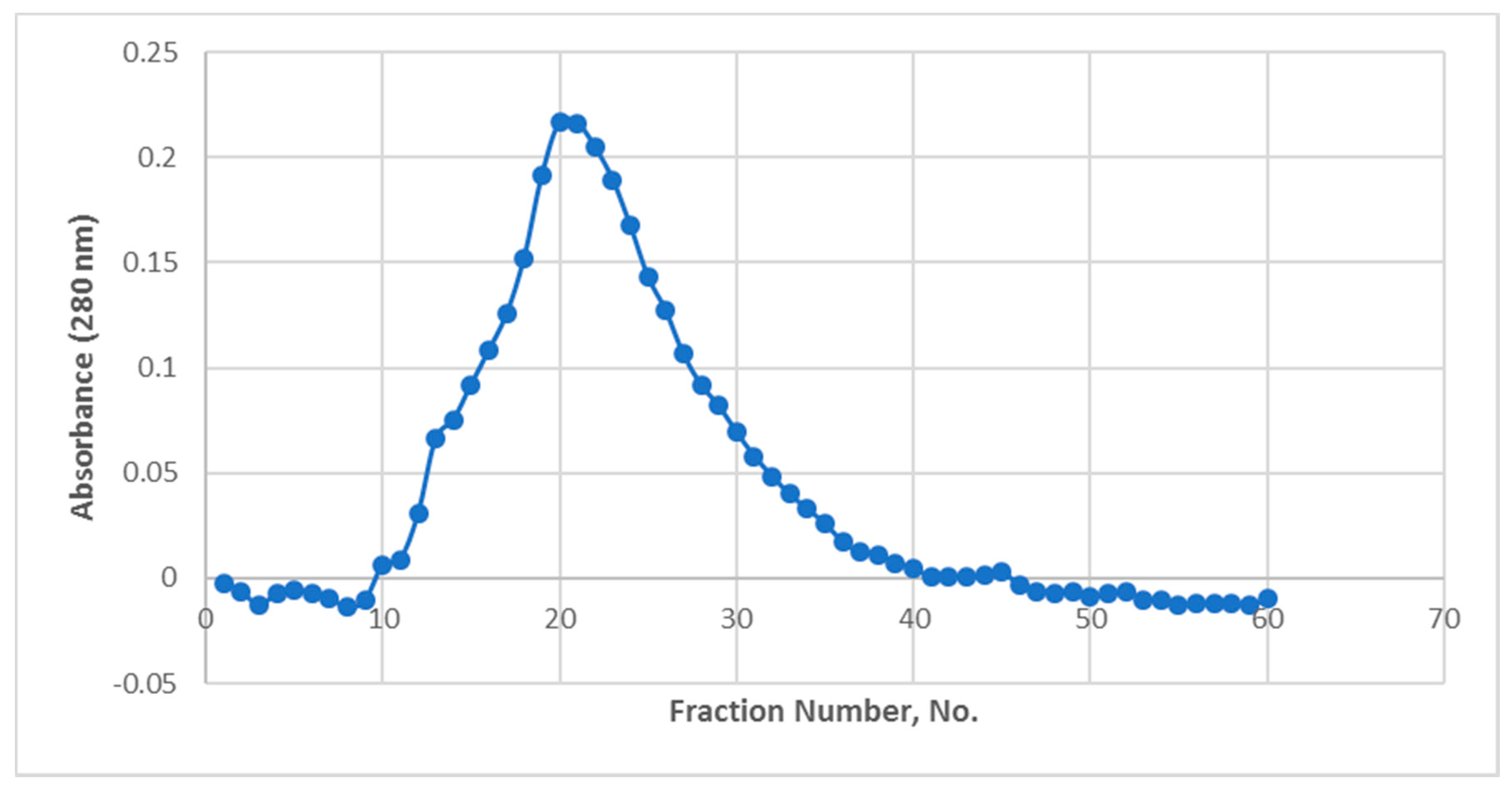
Expounding on the final purification procedure, Gel Filtration Chomatography (GFC),
Figure 2 shows that there was only one observable curve which peaked at fractions 20 and 21. An elution profile showing a curve or band rather than being narrowly peaked suggests that the purified protein was distributed in the fractions found under the curve rather than on a single fraction. SDS-PAGE result of these fractions showed the same profile with protein bands becoming more intense from the 19th up to the 23rd fractions (refer to
Supplementary Materials). The fractions outside the curve have no visible protein bands.
