Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Visual Appearance
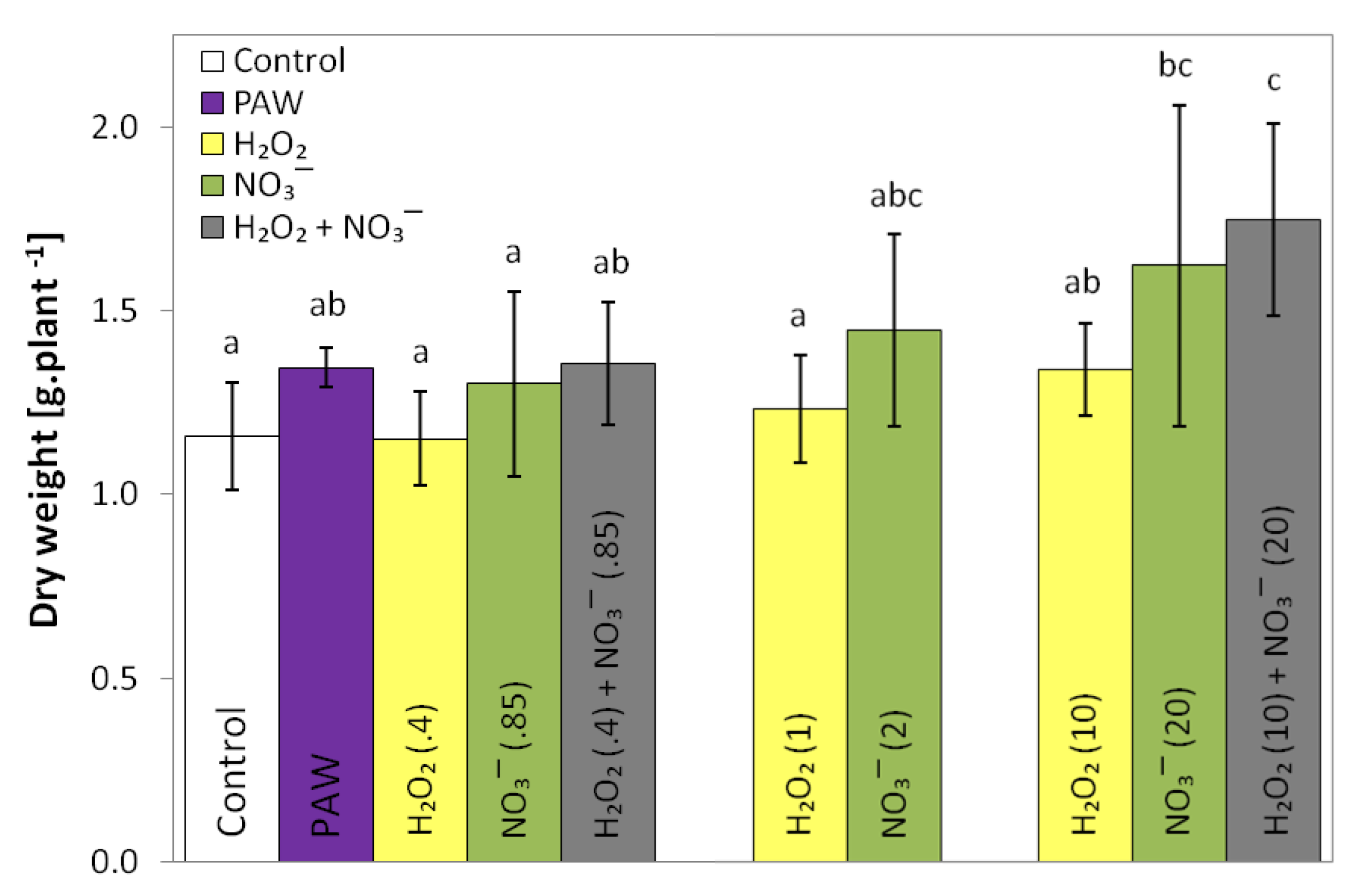
3.2. Fresh and Dry Weight
3.3. Photosynthetic Pigment Content
3.4. Net Photosynthetic Rate
3.5. Activity of Antioxidant Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Chetani, R. A review on the effect of organic and chemical fertilizers on plants. IJRASET 2017, 5, 677–680. [Google Scholar] [CrossRef]
- Sonoda, T.; Takamura, N.; Wang, D.; Namihira, T.; Akiyama, H.; Sonoda, T. Growth control of leaf lettuce using pulsed electric field. IEEE Trans. Plasma Sci. 2013, 42, 1–5. [Google Scholar] [CrossRef]
- Reina, F.G.; Pascual, L.A.; Fundora, I.A. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: Experimental results. Bioelectromagnetics 2001, 22, 596–602. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. D Appl. Phys. 2020, 53, 223001. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef]
- Henselová, M.; Slováková, Ľ.; Martinka, M.; Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 2012, 67, 490–497. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2015, 36, 397–414. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process. Polym. 2018, 15, 1700064. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, e1700076. [Google Scholar] [CrossRef]
- El Shaer, M.; El Welily, H.; Zaki, A.; Arafa, H.; Elsebaei, A.; Eldaly, M.; Mobasher, M. Germination of Wheat Seeds Exposed to Cold Atmospheric Plasma in Dry and Wet Plasma-Activated Water and Mist. Plasma Med. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Starek-Wójcicka, A.; Sagan, A.; Terebun, P.; Kwiatkowski, M.; Kiczorowski, P.; Pawlat, J. Influence of a Helium–Nitrogen RF Plasma Jet on Onion Seed Germination. Appl. Sci. 2020, 10, 8973. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea (Pisum sativum L. cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Terebun, P.; Kwiatkowski, M.; Starek, A.; Reuter, S.; Mok, Y.S.; Pawłat, J. Impact of Short Time Atmospheric Plasma Treatment on Onion Seeds. Plasma Chem. Plasma Process. 2021, 41, 559–571. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Sinclair, A.J.; Cahill, D.M.; Wang, X.; Dai, X.J. Nitrate and Hydrogen Peroxide Generated in Water by Electrical Discharges Stimulate Wheat Seedling Growth. Plasma Chem. Plasma Process. 2017, 37, 1393–1404. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhou, R.; Zhang, X.; Yang, S. Atmospheric-pressure plasma treated water for seed germination and seedling growth on mung bean and its sterilization effect on mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Zhao, Y.; Patange, A.; Sun, D.; Tiwari, B. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef] [PubMed]
