Abstract
The presence of free lime and periclase in converter slag prevents it from directly being utilized as a source of concrete aggregate due to the deleterious expansion caused by reaction of free lime and periclase. In general, a six-month aging period is suggested in order to avoid any potential problems, but it is a tedious process that requires a long period of time. In this work, supercritical CO2 (SCD) was used to reduce the aging period down to one day or so. Since SCD creates a more aggressive environment for reaction, it can directly attack the surface of free lime and periclase to induce expansive reaction before using converter slag as concrete aggregate. According to the experimental results, the surface of converter slag was successfully carbonated by SCD, showing 7.80% of CO2 uptake by weight of converter slag. It was found that the mortar bar made of reacted converter slag reduced the amount of expansion. The 28-day compressive strength of mortar with converter slag was slightly reduced after reaction with SCD, but it was still 32% higher than mortar made of conventional sand.
1. Introduction
According to the World Steel Association, the global crude steel production in 2019 was 1869.9 million tons, and it has been increasing every year [1]. Large amounts of by-product that have been generated from steelmaking business need to be properly utilized/disposed in order to minimize the environmental impact. Blast furnace slag, generated in a blast furnace that manufactures pig iron from iron ore, is pulverized and successfully recycled as a high value-added supplementary cementitious material (Ground granulated blast-furnace slag, GGBFS) [2,3,4,5,6,7]. However, steelmaking slag is mostly used as a low-value-added landfill material such as road pavement and embankment [8,9]. It should be noted that recycling of such waste materials also reduces the burden for CO2 emission from steelmaking industry [10].
Steelmaking slag usually refers to various types of slags that were generated in the process of refining steel from pig iron. Most common types of steelmaking slags are converter slag and electric arc furnace slag. The amount of production and the chemical composition of those slags can vary depending on the properties of raw materials to be injected, properties (or types) of steel that will be produced, and the processing technology that was applied [11]. In most cases, since the steelmaking slag undergoes a rapid cooling process, there is not enough time for cations such as Ca and Mg present in the slag to be stably fixed inside the silicate structure during the smelting process. This means that some amount of Ca and Mg exists as in the form of free lime (CaO) and periclase (MgO), and thus causes harmful expansion by formation of portlandite (Ca(OH)2) or brucite (Mg(OH)2) when used as aggregates for concrete [11,12]. Converter slag and electric arc furnace oxidation slag are those that are known to cause harmful expansion, so they are restricted from being utilized as an aggregate for concrete.
Previous studies that evaluated the stability of converter slag as concrete aggregate reported that converter slag could be used as an aggregate if the reaction of the expandable material was induced in advance [13,14]. There was a report that recommends a minimum of three months for aging period in order to be utilized as aggregates for bituminous materials [15]. According to United States Geological Survey (USGS), it is recommended to undergo an aging process of six months to use steelmaking slag as a road-based aggregate. [16,17]. From the earlier works, three months of aging period was not found to be enough to provide stability of steelmaking slag (combination of electric arc furnace oxidation slag and converter slag) as concrete aggregate [18]. Considering all earlier findings, it is necessary for converter slag to spend six months of aging process in order to avoid any future problem associated with expansive reaction. However, such process takes too long time, and requires additional space for extended storage period.
In this work, a method that can process converter slag in shorter period of time is investigated. It is to utilize carbonation by CO2 in supercritical condition. Since the viscosity and density of supercritical carbon dioxide (SCD) are similar to those of the gaseous state, and the mobility is higher than that of the liquid state, it is known to cause a more aggressive carbonation process [19]. In fact, utilizing carbonation for enhancing properties of recycled aggregate has been an interesting topic for some time [20,21], but the application of supercritical CO2 was limited. In earlier works on the reaction of recycled aggregate and waste concrete with SCD [19,22,23,24], it was reported that the calcium hydroxide inside the concrete reacts completely within a short period (within 1 day), and calcium carbonate was also formed to be produced from dissociation of calcium ions present in the C-S-H [19,22,23,24]. It is proposed that similar mechanism can be applied to stabilize converter slag because most of its components are alkali oxides and silicates.
Utilization of SCD has two significant advantages: (1) permanent mineralization of greenhouse gas to contribute global warming issue and (2) rapid attack on structure of silicates as well as that of alkali oxides and hydroxides to form alkali carbonates. Due to the latter characteristic, moisture and CO2 can penetrate into the spaces generated by the elution of metal cations, and SCD will directly attack the surface of free lime and periclase. Either the hydration or direct carbonation of free CaO (lime) and MgO (periclase) can be induced and cause expansion [25,26], which will eliminate the delayed expansion of the converter slag when used as concrete aggregate. The application of SCD will also significantly reduce the six-month aging period down to one day or so.
Considering the recent depletion of natural aggregates in the construction industry and a strong demand for finding alternative resources, converter slag that was reacted with SCD can be a good option to provide a stable source of supply for the aggregate. In order to meet such objective, an experimental program has been prepared. Converter slag was reacted with SCD, and changes in mineralogical compositions as well as microstructure was investigated. In order to prove contribution towards global warming, the amount of CO2 uptake was calculated from the results of thermogravimetric analyses. Mortar specimens were prepared to test (1) stability against expansion and (2) strength development to prove applicability of SCD for stabilizing converter slag.