Reconsidering
Figure 1, the distinct protein band found at 10 kDa of the crude and partially purified protein extracts did not appear at the purified extract. Moreover, the said protein band did not also appear at any GFC fraction even though there were already 60 fractions collected. This protein band did not elute out of the column as the resin only effectively separated proteins with MW between 10 kDa and 100 kDa. Proteins with molecular weight smaller than the given range would be trapped and totally included in the pores of the gel beads with the possibility of not being eluted out by merely using the same elution buffer.
3.3. Enzymatic Hydrolysis of the Isolated Proteins
There are known isolated proteins, that even in their intact form, can exhibit biologically related activities. However, protein consumed undergo digestion before it can be fully metabolized and utilized by the body as source of energy and amino acids. Moreover, studies by Aluko [
7] and Shahidi and Zhong [
8], suggest that the physiological and functional properties of proteins are from the encrypted bioactive peptides. Hence, enzymatic hydrolysis of the protein extracts was done to obtain the target peptides.
The SDS-PAGE profiles of the crude, partially purified and purified extracts showed that the major protein bands present in the undigested sample disappeared after digestion (refer to
Supplementary Materials). This implies that the proteins in the undigested sample were hydrolyzed up to a certain extent resulting to smaller peptide fragments which were not resolved during gel electrophoresis. No significant thick band appeared at the bottom of the electrophoretogram (refer to
Supplementary Materials) which should have resulted from the accumulation of low MW proteins and/or peptides suggesting that these fragments could have directly run out of the gel system during the electrophoretic run.
Densitometric analysis, used to determine the extent of protein hydrolysis through the reduction in peak height and area of each observable protein bands, showed that there were no significant peaks in the 3 h, 4 h, 12 h and 24 h sample digests proving that the proteins were successfully hydrolyzed into smaller peptide fragments (refer to
Supplementary Materials).
3.4. In Silico Analysis of the Isolated Major Protein from ‘Señorita’ Banana Extracts
UniProt KB [
20] Protein Search query returned more than 2000
Musa acuminata proteins which have a MW of 15 kDa, the approximate MW of the major protein. Some of the proteins returned in the query includes profilin, histone, thioredoxin, ferredoxin, ribosomal proteins, lectin and various enzymes such as kinases, lyases and chitinase. Lectin was used as the putative protein for its sugar binding property and is found in both inter- and intracellular space allowing them to be easily extracted compared to other proteins in the search query which can be found in the cellular organelles requiring a complicated extraction procedure. A study by Al-Alwani [
27] showed that lectin is easily extracted from white kidney beans by using 0.15 M NaCl.
Figure 3 shows the lectin protein sequence after hydrolysis using the enzymes pepsin, trypsin, chymotrypsin, and thermolysin obtained through BIOPEP-UWM [
21] protein analyzer. The highlighted peptide fragments are known to exhibit ACE inhibitory activity and/or antioxidant activity.
3.5. Determination of the Percent (%) Angiotensin Converting Enzyme (ACE) Inhibition as a Basis of Antihypertensive Activity
The results in
Table 2 confirmed that the sample extracts have ACE inhibitory activity even though some values were small. The percent ACE inhibition of the samples were all significantly different to that of captopril (standard), even though the concentration of the drug was leveled to the concentration of the samples (50 μg/mL). A study by Rhajbar et al. [
28] showed that the activity of ACE inhibitory compounds depends on the dosage and identity of the compound. Hence, it could be that the low concentration of the samples during the testing have led to the observed low activities.
