Abstract
One of the new methods of protecting and supporting plant growth is the use of low-temperature plasma. The aim of this study is to evaluate the feasibility of using plasma activated water produced in an atmospheric pressure gliding arc reactor for germination of beetroot (Beta vulgaris) and carrot (Daucus carota) seeds. The study was carried out for different plasma treatment times of water (5, 10 and 20 min) and with fixed geometry and power of the discharge system, using air as the working gas. The effect on germination was evaluated based on the fraction of germinated seeds and their length at 7 and 14 days after treatment. Analysis of fungi present on the seed surface and imaging of the seed surface using scanning electron microscopy (SEM) were auxiliary methods to evaluate the type of treatment effect. In the case of beetroot, a positive effect on the number and length of germinated seeds was observed, which increased with increasing treatment time. This effect can be attributed, among other things, to the surface changes observed on microscopic photographs. In the case of carrot seeds, a more significant positive effect on germination was observed. Fungal decontamination effect was relatively weaker than with the use of the chemical method with sodium hypochlorite.
1. Introduction
Currently, one of the main tasks of plant protection is the implementation of innovative and safe methods to reduce the occurrence of agrophages in crops [1,2]. This is related to the implementation of the concept of sustainable agriculture, promoting the production of high quality food in a socially responsible manner, rational use of natural resources and a reduction in the use of chemical plant protection products [3,4,5,6]. Excessive and careless use of pesticides contributes not only to resistant breeds of agrophages, but also to environmental pollution and residues in the raw materials produced [7]. One alternative method of pesticide-free crop protection may be plasma treatment. Plasma can affect living cells through the action of active particles generated in the plasma (mainly reactive oxygen and nitrogen species—RONS), the action of charged plasma particles, radiation over a wide range of wavelengths, and shear stresses and drying [8,9,10]. The mechanism of plasma action on harmful microorganisms is multistage and mainly involves permanent damage of the cell wall, cytoplasmic membrane, and then intracellular structures, genetic material and the enzyme apparatus [11,12,13]. Plasma can be used to decontaminate seed and seedling material [14,15,16,17,18,19,20,21,22] and have positive effects on physiological processes in plants and plant seedlings [23,24,25,26,27,28,29,30,31,32]. For example, the work of Jiang et al. indicated the effects of low-temperature plasma on seed germination, seedling growth, root morphology, and nutrient uptake in tomato [33]. Many researchers confirm the positive effect of plasma on the process of plant rooting especially on increasing the length and number of roots formed [34,35].
One method of using plasma treatment may be to use plasma activated water (PAW), which may contain active particles (such as dissolved ozone, hydrogen peroxide, nitrates, nitrites, peroxynitrites, OH radicals, etc.) produced directly in the discharge or by their reaction with water, depending on the type of discharge and the working gas used [36]. Then, PAW can be used to remove harmful organisms [37,38,39,40,41,42,43,44,45], either directly on growing plants [46,47] or by aiding in the germination process [48,49]. From the perspective of stability over time, hydrogen peroxide and lower pH in PAW are probably the most influential factors in fungicidal activity. The treatment effect depends on both the discharge parameters and the type of plant being treated. The purpose of this study is to evaluate the feasibility of using PAW generated by a gliding arc discharge (GAD) to aid in the germination of carrot and beetroot seeds. In addition to the results on the fraction of germinated seeds and the length of sprouts, those obtained from surface imaging and the analysis of the type and amount of fungi present on the seeds, conducted via scanning electron microscopy (SEM), were also used to evaluate the effect of plasma. The results were compared with one of the classic methods of plant decontamination—treatment with sodium hypochlorite (NaOCl).
2. Materials and Methods
2.1. Treated Seeds
Edible carrot seeds of the AFALON F1 variety (Daucus carota, Moravo Seed Ożarów Mazowiecki, Poland) and beetroot seeds of the CYLINDRA variety (Beta vulgaris, W. Legutko, Jutrosin, Poland) were used in the research (Figure 1a). For each treatment condition, 10 seeds were randomly selected, and the measurements were repeated 5 times.
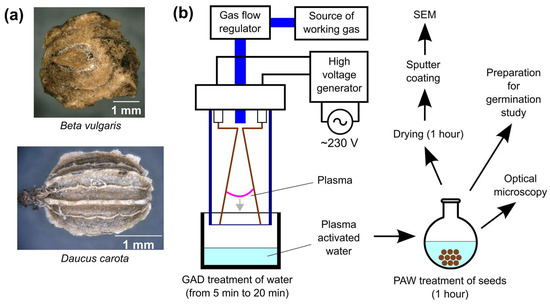
Figure 1.
(a) Photographs of treated seeds, (b) schematic of the experiment.
2.2. Plasma Activated Water
Plasma was generated in a single-phase gliding arc reactor (GAD) operating at atmospheric pressure; this process has been described in greater detail in previous works [50,51]. The discharge system consisted of two copper electrodes, 2 mm thick and 80 mm long, with an angle of 12° between them, placed in a glass tube with an inner diameter of 50 mm. The working gas (air) was blown out through the glass nozzle near the ignition area with a flow rate of 7.33 dm3/min, adjustable with glass tube variable area flow meter (Zakłady Automatyki “ROTAMETR”, Gliwice, Poland). The reactor was powered by an RMS (root-mean-square) voltage of 680 V (3.8 kV peak voltage), frequency of 50 Hz and apparent power of 40 VA. To obtain plasma activated water, 20 mL of distilled water, at 22.6 °C, was placed in a glass vessel with a diameter of 60 mm and with a distance of 20 mm between the electrodes and the water surface (Figure 1b).
The water temperature after plasma treatment of 5, 10 and 20 min, measured with a K-type thermocouple connected to the DT-847U meter (Yu Ching Technology, Taipei, Taiwan), was 27 °C, 29.1 °C and 30.7 °C, respectively.