2. Experimental Procedures
2.1. Materials
The cement used was ASTM Type I Ordinary Portland Cement (OPC). The converter slag with unknown degree of aging was used for this work. Table 1 shows the chemical compositions of OPC and converter slag.

Table 1.
Chemical compositions of Ordinary Portland Cement (OPC) and Converter slag (%).
The CaO content of converter slag was found to be 54.11%. Since there is no standard available for converter slag to be used as aggregate for concrete, the standard specification established for electric arc furnace oxidation slag was used for evaluation on chemical stability of converter slag. According to the Korean industrial standard KS F 2527 “Concrete Aggregate” [27], the electric furnace oxidation slag needs to contain a CaO content of 40% or less and a basicity (CaO/SiO2) ratio of 2.0 or less in order to be used as concrete aggregate. It was found that the converter slag used in this study did not satisfy both criteria. This indicates the converter slag that is used in this work is considered dangerous to be used as aggregate for concrete if no additional separate treatment process is applied. Photographic image of converter slag is shown in Figure 1a.

Figure 1.
Photographic images of converter slag (a) before and (b) after reaction with supercritical carbon dioxide (SCD).
2.2. Supercritical Carbon Dioxide Reaction
It is known that carbon dioxide becomes a supercritical state at 31.1 °C and 73.8 bar [28,29]. In the earlier works, a testing condition of 50 °C and 100 bar was mostly used [19,22,30], but in this work, testing condition of 80 °C and 100 bar was used because higher temperature is expected to raise degree of carbonation from converter slag. Figure 2 shows the reaction process with converter slag and SCD. After placing 10 g of converter slag with 20 g of ultrapure water (18.3 MΩ·cm) into a pressure cell, the cap of the cell was tightly closed and CO2 was injected to meet the pressure level of 100 bar using a syringe pump (Teledyne Isco, Lincoln, NE, USA). The cell was then stored at 80 °C oven for 24 h. After 24 h, the converter slag was removed from the pressure cell and dried in an oven at 50 °C for 24 h. Photographic image of converter slag after reaction with SCD is shown in Figure 1b. There were no clear changes observed in the shape of converter slag after reaction with SCD.
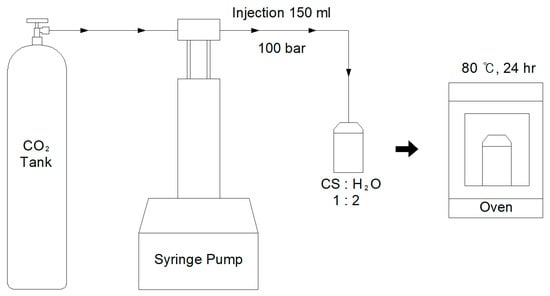
Figure 2.
Experimental setup for reaction between converter slag and supercritical CO2. CS in figure indicates converter slag.
2.3. X-ray Powder Diffraction (XRD)
Changes in mineralogical compositions of converter slag before and after the reaction with SCD were analyzed by X-ray powder diffraction (XRD). Converter slag samples were placed in the X’Pert3-Powder X-ray diffractometer (PANalytical, Malvern, UK) equipped with Cu-Kα radiation at working voltage 40 kV and current 30 mA. Scan was performed using X’Celerator detector from 5° to 70° (2θ) with a step size of 0.026°. Total time required to scan the sample was 1 h.
2.4. Density and Absorption Capacity
The physical properties of the converter slag before and after the reaction with SCD were measured by density (skeletal and apparent density) and absorption capacity. The skeletal density was measured using AccuPyc II 1340 Gas Pycnometer (Micromeritics, Norcross, GA, USA). Prior to the measurements, the apparatus was calibrated with two NIST traceable steel ball standards. The 3.5 g of converter slag samples were placed in a 10 cm3 chamber and 1.345 bar helium was introduced. The mass of the specimen was divided by the average of 10 volume runs to calculate the skeletal density of the specimens.
Apparent density and absorption capacity of the converter slag were measured according to ASTM C 128 Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate [31]. The absorption capacity of converter slag was calculated by measuring the amount of water required to meet saturated surface-dry (SSD) condition from oven dry(OD) condition. The apparent density was measured using a pycnometer, and the measurement method is as shown in Equation (1).
where Wod is mass of oven dry specimen (g), Wpyc is mass of pycnometer filled with water to calibration mark (g), and W*pyc is mass of pycnometer filled with specimen and water to calibration mark (g).
2.5. Thermogravimetric Analyses (TGA)
Thermogravimetric analysis was performed using Thermo Gravimetric-Differential Thermal Analyzer (Bruker axs, Karlsruhe, Germany, TG-DTA 2020). It is used to calculate the amount of portlandite and calcium carbonate since calcium hydroxide decomposed in the range of 450~500 °C [32,33] and calcium carbonate decomposed in the range of 600~800 °C [32]. Approximately 10 mg of the converter slag sample was placed in the platinum crucible and temperature was raised to 1000 °C at the rate of 10 °C/min. The amounts of calcium hydroxide and calcium carbonate in the samples were quantified by the tangential method as suggested by Kim and Olek [34].