Among the samples tested, the undigested crude protein extract showed the highest activity with 85.20% while the remaining samples have roughly 10% to 40%. The undigested crude extract was composed of various proteins in their native conformation as well as traces of other easily extractable compounds such as polyphenols, which could account for the observed high activity. For instance, a study by Geng et al. [
29] and Lau et al. [
30], showed that crude protein extracts from various mushroom samples inhibited ACE by up to 90% depending on the kind of mushroom and extraction methods employed. On the other hand, a study conducted by Rupasinghe [
31] showed that flavonoids, a subclass of polyphenols, extracted from fruit tissues can inhibit ACE with results dependent on the sugar moiety attached to the flavonoid ring. Other documented bioactive compounds that can inhibit ACE include polysaccharides, sterols, saponins, fiber, vitamins C and E, and other minerals.
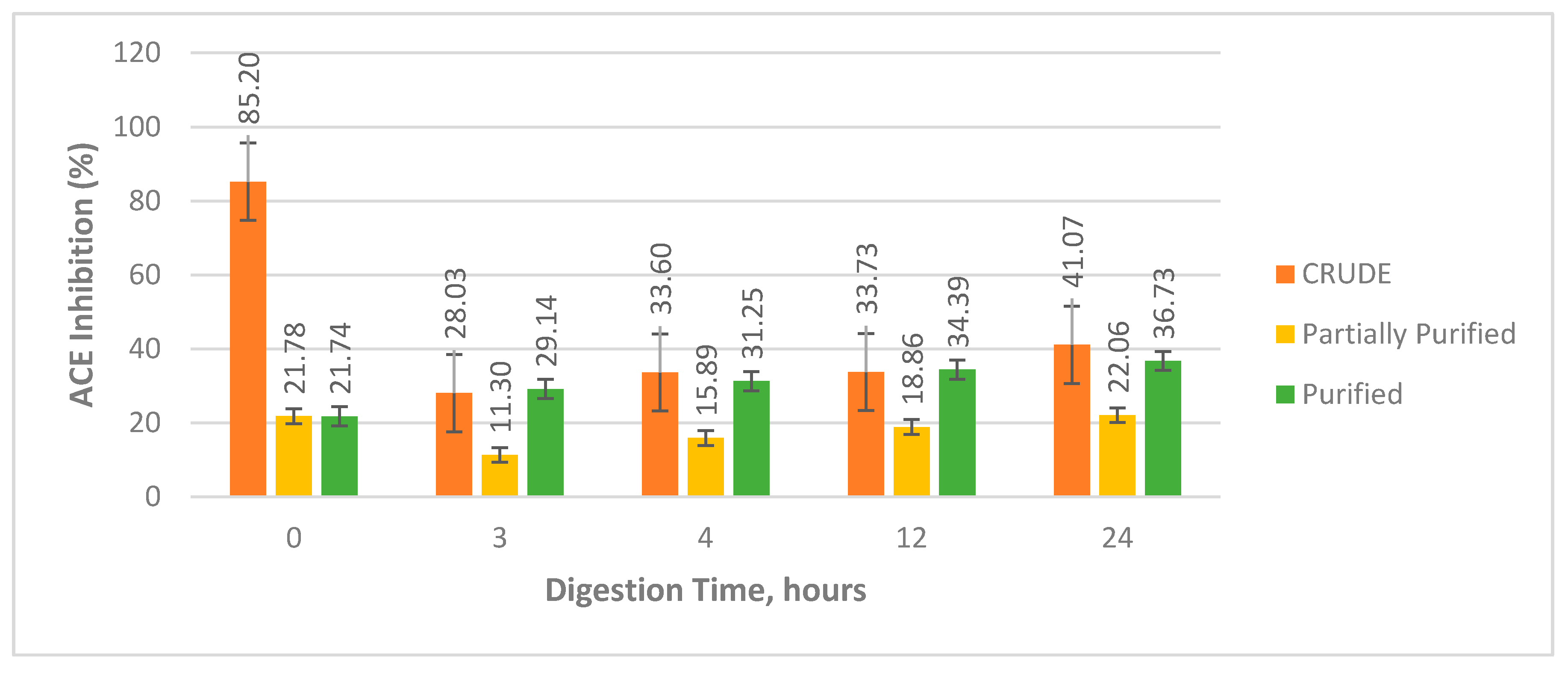
Statistical analysis on the crude sample at different digestion hours showed that there was a significant decrease in the activity of undigested crude sample after the three-hour digestion followed by a subsequent increase in the activity until the 24-h digestion period (
Figure 4). This implies that the initial hydrolysis of the proteins from the crude extract led to denaturation and loss of the active native conformation, resulting to the observed decrease in activity. On the other hand, the observed increase in activity as the digestion period is increased could mean that the precursor protein(s) were continuously broken down to bioactive peptides inhibiting ACE. However, since the activities of 12-h and 24-h digests are not significantly different, the number of bioactive peptides released after 12 h of digestion did not significantly increase.