The colorimetric method using TiOSO4 (Titanium(IV) oxysulfate) solution (Merck, Darmstadt, Germany) and colorimetric Griess assay (Cayman Chemicals, Ann Arbor, USA) were used for the measurement of hydrogen peroxide (H2O2) and nitrites (NO2−), respectively. The obtained concentrations of active species increased with the plasma treatment time and are summarized in Table 1. The GAD plasma source is able to generate high concentrations of nitrogen reactive species but H2O2 concentration was low, which is in a good accordance with previous data [50].

Table 1.
Concentration of selected RONS.
2.3. Germination Rate
Immediately after the plasma treatment of water, the experiment with seeds was carried out in three parts: first, the seeds were treated with PAW, e.g., water was poured over 10 seeds of each species placed in glass flasks and left to soak for one hour. Then, seeds were transferred to the Petri dishes with the mineral nutrient medium solution (the composition of the solution is shown in Table 2). In the last step, mycological analysis was performed.

Table 2.
Composition of mineral medium.
In order to compare plasma treatment with traditional decontamination methods, a series of measurements was performed using a sodium hypochlorite. For this purpose, 10 seeds were sterilized in 10% NaOCl solution for 60 s and then washed 3 times for 3 min in distilled water. NaOCl (POCH Odczynniki Chemiczne, Avantor, Poland) was diluted to proper concentration on-site immediately before application. Seed samples from the same batch were used for the tests. Then, the seeds were placed on the plates, similarly to the plasma treatment and control. All seeds were in sterile growth mineral medium, in the cultivation chamber with controlled atmosphere, under the following conditions: 20–22 °C without light with 65% humidity. The amount of germinated beet and carrot seeds was measured after 7 days for germination energy GEN and after 14 days for germination capacity GC [52]. Both coefficients were then calculated from the following equation:
where: n—the number of seeds germinated at time t; nT—the total number of sown seeds.
2.4. Fungi on the Seed Surface
For additional series with the same treatment conditions, in the germination study a mycological analysis was performed using the method of artificial cultures [53]. The medium with the composition presented in Table 1 was supplemented with distilled water to the volume of 1000 mL, and then sterilized for 20 min in an autoclave, at a temperature of 121 °C and a pressure of 1 atmosphere. The dishes with the seeds lined up were placed at 20–22 °C for 7 days without light. After one week, the plates were inspected, and the grown fungus colonies were cleaved on slants with potato-glucose agar (PDA). Then, segregation was performed on the basis of microscopic and macroscopic features. The obtained fungal isolates were sorted and labeled according to species using studies, keys and monographs [54,55,56,57].
2.5. Surface Imaging Using an Optical Microscope and SEM
Morphology of the seeds was evaluated using a KEYENCE VHX-5000 digital microscope (Osaka, Japan). Images of the samples were taken immediately after one hour of the seeds’ imbibition in plasma activated water.
For more detailed visualization of the surface of Beta vulgaris seeds after PAW treatment, the treated samples were naturally dried for one hour and applied to the mounting surface (carbon disc). A layer of gold (~15 nm) was then sputtered onto the samples for imaging using a Phenom SEM microscope (Thermo Fisher Scientific, USA) at a voltage of 10 kV.
2.6. Statistical Analysis
StatSoft’s Statistica 8.0 software was used for the analysis of the experimental data. Statistical differences between groups were examined with use of two-way analysis of variance (ANOVA). Tukey’s test was used to analyze the significance of differences between mean values (α ≤ 0.05). For the data obtained with measurement of the same objects at different times, the results are correlated due to the use of the ANOVA Repeated Measures Analysis of Variance.
3. Results and Discussion
3.1. Germination Rate
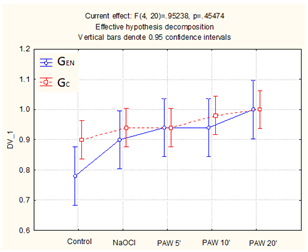
Beta vulgaris germination results for control, NaOCl and PAW (using 5, 10 and 20 min of GAD treatment of water as PAW5′, PAW10′ and PAW 20′) are summarized in Figure 2. Compared to the control, which had a rather high variability within samples, PAW treatment allowed for an increase in the average value of both GEN and GC. The difference in germination energy was evident for PAW after 5 min of plasma treatment, for which the fraction of germinated seeds increased by 16%, performing better than sodium hypochlorite treatment. Similar GEN results were obtained for a time of 10 min plasma treatment of water; however, this treatment time also allowed for an increase in GC, as opposed to PAW5’, which had a shorter treatment time that accelerated initial growth followed by no germination of the remaining seeds. The best results were obtained for PAW with a treatment time of 20 min, for which all seeds from all samples germinated after 7 days. In the case of beetroot seeds, the highest ratio was obtained with the application of water treated with plasma for 20 min; this was also the case for carrot seeds in combination with sodium hypochlorite.
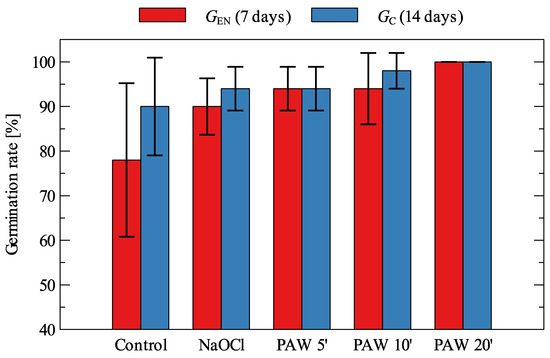
Figure 2.
Germination of Beta vulgaris (mean values and standard deviation).