2.6. pH Measurements
After reaction with SCD, converter slag was dried at 23 ± 2 °C laboratory. The 5 g of hydrated cement paste was placed in 50 ml beaker, and 25 g of deionized water (18.3 MΩ·cm) was poured into beaker to maintain the ratio of converter slag to water at 1:5. The pH measurement was performed on leachate solution every 24 h using Orion Star A211 pH meter (Thermo Fisher Scientific, Waltham, MA, USA). During measurements, the top portion of beaker was covered using paper lid in order to minimize evaporation of water as well as carbonation by ambient air.
2.7. Scanning Electron Microscopy (SEM)
In order to verify whether calcium carbonate minerals were actually produced or not, the converter slag before and after the reaction with SCD was analyzed using the MIRA Field Emission Scanning Electron Microscope (TESCAN, Brno, Czech Republic) equipped with MIRA 3 LMH In-Beam Detector. Prepared converter slag samples were dried at 50 °C for 3 days and then mounted on an aluminum specimen holder. Samples were gently spread onto carbon tape that was attached on the specimen holder. Specimens were then sputtered coated using platinum (Pt). Energy dispersive X-ray spectroscopy (EDS) was also utilized in order to verify chemical compositions of a designated object in SEM image.
2.8. Mortar Bar Expansion Test
In order to evaluate the stability of the converter slag after reaction with SCD, mortar bar expansion test was performed according to ASTM C 1260 Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method) [35]. Although this test procedure is designed to separate alkali reactive aggregate, the applicability of this test procedure to examine the expansion caused by free lime and periclase was proven by the earlier work [18] because the testing temperature is so high that it can accelerate the reaction of CaO and MgO.
For this purpose, mortar bar specimens were prepared using the mix proportions shown in Table 2. Converter slag samples were sieved to meet the gradation requirements as specified in ASTM C 1260 [35]. Gradation of converter slag used in this work is shown in Table 3. The water-to-cement ratio was set at 0.47, and cement-to-fine-aggregate (here, converter slag) ratio was adjusted to 1:2.5 because, according to ASTM C 1260 [35], cement to fine aggregate (here, converter slag) ratio needed to be determined by the densities of the fine aggregate, considering the density of standard sand was 2.65 g/cm3. The measured apparent density was used to calculate the cement to converter slag ratio.

Table 2.
Mix proportions of mortar specimen (kg/m3).

Table 3.
Gradation of converter slag used in this work (as specified in ASTM C 1260).
To prepare mortar specimens, materials were mixed by following ASTM C 305 Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency [36]. Cement and converter slag were dry-mixed at low speed for 30 s using a planetary paddle mixer. After that, the water was added, and materials were mixed for 60 s. The mixture was allowed to rest for 30 s to scrape down the side and bottom of the mixing bowl. The mortar was mixed at medium speed for 60 s, and mixing was finished.
After mixing, mortar was filled into 25.4 mm × 25.4 mm × 295 mm size brass mold. The mold was covered by plastic wrap in order to prevent evaporation of moisture for a day. After demolding, specimen was cured in tap water at 80 °C for a day. Zero reading was performed on 2-day-old specimen, and mortar bar specimens were immersed in 80 °C 1M NaOH solution until measurement was terminated. Mortar bar expansion was recorded every day until failure.
2.9. Compressive Strength
Compressive strength was measured in order to investigate the effect caused by the reaction with SCD on converter slag. Mortar specimens were prepared by following ASTM C 109 Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens) [37]. Mortar samples with ISO-679 [38] standard sand (produced by Société Nouvelle du Littoral (SNL), Leucate, France) was also prepared in order to investigate the effect on compressive strength caused by converter slag. The mix proportion of mortar is presented in Table 2. The mixing procedure of mortar was carried out in the same procedure as described in earlier chapter.
The mortar specimens were cast into a 50 mm × 50 mm × 50 mm cube mold, covered by plastic wrap and demolded after 24 h, and immersed in a 21 ± 0.5 °C saturated calcium hydroxide solution for 27 days for curing. Compressive strength measurement was performed on 28 days of age and carried out using a UH-F100A universal testing machine (Shimadzu Corporation, Kyoto, Japan), and the loading speed was set to 2 mm/min.
3. Results and Discussion
3.1. Effect of Carbonation by SCD on Converter Slag
3.1.1. Mineralogical Properties
XRD analysis was performed to investigate the effect of SCD on mineralogical changes of converter slag after reaction. Figure 3 shows the XRD patterns of the converter slag before and after the reaction with SCD. Portlandite was observed in the XRD pattern of as received converter slag. This indicates that some amount of free lime in converter slag has already reacted. The converter slag used in this work has experienced some period of aging, but it is not considered so significant because there was only small indication of calcite from as received converter slag due to the carbonation by atmospheric CO2. It is also noticeable that a small hint of free lime was observed. Periclase peaks were also clearly observed from XRD pattern.
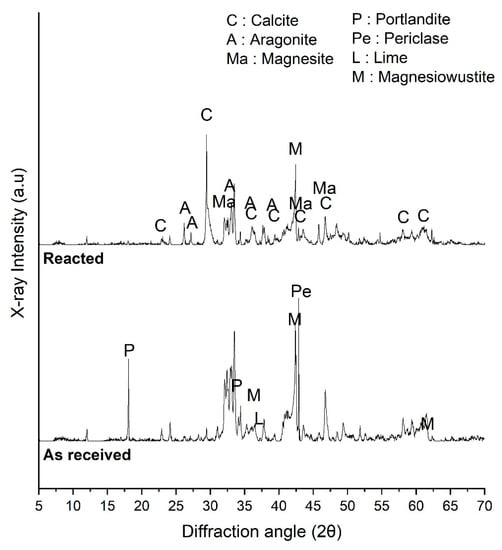
Figure 3.