For the partially purified extract samples, the same trend in the activities of the crude extract samples was observed. However, the observed activities were found to be the lowest among the three sample groups.
Table 2 shows that there was an observable significant three-fold decrease in the activity of the undigested crude sample when partially purified. Partial purification removed non-protein contaminants and unprecipitated proteins from the crude extract. The removal of these components, which might have ACE inhibitory activity, decreased significantly the activity of the undigested partially purified sample. On the other hand, the increase in the activity of the purified sample could be associated to the peptides inhibiting the ACE to have more effective interaction due to the decrease in the sample components that have prevented otherwise [
32].
For the purified extract samples, there was an increase in activity starting from the undigested sample up to the 24-h digest. This trend was different to the other two sample groups. Purified samples were primarily composed of the major protein, as a result, the activities observed had only reflected the characteristic activity of the major protein. In silico analysis of the putative protein showed that enzymatic hydrolysis of the protein will release bioactive peptides that can inhibit ACE, thus, prolonging the hydrolysis of the protein through increased digestion period released more bioactive peptides resulting to an observable increase in activity.
3.6. Determination of the Radical Scavenging Activity by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay as a Basis of Antioxidant Activity
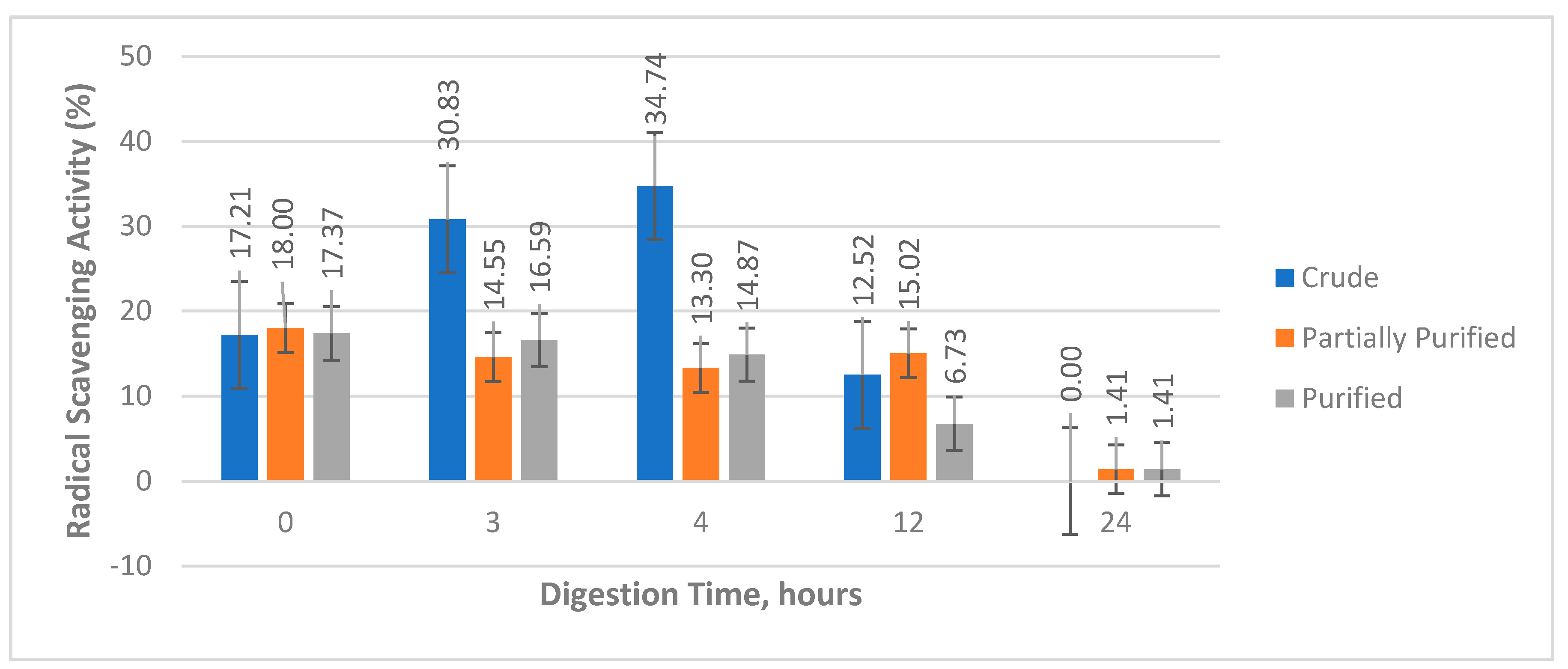
For the effect of digestion time on antioxidant activity of the protein extracts, a decrease in the activity of the partially purified and purified samples was observed as the digestion time was increased, whereas the activity of the crude samples continued to increase up to the four hour digestion time before it significantly decreased at the 12 h and 24 h digestion time (
Figure 5). This trend in antioxidant activity, where there is an initial increase in activity followed by a gradual decline has also been observed to other studied [
33,
34]
Table 3 showed that the activity of the undigested crude sample after three hours and four hours of digestion increased and found to be not significantly different with the activity of ascorbic acid (positive control). This implies that the initially active proteins found in the undigested crude sample released peptides with high antioxidant activity However, further hydrolysis of these peptides decreased the activity of the sample as observed in the activities of the 12 h and 24 h digests, showing that the peptides with high activity might have been degraded to smaller peptides with lower activity.