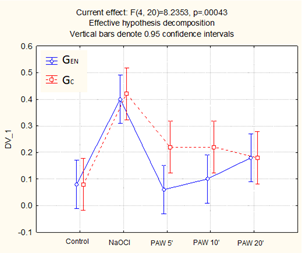
The germination results obtained for Daucus carota are shown in Figure 3.
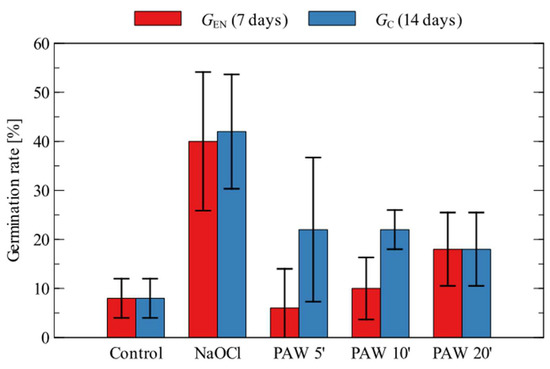
Figure 3.
Germination of Daucus carota (mean values and standard deviation).
For the tested seeds, the germination of the control samples was low and did not exceed 10% even after 14 days. As the plasma treatment time increased, an increase in GEN was evident, which more than doubled for a time of 20 min. However, the best results at the end of the test (14 days) were obtained for PAW, which undergone plasma treatment for shorter times (5 and 10 min), where the average GC value was almost three times higher than the control sample. For the tested seeds, the best results were obtained for the sodium hypochlorite treatment, although the results obtained from the different samples varied quite significantly.
3.2. Sprout Length
In the case of the total length of sprouts, the highest results were obtained in both plant species for seeds treated with sodium hypochlorite.
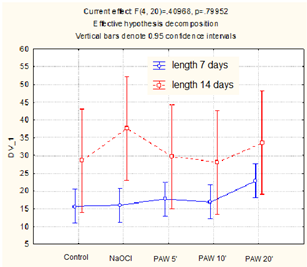
The average sprout lengths from each experimental condition for Beta vulgaris are shown in Figure 4.
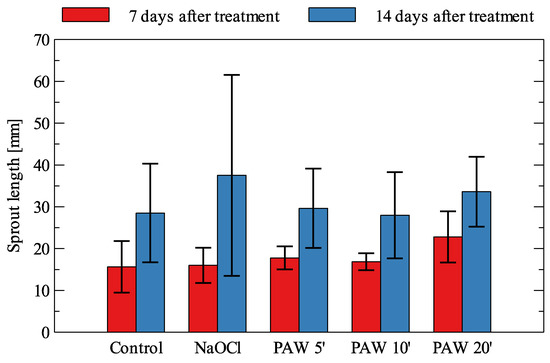
Figure 4.
Sprout length of Beta vulgaris (mean values and standard deviation).
When comparing mean sprout lengths, after 7 days, each treatment type allowed greater lengths, with a slightly greater advantage for PAW with 20 min plasma treatment (a 46% increase over the control). After 14 days, the differences for the plasma treatment are less noticeable (4 to 17% increase over the control), with a large increase in length for the sodium hypochlorite treatment (32% increase with a relatively large difference between the individual samples). As for the sprouts’ production at the 7th day of observation, application of PAW5’ produced a visible improvement in the germination process. With an extension in the observation time, the stimulation effect became weaker, and for 14 days was clearly visible only for 20 min-treated PAW and NaOCl.
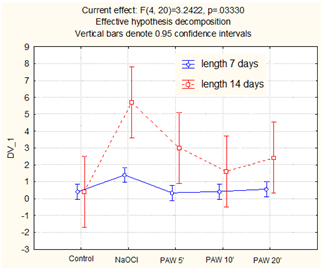
The results obtained for Daucus carota are shown in Figure 5.
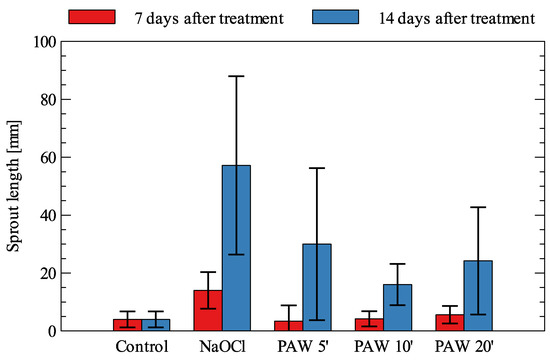
Figure 5.
Sprout length of Daucus carota (mean values and standard deviation).
These seeds react in a slightly different manner than Beta vulgaris as a very weak stimulative effect can be observed after 7 days of treatment; the best results were obtained for sodium hypochlorite (250% increase), while PAW treatment was again the most effective, with the longest water treatment time with plasma (40% increase compared to the control). Such a PAW still had relatively low concentration of H2O2, with decontaminative properties and a reasonably high concentration of nitrites with known antimicrobial properties, especially at the low pH, which together boosted plant’s growth and inhibited the development of certain fungal colonies. Nitrites are known for their fertilizing properties and support further plant development. After 14 days, a very significant increase in sprout length was noticeable for all types of treatment. For NaOCl, this was more than a thirteenfold increase over the control. For PAW treatment, the best effect was obtained for the shortest time of PAW treatment with plasma (650% increase), followed by 20 and 10 min of treatment (505% and 300% increase, respectively), which could be explained by the attaining of a kind of balance between RONS and pH, with the location of the above factors in a plant environmental tolerance zone. On the other hand, the germination boost that was clearly visible for NaOCl treatment could be explained by a strong correlation between the germination process and fungal decontamination, which is described in the next chapter.