XRD pattern of converter slag reacted with supercritical carbon dioxide.
After reaction with SCD, the lime hump that was observed from as received converter slag was disappeared, and the periclase peak was significantly reduced. Indeed, magnesite (MgCO3) formation was observed, which can be the reaction product between MgO and SCD. Portlandite disappeared from the XRD pattern. Calcite peak increased and aragonite was observed after reaction with SCD. Calcium carbonate minerals were produced as results of reaction between portlandite and SCD or lime and SCD. This means that the converter slag has undergone significant level of carbonation during the reaction with SCD.
3.1.2. Absorption and Density
Table 4 summarizes the absorption capacity, apparent density, and skeletal density of converter slag before and after the reaction with SCD. The absorption capacity of as received converter slag was 1.78%, the apparent density was 3.07 g/cm3, and the skeletal density was 3.41 g/cm3. The absorption capacity of reacted converter slag was 2.52%, the apparent density was 3.15 g/cm3, and the skeletal density was 3.54 g/cm3.

Table 4.
Absorption, apparent density, and skeletal density of converter slag reacted with supercritical carbon dioxide.
The reaction with SCD increased absorption capacity of converter slag. Such result indicates that the volume of porous network (most likely crack in this case) has been increased by the reaction with SCD. It is due to the expansive reaction with SCD from free lime and periclase that is similar to the case from crystallization of portlandite or brucite in pore structure [39]. As water and dissolved CO2 migrates into the site where free lime or periclase is located, reaction occurs with SCD, and CaCO3 or MgCO3 is produced either from direct reaction or through secondary reaction, which takes two separate processes by (1) earlier formation of Ca(OH)2 and Mg(OH)2 and (2) their later conversion into CaCO3 and MgCO3. Since density of the portlandite, calcite, aragonite, brucite, and magnesite are 2.23, 2.71, 2.93, 2.39, and 3.00 g/cm3, respectively, all these reaction products occupy more space than free lime and periclase whose densities are 3.34 and 3.78 g/cm3, respectively, thereby causing expansion in converter slag after reaction with SCD. The increase in apparent and skeletal densities after reaction with SCD is mainly associated with transition from portlandite to calcite and aragonite.
3.1.3. Thermogravimetric Analysis
Figure 4 shows the TG/DTA analysis results of converter slag before and after reaction with SCD. Figure 4a is a TG graph obtained from as received converter slag, and a weight loss of 0.34% was observed at around 450 °C [34]. This peak is associated with the decomposition of portlandite. Same to the results from XRD patterns, the presence of portlandite in converter slag was also verified from TG. The presence of calcite was also verified by TG. There was 98.02 (before decomposition) − 97.61 (after decomposition) = 0.41% weight loss event associated with decomposition of CaCO3.
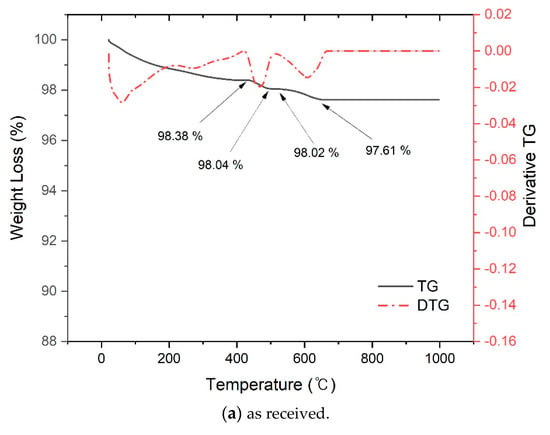
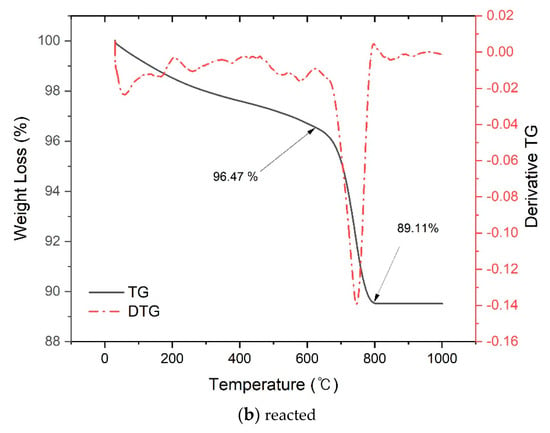
Figure 4.
Thermogravimetric analysis of converter slag reacted with supercritical carbon dioxide.
Figure 4b shows the TG/DTA graph of converter slag after reaction with SCD. From the TG graph of reacted specimens, the weight loss in the 450 °C region observed from as received converter slag disappeared due to the carbonation by SCD, and a weight loss of 96.47 (before decomposition) to 89.11 (after decomposition) = 7.36% was observed in the 600–800 °C region, which is associated with the decomposition of CaCO3 polymorphs [32].