- Yemeli, G.B.N.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The effect of water activated by nonthermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process. Polym. 2021, 18, 2000205. [Google Scholar] [CrossRef]
- Sergeichev, K.F.; Lukina, N.A.; Sarimov, R.M.; Smirnov, I.G.; Simakin, A.V.; Dorokhov, A.S.; Gudkov, S.V. Physicochemical Properties of Pure Water Treated by Pure Argon Plasma Jet Generated by Microwave Discharge in Opened Atmosphere. Front. Phys. 2021, 8, 614684. [Google Scholar] [CrossRef]
- Belov, S.V.; Danyleiko, Y.K.; Glinushkin, A.P.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Konchekov, E.M.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; et al. An Activated Potassium Phosphate Fertilizer Solution for Stimulating the Growth of Agricultural Plants. Front. Phys. 2021, 8, 618320. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.S.; Huffaker, R.C. The Uptake of NO3−, NO2−, and NH4+ by Intact Wheat (Triticum aestivum) Seedlings. Plant Physiol. 1986, 82, 1051–1056. [Google Scholar] [CrossRef]
- Forde, B.G. Nitrate transporters in plants: Structure, function and regulation. Biochim. Biophys. Acta 2000, 1465, 219–235. [Google Scholar] [CrossRef]
- Lee, R.B. The effect of nitrite on root growth of barley and maize. New Phytol. 1979, 83, 615–622. [Google Scholar] [CrossRef]
- Kang, M.H.; Jeon, S.S.; Shin, S.M.; Veerana, M.; Ji, S.-H.; Uhm, H.-S.; Choi, E.-H.; Shin, J.H.; Park, G. Dynamics of nitric oxide level in liquids treated with microwave plasma-generated gas and their effects on spinach development. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene expression in Tomato seedlings. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gierczik, K.; Vukušić, T.; Kovács, L.; Székely, A.; Szalai, G.; Milošević, S.; Kocsy, G.; Kutasi, K.; Galiba, G. Plasma-activated water to improve the stress tolerance of barley. Plasma Process. Polym. 2020, 17, 1900123. [Google Scholar] [CrossRef]
- Schnabel, U.; Schmidt, C.; Stachowiak, J.; Bösel, A.; Andrasch, M.; Ehlbeck, J. Plasma processed air for biological decontamination of PET and fresh plant tissue. Plasma Process. Polym. 2018, 15, 1600057. [Google Scholar] [CrossRef]
- Schnabel, U.; Sydow, D.; Schulter, O.; Andrasch, M.; Ehlbeck, J. Decontamination of fresh-cut iceberg lettuce and fresh mung bean sprouts by non-thermal atmospheric pressure plasma processed water (PPW). Mod. Agric. Sci. Technol. 2015, 1, 23–39. [Google Scholar] [CrossRef]
- Fröhling, A.; Ehlbeck, J.; Schlüter, O. Impact of a Pilot-Scale Plasma-Assisted Washing Process on the Culturable Microbial Community Dynamics Related to Fresh-Cut Endive Lettuce. Appl. Sci. 2018, 8, 2225. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Kim, Y.-J. Inactivation mechanism of Salmonella Typhimurium on the surface of lettuce and physicochemical quality assessment of samples treated by micro-plasma discharged water. Innov. Food Sci. Emerg. Technol. 2019, 52, 17–24. [Google Scholar] [CrossRef]