3.3. Statistical Analysis
Results of statistical analysis are depicted in Table 3 and Table 4. The best results, with a statistically significant change in the case of Beta vulgaris, were obtained for PAW, with a treatment time of 20 min, for which all seeds, from all samples, germinated after 7 days. For the tested Daucus carota seeds, the best results, with a statistically significant change, were obtained for the sodium hypochlorite treatment, although the results obtained from the different samples varied quite significantly.

Table 3.
Statistical analysis of germination results. The letter indicators next to the means determine the statistically homogeneous groups.

Table 4.
Results of statistical analysis.
3.4. Fungi on the Seed Surface
Fungal infestations constitute a persistent problem for improperly stored seeds, which brings losses of the seeding material, generates additional costs and is inappropriate from a sustainable ecology point of view. During our observation of basic microbiota present in the examined seeds, certain trends were observed when assessing the infestation of seeds of selected species by fungi. The seeds were highly contaminated. With longer GAD application times to water, a reduction in the number of fungi inhabiting the seeds can be observed. A total of 13 fungal species were identified in Beta vulgaris. Colony counts for each species and treatment condition are summarized in Table 5.

Table 5.
Fungal species on Beta vulgaris.
In total, the largest numbers of colonies were observed for Epicoccum nigrum, Clonostachys rosea and Alternaria alternata. Compared to the control, all PAW treatments eliminated fungi such as Botrytis cinerea, Fusarium solani and Truncatella truncata with the same efficiency as the sodium hypochlorite treatment. For Aspergillus niger, the reduction occurred only for PAW with a treatment time of 10 min. On the other hand, for some treatment times, more colonies of Alternaria alternata, Epicoccum nigrum and Penicillium expansum were observed.
The results obtained for Daucus carota are summarized in Table 6.

Table 6.
Fungal species on Daucus carota.
The highest number of colonies was observed for the Alternaria alternata species, which was completely resistant to PAW treatment but was entirely removed by NaOCl. On the other hand, high numbers of colonies were observed for the species Cladosporium sp. and Stemphylium botryosum, which were only detected for the treatment with sodium hypochlorite. This may result from the surface effect of PAW, the relatively low concentration of disinfective hydrogen peroxide, and also from high primary contamination of seeds, especially carrots, as a result of improper storage. PAW treatment resulted in a complete reduction in Alternaria radicina and Trichoderma harzianum, which were present in both control and NaOCl treated samples. It also caused a slight increase in Chaetomium cochliodes, Fusarium avenaceum, Mucor mucedo, Penicillium expansum and Trichoderma koningii, which usually occurred for longer processing times. In the case of the cheapest treatment option for the feed gas (air), the RONS composition present in plasma activated water had mild fungitoxic effects. Such a treatment strongly depended on the fungal species; thus, positive point is a kind of treatment selectivity, which can be achieved using PAW. It has to be pointed out that PAW reveals basic surface activity. However, in some cases, high contamination, with the presence of pathogens that are located in the deeper zones of seeds, also cannot be removed by traditional chemical treatment techniques such as NaOCl.
3.5. Surface Imaging Using an Optical Microscope and SEM
In order to emphasize the differences between two types of tested seeds and also the differences visible in the obtained results, the seeds’ structures were described on the basis of the literature data, and microscopic analyses were performed. Beta vulgaris belongs to the complex Eudicots, with a perispermic seed structure. In this case, water and oxygen penetration can be limited by thick, hard pericarp tissue surrounding the internal botanical seed. Morphologically, an ovary cap with the remnants of the stigma covers the pericarp’s upper part. On the opposite side of the seed, the basal pore is located in a position that allows for water intake. Pericarp consists of three layers with different sizes of crystalized chemical compounds, which tend to be bigger with depth. The first of two dense internal sclerenchymal layers is formed of small, multilayer-wall sclereids, followed by the second layer of thinner cell wall sclereids with crystal clusters inside, and the rather loose parenchyma cells located externally. The analysis of water extract from the pericarp revealed predominating cations such as potassium, sodium, magnesium, calcium, chlorine, sulphur and anions with nitrate, phosphate, chloride and sulphate oxalate ions dominating, which form the osmotic potential within pericarp, which may cause delays in the seed germination process [58,59,60,61,62,63].
When using both the optical microscope and the scanning electron microscope (Figure 6 and Figure 7), imaged samples are characterized by oval shaped parenchymal cells, of random size, with a very developed, concaved structure. The cells are not densely packed and intercellular spaces can be observed. After the PAW treatment, the structure became folded and wrinkled, and also revealed signs of rupture in comparison to the control sample. Changes are noticeable for PAW with 5 min of plasma treatment. On the other hand, besides the undulating structure, longer times resulted in the flattening of the outer edges of the cells.
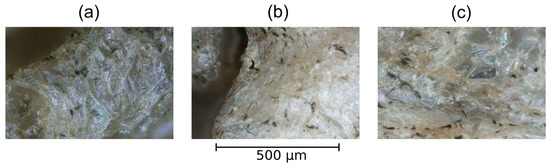
Figure 6.
Optical microscope images of Beta vulgaris at 500× magnification. (a) Control (water); (b) PAW 5′; (c) PAW 20′.
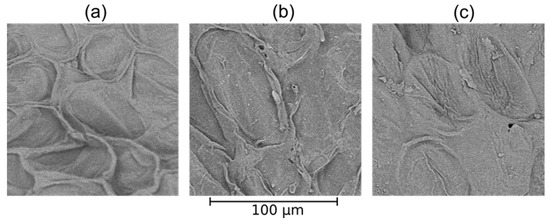
Figure 7.
SEM images of Beta vulgaris at 1000× magnification. (a) Control (water); (b) PAW 5′; (c) PAW 20′.