It was found that the amount of CO2 in CaCO3 produced by reaction with SCD was 7.36 (after reaction) to 0.41 (before reaction) = 6.95%. Considering the final weight after TGA as a weight of unreacted and uncarbonated converter slag, the amount of CO2 uptake that converter slag can provide by the reaction with SCD can be calculated. It was found to be 7.80 g CO2 per 100 g converter slag. If there was no carbonation on as received converter slag, this could have increased to 8.26 g CO2 per 100 g converter slag. It would be 10.40 g CO2 per 100 g fresh converter slag supposing that there was neither reaction nor carbonation.
According to the work of Ukwattage et al. [40], CO2 uptake by blast furnace slag varied from changes in testing condition. They tested under a pressure range of 10 to 60 bar and temperature range of 20 to 80 °C, and the maximum amount was reported to be 2.95 g CO2 per /100 g blast furnace slag at 50 °C 30 bar. The amount of CO2 uptake by converter slag after reaction with SCD was greater than what was found earlier from the blast furnace slag. Such difference might be related to higher CO2 bearing capacity of converter slag than blast furnace slag because converter slag used in this work has higher amount of CaO than typical blast furnace slag. However, it is also strongly associated with increased reactivity of CO2 in supercritical condition.
It should be also noted that particle size of the steelmaking slag can significantly affect the amount of CO2 uptake. Poletini and Stramazzo [41] reported that basic oxygen furnace can absorb CO2 up to 0.47~46.5 g of its original weight depending on its particle size. In their other work [42], total CO2 uptake was reported to be around 6.7~53.6% CO2 depending on the changes in temperature, pressure, and particle sizes. In this work, the size of the aggregate had to be controlled for mortar bar expansion tests, and the temperature and pressure condition was set to a single parameter. Such factors will need to be considered for further experimental works because considering the results from earlier works, it is reasonable to expect that CO2 uptake by converter slag could have been increased if particle size was reduced.
3.1.4. pH Analysis
Figure 5 shows the pH measurements of leachate from the converter slag before and after reaction with SCD. The initial pH of leachate from as received converter slag was 11.37. As time passed, the pH slightly decreased, but it was stabilized at around 11.14 after 10 days. The initial pH of leachate from reacted converter slag was measured to be 9.80 due to the carbonation from SCD. The drop in pH was 1.49 compared to the unreacted sample. The pH of leachate from converter slag decreased continuously over time and stabilized at around 9.35 after 10 days of measurement. The result indicates converter slag can be safely utilized for land reclamation material after the reaction with SCD.
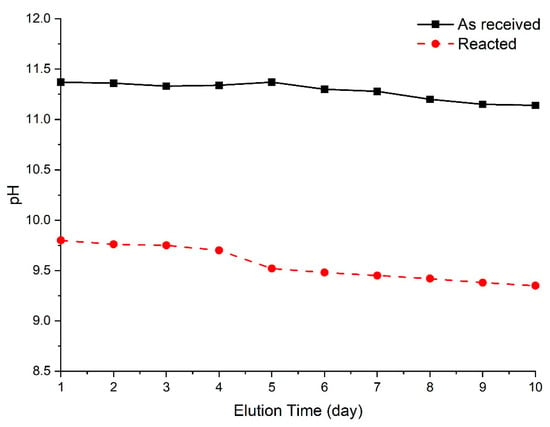
Figure 5.
pH of converter slag reacted with supercritical carbon dioxide.
3.1.5. SEM Analysis
Figure 6 shows an SEM images of as received converter slag, and carbonate minerals such as calcite or aragonite were not clearly observed on the surface of the converter slag. However, according to Figure 7, clear evidence of calcite formation was observed after reaction with SCD. Numbers of euhedral calcite crystals (similar to cubic shape) [43] were produced on the surface of converter slag, and were widely distributed over the surface. EDS analysis (Figure 6b) on a cubic shaped crystal verifies the successful calcite formation on converter slag after reaction with SCD.
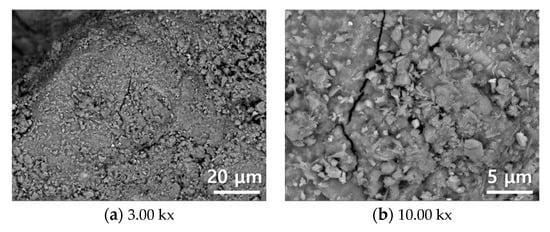
Figure 6.
SEM image of as received converter slag.
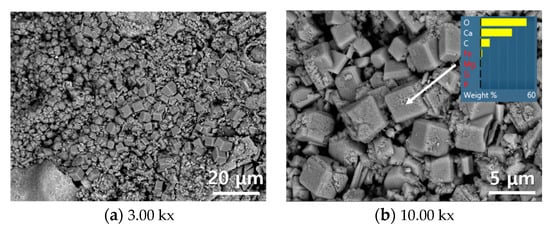
Figure 7.
SEM image of converter slag reacted with supercritical carbon dioxide.