On the other hand, the observed general decrease in activity of the partially purified and purified protein samples as the digestion period was increased, could mean that during hydrolysis, the initially active proteins present in the undigested samples did not release any peptides with high antioxidant activity, rather they were continuously degraded to peptides with lower activity. This observation is contrary to the result of in silico analysis.
Statistical analysis of the crude, partially purified, and purified sample activities at a specific digestion period showed that the activities of undigested samples where not significantly different from each other, whereas, for most digested samples, only the activity from the digested crude samples were found to be significantly different. For the undigested samples, it could be that the activities observed were solely from the extracted and purified major protein, hence, the activities were not significantly different from each other. Meanwhile, the observed significantly different activity of the crude digested samples compared to other digested samples could be the result of the activity of peptides released from the non-major proteins removed during purification. In silico analysis of the putative major protein (
Figure 3) showed that only few antioxidant peptides will be released upon hydrolysis justifying the low activities of the digested partially purified and purified samples
4. Conclusions
The study extracted, purified and hydrolyzed protein from ‘Señorita’ banana pulp with the major protein having an approximate molecular weight of 15 kDa. The low concentration of the crude extract (167.32 µg/mL buffer) was associated to the characteristic low protein content of the sample. Furthermore, these extracts and the peptides released during the enzymatic hydrolysis were tested for ACE inhibitory and antioxidant activities. The undigested crude sample had the highest ACE inhibitory activity (85.20%), but it was found to be significantly different from the activity of the standard, captopril (99.72%). On the other hand, the 3 h and 4 h crude digests had the highest DPPH radical scavenging activity (30.83% and 34.74%, respectively) that were found to be not significantly different to the activity of the standard, ascorbic acid (36.31%). For the effect of digestion period, longer digestion time results to higher ACE inhibitory activity while for the DPPH radical scavenging activity, there is an initial increase followed by a decline at longer digestion time. These are expected based on the result of in silico analysis of the putative major protein, lectin, and the available literatures similar to the study. Over-all, this study can serve as an additional reference to the very limited banana protein and peptide studies.