Carrot seeds are relatively small and they belong to the schizocarpic fruits. Their external layer is formed by a thin seed coat and it has overhanging beards. Pericarp is partially joined to the seed and consists of the epicarp, mesocarp and endocarp. The bulk of the seed incorporates thick-walled endosperm tissue embodying an embryo [64,65,66].
As shown in Figure 8, on the weavy-ribbed top structure of the coat, the distinctive elongating beards adjoined to the ribs are visible in the control sample. PAW treatment caused visible changes such as enhanced swelling of the ribs and removal of beards. Beard removal is also a common process during the scarification process of the carrot seeds, which takes place in order to enhance germination and to save space during the seed packaging process.
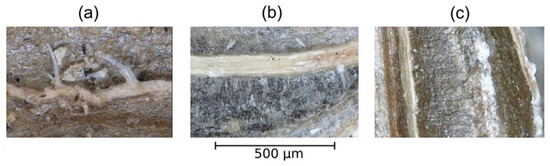
Figure 8.
Optical microscope images of Daucus carota at 500× magnification. (a) Control (water); (b) PAW 5′; (c) PAW 20′.
Previous studies with the GAD reactor indicated that high concentrations of NOx can be generated in the gas phase and further transported to the liquid phase, where compounds such as H2O2 also appear through secondary reactions [50]. In the case of PAW treatment of seeds, these compounds can cause an increase in germination, e.g., through H2O2 accumulation [48,49]. The increase in germination may also be related to other effects of plasma treatment on seeds, such as surface etching and increased water absorption [67,68,69], or inactivation of harmful organisms [16,70,71]. Many of these effects can also affect seeds through active particles found in PAW, e.g., via H2O2 activating the catalase enzyme for the synthesis of new proteins [26], increases in chlorophyll content [72], the cracking of hard seeds, thus allowing them to absorb moisture [73] or the enhancement of nitrogen contents in the seeds through adsorption, diffusion and trapping of RNS produced in plasmas [74]. Their effect can not only be an increase in germination, but also an increase in the quality of the plants grown and their levels of disease tolerance [75,76].
We speculate that the results of seeds’ imbibition in PAW, especially with the presence of RONS and low pH, changed the osmotic potential and made the seed surface more prone to structural changes.
On the basis of the results, it can be concluded that when using the same reactor and the same treatment conditions, the results largely depend on the type of treated seeds. In the case of Beta vulgaris, the effect of PAW treatment is evident in both the change of microflora, germination parameters and surface structure of the tested seeds. Due to the higher rate of germination (GEN), sprouts of greater length were obtained in addition to a larger fraction of germinated seeds. Because of the relatively small decontamination effect of fungi whose specific species were restricted or stimulated, the gradual increase in treatment efficiency with increasing treatment time may be related to the change in surface structure observed via optical and scanning electron microscopy. In the case of Daucus carota, characterized by other types of fungi, a complete reduction in Alternaria radicina and Trichoderma harzianum species, which were not removed during the classical decontamination method with sodium hypochlorite, can be observed. Compared to the control, significantly better germination and sprout length parameters were obtained even for shortest time, but these were still worse than the results obtained for traditional treatment with NaOCl. In combination with the overall better decontamination effect of sodium hypochlorite, it can be thought that for carrot seeds, the decisive influence on germination was connected with fungi on the surface, which is better removed by the traditional method. However, the use of PAW may still offer a competitive alternative due to the ecology and economy of plasma treatment with the investigated device, for which only electricity and commonly available air are needed. PAW can be also generated in larger quantities in devices of different constructions [77,78,79], which can further improve the cost factor.
4. Conclusions
Plasma activated water positively influenced the seeds germination process; however, differences between the plant species, in terms of the seeds’ response, were observed. Longer PAW imbibition times resulted in better germination parameters in comparison to the control sample. For Beta vulgaris, treatment times of 10 and 20 min also allowed a higher fraction of germinating seeds than NaOCl treatment, with only slightly shorter average sprout lengths. For Daucus carota, the germination rate and sprout length were both noticeably lower than for sodium hypochlorite solution, but still several times higher compared to the control.
Plasma activated water had an impact on the composition of fungal species inhabiting Beta vulgaris and Daucus carota seeds. Fungal species responded to the PAW treatment in different manners; some of them were unaffected. The highest counts isolated were assigned to the harmful producers of mycotoxins such as Alternaria alternata and moulds of the genus Penicillium. The beneficial species were dominated by Epicoccum nigrum, Clonostachys rosea and Trichoderma sp.