3.2. Aggregate Stability Evaluation
Figure 8 shows the expansion rate of mortar bar using converter slag before and after reaction with SCD. According to Figure 8, for the first 10 days, the expansion rate of mortar using as received converter slag showed very similar expansion rate to that of mortar bar made of reacted converter slag. However, at 12 days, a crack was found from the surface of the mortar bar made of as received converter slag. Although the specimen did not show significant amount expansion during first 14 days of measurement, a large number of cracks was produced at 17 days accompanied by rapid increase in expansion rate. Finally, at 19 days, mortar bar was completely destroyed showing expansion rate of 0.13%. The photographic images of mortar bar made of as received converter slag are shown in Figure 9. The result proves instability of unreacted converter slag for its application as source of aggregate in concrete.
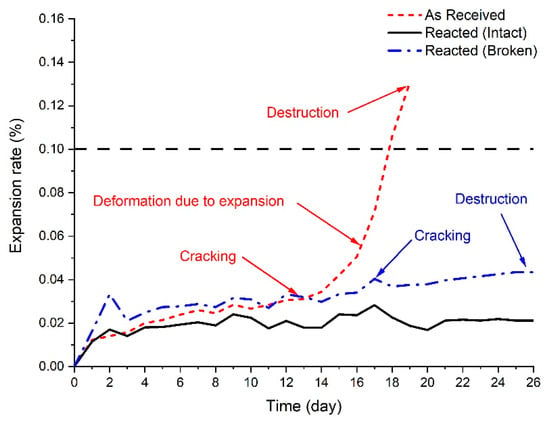
Figure 8.
Expansion rate of mortar bar using converter slag as fine aggregate before and after SCD reaction.
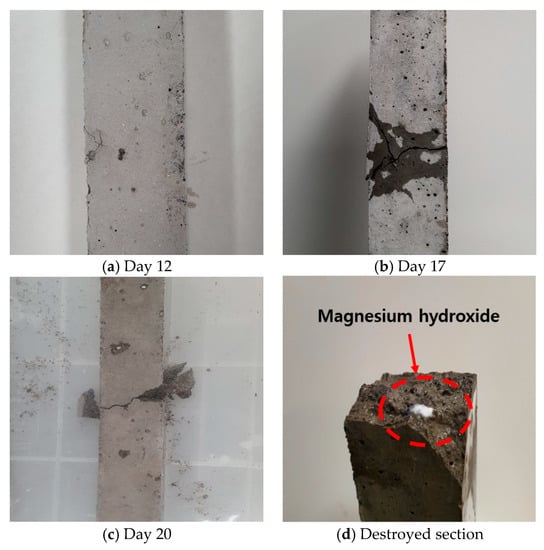
Figure 9.
Destruction process during expansion test of mortar bar using converter slag as fine aggregate.
From expansion of mortar bar made of reacted converter slag, two mortar bar specimens were not damaged whereas the last one was broken during expansion test. With respect to failed mortar bar, the first crack was observed at 17 days of measurement. The specimen was broken at 26 day, but showing only 0.04% of expansion. It was found that the reaction with SCD either removed the failure or delayed the time of failure, but it did not completely prevent the failure of the mortar bar. It is considered that the failure of mortar bar is mainly associated with periclase that was observed from XRD pattern of reacted specimen.
The expansion seemed to be generated at the place where free periclase was located. It is evidenced by the observation of white powder, the color of magnesium hydroxide (brucite), on the surface of converter slag (Figure 9d). It can be concluded that due to the expansive reaction that occurred within converter slag, the mortar bar expanded, and a crack was generated. However, failure mode of the mortar bar with converter slag was somewhat different from what was generally observed from reactive aggregate. It should be noted that the failure of the mortar bar occurred much earlier than the expansion rate designated for reactive aggregate criterion (0.2%) in ASTM C 1260 [35]. Some samples were even broken before potentially reactive aggregate criterion (0.1%), which is set to classify less reactive aggregate.
In general, mortar bar expansion from reactive aggregate can easily be higher than expansion rate that was designated for reactive aggregate criteria [44,45,46,47]. Such a difference is associated with the fact that the reaction of reactive aggregate occurs mostly at the outside rim of the reactive aggregate (only small portion of reaction occurs inside the aggregate) [45,47] whereas all the reaction from free lime and periclase occurs inside the converter slag. When free lime or periclase reacts inside converter slag, it expands and generate cracks on converter slag (local expansion), which eventually led to the breakage of the mortar bar without matter of how much reaction has taken place. This can explain how the mortar bar with reacted converter slag was destroyed without showing any notable amount of expansion. The only difference between mortar bar with and without reaction with SCD was on the fact that expansion with as received converter slag occurred at many different locations whereas expansive reaction with reacted converter slag occurred only at some limited spaces showing very small amount of expansion.
The results from mortar bar expansion test clearly state a very important fact that any presence of free lime or periclase remaining on the steel slag, even in very small amount, can cause breakage of concrete specimen due to the expansion within converter slag. A complete aging or carbonation treatment is necessary in order to avoid such problem. It should be noted that parameters such as increasing (1) the amount of time for reaction, (2) temperature, (3) pressure, or (4) control of particle size (to a finer size) will definitely increase the efficiency of SCD treatments on converter slag.
3.3. 28 Days Compressive Strength
Figure 10 shows the compressive strength of mortar using converter slag at 28 days of age. The compressive strength of the mortar using as received converter slag was 53.12 MPa, and the compressive strength of the mortar using reacted converter slag was measured to be 50.16 MPa. A slight decrease in compressive strength was observed, and it is primarily associated with the increase in absorption capacity associated with generation of cracks from the converter slag after reaction with SCD.