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.; Bourke, P. Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, U.; Andrasch, M.; Stachowiak, J.; Weit, C.; Weihe, T.; Schmidt, C.; Muranyi, P.; Schlüter, O.; Ehlbeck, J. Sanitation of fresh-cut endive lettuce by plasma processed tap water (PPtW)—Up-scaling to industrial level. Innov. Food Sci. Emerg. Technol. 2019, 53, 45–55. [Google Scholar] [CrossRef]
- Min, W.; Chen, Q.; Chen, G.; Yang, S. Effect of Atmospheric Pressure Plasma on Growth and Development of Lettuce. Acta Agric. Boreali Sin. 2007, 22, 108–113. [Google Scholar] [CrossRef]
- Stoleru, V.; Stratulat, C.; Teliban, G.; Padureanu, S.; Patras, A.; Burlica, R.; Dirlau, D.; Astanei, D.; Beniuga, O. Morphological, Physiological and Productive Indicators of Lettuce under Non-thermal Plasma. In Proceedings of the 2018 International Conference and Exposition on Electrical and Power Engineering (EPE), Iasi, Romania, 18–19 October 2018; pp. 0937–0942. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Martišovitš, V.; Machala, Z. Transient spark: A dc-driven repetitively pulsed discharge and its control by electric circuit parameters. Plasma Sources Sci. Technol. 2011, 20, 035015. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen”. Eur. J. Inorg. Chem. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Moorcroft, M.J. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Kučerová, K.; Machala, Z.; Hensel, K. Transient Spark Discharge Generated in Various N2/O2 Gas Mixtures: Reactive Species in the Gas and Water and Their Antibacterial Effects. Plasma Chem. Plasma Process. 2020, 40, 749–773. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Weber, W.J.; Strumm, W. Buffer System of Natural Fresh Water. J. Chem. Eng. Data 1963, 8, 464–468. [Google Scholar] [CrossRef]
- Takaki, K.; Takahata, J.; Watanabe, S.; Satta, N.; Yamada, O.; Fujio, T.; Sasaki, Y. Improvements in plant growth rate using underwater discharge. J. Phys. Conf. Ser. 2013, 418, 012140. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Zhang, L.; Xu, Y.; Fu, M.; Luo, Z. Hydrogen peroxide accelerated the lignification process of bamboo shoots by activating the phenylpropanoid pathway and programmed cell death in postharvest storage. Postharvest Biol. Technol. 2019, 153, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013, 4, 220. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate photosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Espinoza, F.H.; Murillo-Amador, B.; García-Hernández, J.L.; Fenech-Larios, L.; Rueda-Puente, E.O.; Troyo-Diéguez, E.; Kaya, C.; Beltrán-Morales, A. Field evaluation of the relationship between chlorophyll content in basil leaves and a portable chlorophyll meter (spad-502) readings. J. Plant Nutr. 2010, 33, 423–438. [Google Scholar] [CrossRef]