Author Contributions
Conceptualization: J.P., M.K. (Marek Kopacki) and K.H.; methodology: J.P., M.K. (Michał Kwiatkowski), P.T., M.K. (Marek Kopacki) and K.H.; investigation: J.P., P.T., M.K. (Michał Kwiatkowski), M.K. (Marek Kopacki) and K.H. data curation: P.T., M.K. (Marek Kopacki) and J.P., writing—original draft preparation: P.T. and J.P.; writing—review and editing: P.T., J.P. and M.K. (Marek Kopacki); visualization: J.P., M.K. (Michał Kwiatkowski) and P.T.; supervision: J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Polish–Slovak Bilateral Cooperation Programme (PlasmaBioAgro) PPN/BIL/2018/1/00065+SK-PL-18-0090 and the LUT research fund (FD-20/EE-2/418).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Eng. Łukasz Kaca for the technical assistance during selected research tasks and Łukasz Remez from PIK INSTRUMENTS Sp.z.O.O for the SEM documentation. We acknowledge fruitful discussion with COST Action PlAgri CA19110 and CEEPUS CIII-AT-0063 members.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight Principles of Integrated Pest Management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Jamiolkowska, A.; Hetman, B.; Skwarylo-Bendarz, B.; Kopacki, M. Integrowana ochrona roślin w Polsce i Unii Europejskiej oraz prawne podstawy jej funkcjonowania. Praca przeglądowa. Ann. Univ. Mariae Curie-Skłodowska Sect. E Agric. 2017, 72. [Google Scholar] [CrossRef]
- Mazur-Wierzbicka, E. The Application of Corporate Social Responsibility in European Agriculture. Misc. Geogr. 2015, 19, 19–23. [Google Scholar] [CrossRef]
- Gamliel, A. Application Aspects of Integrated Pest Management. J. Plant Pathol. 2010, 92, S23–S26. [Google Scholar]
- Brühl, C.A.; Zaller, J.G. Biodiversity Decline as a Consequence of an Inappropriate Environmental Risk Assessment of Pesticides. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Valiuškaitė, A.; Uselis, N.; Kviklys, D.; Lanauskas, J.; Rasiukevičiūtė, N. The Effect of Sustainable Plant Protection and Apple Tree Management on Fruit Quality and Yield. Zemdirb. Agric. 2017, 104. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide Productivity and Food Security. A Review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for Medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive Species in Non-Equilibrium Atmospheric-Pressure Plasmas: Generation, Transport, and Biological Effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, L.; Medvecká, V.; Zahoranová, A.; Ševčovičová, A.; Gálová, E. Genotoxic Effect of Low Temperature Plasma Treatment on Plant Seeds. Toxicol. Lett. 2017, 280, S119. [Google Scholar] [CrossRef]
- Rodriguez, C.; Wandell, R.J.; Zhang, Z.; Neurohr, J.M.; Tang, Y.; Rhodes, R.; Kinsey, S.T.; Locke, B.R. Escherichia Coli Survival in Plasma-Treated Water and in a Gas–Liquid Plasma Reactor. Plasma Process. Polym. 2020, 17, 2000099. [Google Scholar] [CrossRef]
- Sera, B.; Špatenka, P.; Šerý, M.; Vrchotova, N.; Hrušková, I. Influence of Plasma Treatment on Wheat and Oat Germination and Early Growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Matra, K. Atmospheric Non-Thermal Argon–Oxygen Plasma for Sunflower Seedling Growth Improvement. Jpn. J. Appl. Phys. 2017, 57, 01AG03. [Google Scholar] [CrossRef]
- Kordas, L.; Pusz, W.; Czapka, T.; Kacprzyk, R. The Effect of Low-Temperature Plasma on Fungus Colonization of Winter Wheat Grain and Seed Quality. Pol. J. Environ. Stud. 2015, 24, 433–438. [Google Scholar]
- Ndiffo Yemeli, G.B.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The Effect of Water Activated by Nonthermal Air Plasma on the Growth of Farm Plants: Case of Maize and Barley. Plasma Process. Polym. 2020, 18, 2000205. [Google Scholar] [CrossRef]
- Takaki, K.; Hayashi, N.; Wang, D.; Ohshima, T. High-Voltage Technologies for Agriculture and Food Processing. J. Phys. Appl. Phys. 2019, 52, 473001. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Žigon, D.; Kovač, J.; Jurov, A.; Dickenson, A.; Walsh, J.L.; Cvelbar, U. Cold Atmospheric Pressure Plasma-Assisted Removal of Aflatoxin B1 from Contaminated Corn Kernels. Plasma Process. Polym. 2021, 18, 2000163. [Google Scholar] [CrossRef]
- Dufour, T.; Gutierrez, Q.; Bailly, C. Sustainable Improvement of Seeds Vigor Using Dry Atmospheric Plasma Priming: Evidence through Coating Wettability, Water Uptake, and Plasma Reactive Chemistry. J. Appl. Phys. 2021, 129, 084902. [Google Scholar] [CrossRef]
- Măgureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-Thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Judée, F.; Dufour, T. Seed-Packed Dielectric Barrier Device for Plasma Agriculture: Understanding Its Electrical Properties through an Equivalent Electrical Model. J. Appl. Phys. 2020, 128, 044901. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Groot, G.J.J.B. de Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Hou, J.; Shao, H.; Dong, Y.; Jiang, J. Improving Seed Germination and Peanut Yields by Cold Plasma Treatment. Plasma Sci. Technol. 2016, 18, 1027–1033. [Google Scholar] [CrossRef]
- Silva, D.L.S.D.; Farias, M.D.L.; Vitoriano, J.D.O.; Alves Júnior, C.; Torres, S.B. Use of Atmospheric Plasma in Germination of Hybanthus Calceolaria (l.) Schulze-Menz Seeds. Rev. Caatinga 2018, 31, 632–639. [Google Scholar] [CrossRef]
- Puač, N.; Škoro, N.; Spasić, K.; Živković, S.; Milutinović, M.; Malović, G.; Petrović, Z.L. Activity of Catalase Enzyme in Paulownia Tomentosa Seeds during the Process of Germination after Treatments with Low Pressure Plasma and Plasma Activated Water. Plasma Process. Polym. 2018, 15, 1700082. [Google Scholar] [CrossRef]