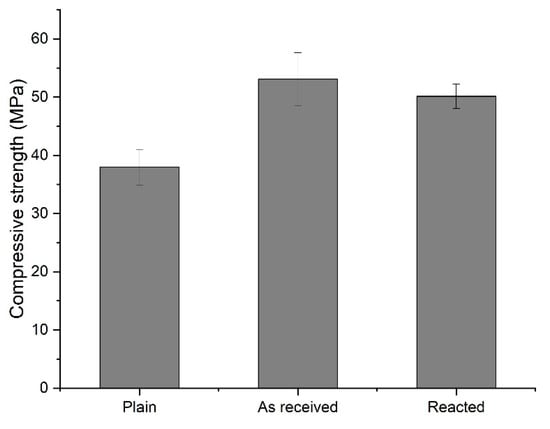
Figure 10.
The 28 days compressive strength of mortar using supercritical carbon dioxide-reacted converter slag as fine aggregate.
Considering that the compressive strength of the mortar using standard sand (plain) at 28 days of age was measured to be 37.99 MPa, it was found that the converter slag after reaction with SCD can be a good source of fine aggregate because the strength was still more than 32% higher than mortar made of standard sand. Although a slight decrease in compressive strength is expected on mortar using converter slag after reaction with SCD, completely carbonated converter slag will be away from the worries that may produce potential expansion caused by lime and periclase, and therefore worth more for its recycling with added value of mineralization of CO2 to contribute global warming issue.
4. Conclusions
In this work, SCD was applied in order to maximize the reaction of free lime and periclase that resides in converter slag. Although the amount of reaction with SCD was not sufficient to make periclase completely react, this work has found a potential applicability of SCD for stabilization of converter slag to be used either as land reclamation material or as fine aggregate. According to the results obtained from this work, the following conclusions can be drawn.
(1) After reaction with SCD, density and absorption capacity of converter slag increased. Increase in density was mainly associated with the conversion from portlandite to calcite or aragonite, whereas the increase in absorption capacity was associated with cracks caused by the reaction of free lime and periclase.
(2) CO2 uptake was found to be 7.80 g per 100 g converter slag. Reaction of converter slag with SCD can be an effective source of CO2 mineralization that can reduce greenhouse gas emission.
(3) Mortar bar made of unreacted converter slag was completely destroyed at 19 days showing 0.13% expansion. One of the mortar bars made of reacted converted slag was also broken at 26 days showing 0.04% expansion. It was recommended not to use converter slag as concrete aggregate if there is any lime or periclase present, regardless of its amount. Complete removal of free lime and periclase is necessary in order for its successful use as concrete aggregate.
(4) The compressive strength of the mortar was slightly decreased after reaction of converter slag with SCD. However, completely reacted converter slag can be successfully used as fine aggregate as the strength of mortar was 30% higher than mortar made of standard sand.
It should be noted that experimental condition in this work was fixed at 80 °C 100 bar. Since parameters such as reaction time, temperature, and pressure can significantly affect the reaction with SCD, the relationship between such parameters and reactivity of converter slag should be investigated in the future. The effect of particle size also needs to be quantitatively investigated, although it is certain that a finer size will have a larger amount of reaction with SCD.
Author Contributions
Data curation, H.M. and J.-H.K.; formal analysis, C.-W.C. and M.L.; funding acquisition, C.-W.C.; investigation, H.M. and K.K.; methodology, H.M., K.K., J.-H.K., and M.L.; project administration, J.-H.K.; resources, M.L.; supervision, C.-W.C. and M.L.; writing—original draft, H.M.; writing—review and editing, J.-H.K. and C.-W.C. All authors have read and agreed to the published version of the manuscript.
Funding
Korea Agency for Infrastructure Technology Advancement (Grant 20CTAP-C151965-02).
Informed Consent Statement
Not applicable.
Acknowledgments
This work is supported by the Korea Agency for Infrastructure Technology Advancement (KAIA) grant funded by the Ministry of Land, Infrastructure and Transport (Grant 20CTAP-C151965-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Worldsteel Association. Global Crude Steel Output Increases by 3.4% in 2019; Worldsteel Association: Brussels, Belgium, 2020; Available online: https://www.worldsteel.org/media-centre/press-releases/2020/Global-crude-steel-output-increases-by-3.4--in-2019.html (accessed on 27 January 2020).