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of Nitrogen Deficiency on Gas Exchange, Chlorophyll Fluorescence, and Antioxidant Enzymes in Leaves of Rice Plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A. Salicylic Acid: A Plant Hormone; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; p. 401. [Google Scholar]
- Hamlyn, G.J.; Flowers, T.J.; Jones, M.B. Plants under Stress: Biochemistry, Physiology and Ecology and Their Application to Plant Improvement; Cambridge University Press: Cambridge, UK, 1989; p. 257. [Google Scholar]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Improvement of Photo-synthesis and Photosynthetic Productivity of Winter Wheat by Cold Plasma Treatment under Haze Condition. J. Agr. Sci. Technol. 2019, 21, 1889–1904. [Google Scholar]
- Švubová, R.; Slováková, Ľ.; Holubová, Ľ.; Rovňanová, D.; Gálová, E.; Tomeková, J. Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants 2021, 10, 177. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Liu, D.; Wang, W.; Niu, J.; Xia, Y.; Qi, Z.; Zhao, Z.; Song, Y. Effect of Nonthermal Plasma-Activated Water on Quality and Antioxidant Activity of Fresh-Cut Kiwifruit. IEEE Trans. Plasma Sci. 2019, 47, 4811–4817. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]





| Solution/Variant (Abbrev.) | H2O2 [mM] | NO3− [mM] | pH |
|---|---|---|---|
| Control (tap water) | - | ~0.02 | ~7.5 |
| PAW | ~0.42 | ~0.85 | ~7.5 |
| H2O2 (.4) | 0.4 | - | ~7.6 |
| H2O2 (1) | 1.0 | - | ~7.7 |
| H2O2 (10) | 10.0 | - | ~7.8 |
| NO3− (.85) | - | 0.85 | ~7.9 |
| NO3− (2) | - | 2.0 | ~7.9 |
| NO3− (20) | - | 20.0 | ~7.9 |
| H2O2 (.4) + NO3− (.85) | 0.4 | 0.85 | ~7.9 |
| H2O2 (10) + NO3− (20) | 10.0 | 20.0 | ~7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučerová, K.; Henselová, M.; Slováková, Ľ.; Bačovčinová, M.; Hensel, K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Appl. Sci. 2021, 11, 1985. https://doi.org/10.3390/app11051985
Kučerová K, Henselová M, Slováková Ľ, Bačovčinová M, Hensel K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Applied Sciences. 2021; 11(5):1985. https://doi.org/10.3390/app11051985
Chicago/Turabian StyleKučerová, Katarína, Mária Henselová, Ľudmila Slováková, Michaela Bačovčinová, and Karol Hensel. 2021. "Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters" Applied Sciences 11, no. 5: 1985. https://doi.org/10.3390/app11051985
APA StyleKučerová, K., Henselová, M., Slováková, Ľ., Bačovčinová, M., & Hensel, K. (2021). Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Applied Sciences, 11(5), 1985. https://doi.org/10.3390/app11051985