- Zambon, Y.; Contaldo, N.; Laurita, R.; Várallyay, E.; Canel, A.; Gherardi, M.; Colombo, V.; Bertaccini, A. Plasma Activated Water Triggers Plant Defence Responses. Sci. Rep. 2020, 10, 19211. [Google Scholar] [CrossRef]
- Terebun, P.; Kwiatkowski, M.; Starek, A.; Reuter, S.; Mok, Y.S.; Pawłat, J. Impact of Short Time Atmospheric Plasma Treatment on Onion Seeds. Plasma Chem. Plasma Process. 2021, 41, 559–571. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wannicke, N.; Horn, S.; Weltmann, K.-D.; Brust, H. A Coaxial Dielectric Barrier Discharge Reactor for Treatment of Winter Wheat Seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]
- Veerana, M.; Choi, E.H.; Park, G. Influence of Non-Thermal Atmospheric Pressure Plasma Jet on Extracellular Activity of α-Amylase in Aspergillus Oryzae. Appl. Sci. 2021, 11, 691. [Google Scholar] [CrossRef]
- Watanabe, S.; Ono, R.; Hayashi, N.; Shiratani, M.; Tashiro, K.; Kuhara, S.; Inoue, A.; Yasuda, K.; Hagiwara, H. Growth Enhancement and Gene Expression of Arabidopsis Thaliana Irradiated with Active Oxygen Species. Jpn. J. Appl. Phys. 2016, 55, 07LG10. [Google Scholar] [CrossRef]
- Pawłat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of Atmospheric Pressure Plasma Jet Operating with DBD on Lavatera Thuringiaca L. Seeds’ Germination. PLOS ONE 2018, 13, e0194349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, J.; Dong, Y. Effect of Cold Plasma Treatment on Seedling Growth and Nutrient Absorption of Tomato. Plasma Sci. Technol. 2018, 20, 044007. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of Cold Radiofrequency Plasma with Seeds of Beans (Phaseolus Vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef]
- Li, L.; Guo, H.; Zong, J.; Chen, J.; Wang, Y.; LI, J.; LI, D.; SHAO, H.; LIU, J. Influence of Low-Vacuum Helium Cold Plasma Pre-Treatment on the Rooting and Root Growth of Zoysiagrass (Zoysia Willd.) Stolon Cuttings. Plasma Sci. Technol. 2019, 21, 055504. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–Liquid Interactions: A Review and Roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Kelar Tučeková, Z.; Vacek, L.; Krumpolec, R.; Kelar, J.; Zemánek, M.; Černák, M.; Růžička, F. Multi-Hollow Surface Dielectric Barrier Discharge for Bacterial Biofilm Decontamination. Molecules 2021, 26, 910. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of Plasma-Activated Water with Biofilms: Inactivation, Dispersal Effects and Mechanisms of Action. Npj Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.H.; Noh, H.; Ahn, G.R.; Kim, S.H.; Kaushik, N.K.; Choi, E.H.; Lee, G.J. Influence of Nonthermal Atmospheric Plasma-Activated Water on the Structural, Optical, and Biological Properties of Aspergillus Brasiliensis Spores. Appl. Sci. 2020, 10, 6378. [Google Scholar] [CrossRef]
- Kučerová, K.; Machala, Z.; Hensel, K. Transient Spark Discharge Generated in Various N2/O2 Gas Mixtures: Reactive Species in the Gas and Water and Their Antibacterial Effects. Plasma Chem. Plasma Process. 2020, 40, 749–773. [Google Scholar] [CrossRef]
- Janda, M.; Martišovitš, V.; Hensel, K.; Machala, Z. Generation of Antimicrobial NOx by Atmospheric Air Transient Spark Discharge. Plasma Chem. Plasma Process. 2016, 36, 767–781. [Google Scholar] [CrossRef]
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357. [Google Scholar] [CrossRef]
- Gamaleev, V.; Iwata, N.; Ito, G.; Hori, M.; Hiramatsu, M.; Ito, M. Scalable Treatment of Flowing Organic Liquids Using Ambient-Air Glow Discharge for Agricultural Applications. Appl. Sci. 2020, 10, 801. [Google Scholar] [CrossRef]
- Park, D.; Dobrynin, D.; Fridman, G.; Fridman, A. Effects of Plasma Treated Water on Plants. In Proceedings of the 2012 Abstracts IEEE International Conference on Plasma Science, Edinburgh, UK, 8–13 July 2012. [Google Scholar]
- Laurita, R.; Contaldo, N.; Zambon, Y.; Bisag, A.; Canel, A.; Gherardi, M.; Laghi, G.; Bertaccini, A.; Colombo, V. The Use of Plasma-Activated Water in Viticulture: Induction of Resistance and Agronomic Performance in Greenhouse and Open Field. Plasma Process. Polym. 2021, 18, 2000206. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, S.; Xiang, Q.; Lyu, Y.; Shen, R. Effect of Plasma-Activated Water on the Microbial Decontamination and Food Quality of Thin Sheets of Bean Curd. Appl. Sci. 2019, 9, 4223. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Bačovčinová, M.; Hensel, K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Appl. Sci. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of Plasma-Activated Water Treatment on Seed Germination and Growth of Mung Bean Sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma Activated Water: The next Generation Eco-Friendly Stimulant for Enhancing Plant Seed Germination, Vigor and Increased Enzyme Activity, a Study on Black Gram (Vigna Mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Pawłat, J.; Terebun, P.; Kwiatkowski, M.; Tarabová, B.; Kovaľová, Z.; Kučerová, K.; Machala, Z.; Janda, M.; Hensel, K. Evaluation of Oxidative Species in Gaseous and Liquid Phase Generated by Mini-Gliding Arc Discharge. Plasma Chem. Plasma Process. 2019, 39, 627–642. [Google Scholar] [CrossRef]
- Pawłat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of Atmospheric Pressure Plasma Generated in GlidArc Reactor on Lavatera Thuringiaca L. Seeds’ Germination. Plasma Process. Polym. 2018, 15, 1700064. [Google Scholar] [CrossRef]
- International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2017.
- Kopacki, M.; Parafiniuk, S.; Skwaryło-Bednarz, B. Fungi Colonizing Chrysanthemums Plants Cultivated in the Field in Different Protection Systems. Fresenius Environ. Bull. 2018, 27, 2751–2760. [Google Scholar]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, Surrey, 1971. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 3rd ed.; Burgess Publishing Company: Minneapolis, MN, USA, 1972. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi; Academic Press: London, UK, 1980; Volume 1, ISBN 978-0-12-220401-2. [Google Scholar]
- Marcinkowska, J. Oznaczanie Rodzajów Grzybów Sensu Lato Ważnych w Fitopatologii; Powszechne Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2012. [Google Scholar]
- Chomontowski, C.; Podlaski, S. Impact of Sugar Beet Seed Priming Using the SMP Method on the Properties of the Pericarp. BMC Plant Biol. 2020, 20, 32. [Google Scholar] [CrossRef]
- Hermann, K.; Meinhard, J.; Dobrev, P.; Linkies, A.; Pesek, B.; Heß, B.; Macháčková, I.; Fischer, U.; Leubner-Metzger, G. 1-Aminocyclopropane-1-Carboxylic Acid and Abscisic Acid during the Germination of Sugar Beet (Beta Vulgaris L.): A Comparative Study of Fruits and Seeds. J. Exp. Bot. 2007, 58, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Yamamoto, R. The Growth Inhibitors in Sugar Beet Seed Balls: II. Isolation of Potassium Nitrate as the Germination Inhibitor and Hypocotyl Stimulating Substance. Jpn. J. Crop Sci. 1975, 44, 465–470. [Google Scholar] [CrossRef][Green Version]
- Ignatz, M.; Hourston, J.E.; Turečková, V.; Strnad, M.; Meinhard, J.; Fischer, U.; Steinbrecher, T.; Leubner-Metzger, G. The Biochemistry Underpinning Industrial Seed Technology and Mechanical Processing of Sugar Beet. Planta 2019, 250, 1717–1729. [Google Scholar] [CrossRef]
- Inoue, K.; Yamamoto, R. The Growth Inhibitors in Sugar Beet Seed Balls: I. Isolation of Mono-Sodium Oxalate as a Root Growth Inhibitor. Jpn. J. Crop Sci. 1974, 43, 439–444. [Google Scholar] [CrossRef][Green Version]
- Junttila, O. Germination Inhibitors in Fruit Extracts of Red Beet (Beta Vulgaris Cv. Rubra). J. Exp. Bot. 1976, 27, 827–836. [Google Scholar] [CrossRef]
- Dawidowicz-Grzegorzewska, A. Ultrastructure of Carrot Seeds during Matriconditioning with Micro-Cel E. Ann. Bot. 1997, 79, 535–545. [Google Scholar] [CrossRef][Green Version]
- Miranda, R.M.; Dias, D.C.; Picoli, E.A.; Silva, P.P.; Nascimento, W.M. Physiological Quality, Anatomy and Histochemistry during the Development of Carrot Seeds ( Daucus Carota L.). Ciênc. E Agrotecnologia 2017, 41, 169–180. [Google Scholar] [CrossRef]
- Bercu, R.; Broască, L. Comparative Histoanatomical Aspects of the Fruit of Some Apiaceae Lindl. Fruit Used for Therapeutic Purposes. Ann. Romanian Soc. Cell Biol. 2012, 17, 265–270. [Google Scholar]
- Alves Junior, C.; de Oliveira Vitoriano, J.; da Silva, D.L.S.; de Lima Farias, M.; de Lima Dantas, N.B. Water Uptake Mechanism and Germination of Erythrina Velutina Seeds Treated with Atmospheric Plasma. Sci. Rep. 2016, 6, 33722. [Google Scholar] [CrossRef]
- Kitazaki, S.; Sarinont, T.; Koga, K.; Hayashi, N.; Shiratani, M. Plasma Induced Long-Term Growth Enhancement of Raphanus Sativus L. Using Combinatorial Atmospheric Air Dielectric Barrier Discharge Plasmas. Curr. Appl. Phys. 2014, 14, S149–S153. [Google Scholar] [CrossRef]
- Ji, S.-H.; Choi, K.-H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; kyung Jung, S.; Choi, E.-H.; et al. Effects of High Voltage Nanosecond Pulsed Plasma and Micro DBD Plasma on Seed Germination, Growth Development and Physiological Activities in Spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Starek, A.; Sagan, A.; Andrejko, D.; Chudzik, B.; Kobus, Z.; Kwiatkowski, M.; Terebun, P.; Pawłat, J. Possibility to Extend the Shelf Life of NFC Tomato Juice Using Cold Atmospheric Pressure Plasma. Sci. Rep. 2020, 10, 20959. [Google Scholar] [CrossRef]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea Mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-Activated Water Production and Its Application in Agriculture. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Rashid, M.M.; Reza, M.A.; Talukder, M.R. Combined Effects of Air Plasma Seed Treatment and Foliar Application of Plasma Activated Water on Enhanced Paddy Plant Growth and Yield. Plasma Chem. Plasma Process. 2021, 41, 1081–1099. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Zhou, R.; Zhang, X.; Yang, S. Atmospheric-Pressure Plasma Treated Water for Seed Germination and Seedling Growth of Mung Bean and Its Sterilization Effect on Mung Bean Sprouts. Innov. Food Sci. Emerg. Technol. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The Effects of Plasma on Plant Growth, Development, and Sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Čech, J.; Sťahel, P.; Ráheľ, J.; Prokeš, L.; Rudolf, P.; Maršálková, E.; Maršálek, B. Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria. Water 2020, 12, 3167. [Google Scholar] [CrossRef]
- Ihara, S.; Sakai, T.; Yoshida, Y.; Nishiyama, H. Fundamental Characteristics of Discharge Plasma Generated in a Water Cavitation Field. J. Electrost. 2018, 93, 110–117. [Google Scholar] [CrossRef]
- Ihara, S.; Nishiyama, H.; Matsunaga, T.; Yoshida, Y.; Tokuyama, Y.; Terato, H. Improving the Efficiency of a Water-Treatment System Based on Water Cavitation and Plasma Using a Nozzle-Less Reactor. AIP Adv. 2019, 9, 045005. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).