- Virgalitte, S.J.; Luther, M.D.; Rose, J.H.; Mather, B.; Bell, L.W.; Ehmke, B.A.; Lewis, D.W. Ground Granulated Blast-Furnace Slag as a Cementitious Constituent in Concrete. In American Concrete Institute ACI Report 233R-95; American Concrete Institute: Farmington Hills, MI, USA, 1995. [Google Scholar]
- Shariq, M.; Prasad, J.; Masood, A. Effect of GGBFS on time dependent compressive strength of concrete. Constr. Build. Mater. 2010, 24, 1469–1478. [Google Scholar] [CrossRef]
- Siddique, R.; Kaur, D. Properties of concrete containing ground granulated blast furnace slag (GGBFS) at elevated temperatures. J. Adv. Res. 2020, 3, 45–51. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Usman, M.; Park, C.; Hanif, A. Durability of slag waste incorporated steel fiber reinforced concrete in marine environment. J. Build. Eng. 2021, 33, 101641. [Google Scholar] [CrossRef]
- Kim, Y.; Hanif, A.; Usman, M.; Munir, M.J.; Kazmi, S.M.S. Slag waste incorporation in high early strength concrete as cement replacement: Environmental impact and influence on hydration & durability attributes. J. Clean. Prod. 2021, 172, 3056–3065. [Google Scholar]
- Al-Negheimish, A.I.; Al-Sugair, F.H.; Al-Zaid, R.Z. Utilization of local steelmaking slag in concrete. J. King Saud Univ. Eng. Sci. 2018, 9, 39–54. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Lee, K.-M.; Park, P.J. Estimation of the environmental credit for the recycling of granulated blast furnace slag based on LCA. Resour. Conserv. Recycl. 2005, 44, 139–151. [Google Scholar] [CrossRef]
- McGannon, H.E. The Making, Shaping and Treating of Steel, 8th ed.; Berne and Pan-American International: Pittsburgh, PA, USA, 1964. [Google Scholar]
- Lee, A.R. Blast Furnace and Steel Slag: Production Properties and Uses; Edward Arnold Ltd.: London, UK, 1974. [Google Scholar]
- Moon, H.Y.; Yoo, J.H. Utilization of electric arc furnace slag and converter slag after aging for concrete aggregate. J. Korea Concr. Inst. 2002, 14, 567–607. [Google Scholar]
- Yoo, J.H.; Choi, J.J. A study on the residual expansibility of electric arc furnace slag aggregate. J. Korean Recycl. Constr. Resour. Inst. 2006, 2, 128–135. [Google Scholar]
- Sorlini, S.; Sanzeni, A.; Rondi, L. Reuse of steel slag in bituminous paving mixtures. J. Hazard. Mater. 2012, 209, 84–91. [Google Scholar] [CrossRef]
- United Stated Geological Survey (USGS). Slag-Iron and Steel, Iron and Steel Slag Statistics and Information; Minerals Yearbook; Annual Publication: Reston, VA, USA, 2001; pp. 1–7. [Google Scholar]
- Yildirim, I.Z.; Prezzi, M. Use of Steel Slag in Subgrade Applications; Publication FWA/IN/JTRP-2009/32; Joint Transportation Research Program; Indiana Department of Transportation and Purdue University: West Lafayette, IN, USA, 2009. [Google Scholar]
- Moon, H.; Jang, B.K.; Kim, J.H.; Chung, C.W. Evaluation on Applicability of Copper and Steelmaking Slags for Use of Heavy Weight Aggregates in Marine Concrete Structure. J. Korean Recycl. Constr. Resour. Inst. 2017, 5, 345–352. [Google Scholar]
- Chung, C.W.; Lee, M.; Kim, S.O.; Kim, J. The pH Reduction of the Recycled Aggregate Originated from the Waste Concrete by the scCO2 Treatment. Econ. Environ. Geol. 2017, 50, 257–266. [Google Scholar]
- Kou, S.-C.; Zhan, B.-J.; Poon, C.-S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Hanif, A.; Kim, Y.; Lu, Z.; Park, C. Early-age behavior of recycled aggregate concrete under steam curing regime. J. Clean. Prod. 2017, 152, 103–114. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, J.H.; Lee, M.H.; Chung, C.W. Carbonation Mechanism of Hydrated Cement Paste by Supercritical Carbon Dioxide. J. Korea Inst. Build. Constr. 2018, 18, 403–412. [Google Scholar]
- Park, J.; Lee, J.; Chung, C.W.; Wang, S.; Lee, M. Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process. Minerals 2020, 10, 486. [Google Scholar] [CrossRef]
- Park, S.M.; Moon, H.; Kim, J.H.; Lee, M.H.; Chung, C.W. Reaction of hydrated cement paste with supercritical carbon dioxide. Constr. Build. Mater. 2021, 281, 122615. [Google Scholar] [CrossRef]
- Waligora, J.; Bulteel, D.; Degrugilliers, P.; Damidot, D.; Potdevin, J.L.; Measson, M. Chemical and mineralogical characterizations of LD converter steel slags: A multi-analytical techniques approach. Mater. Charact. 2020, 61, 39–48. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, P.; Wang, D. Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J. Clean. Prod. 2017, 156, 50–61. [Google Scholar] [CrossRef]
- Korea Agency for Technology and Standards. KS F 2527 Concrete Aggregate; Korea Agency for Technology and Standards: Eumseong-gun, Chungcheongbuk-do, Korea, 2018. [Google Scholar]
- Kendall, J.L.; Canelas, D.A.; Young, J.L.; DeSimone, J.M. Polymerizations in supercritical carbon dioxide. Chem. Rev. 1999, 99, 543–564. [Google Scholar] [CrossRef]
- Leitner, W. Supercritical carbon dioxide as a green reaction medium for catalysis. Acc. Chem. Res. 2002, 35, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, J.; Chung, C.W.; Kim, J.; Lee, M.; Kim, S.O. The Neutralization Treatment of Waste Mortar and Recycled Aggregate by Using the scCO2-Water-Aggregate Reaction. Econ. Environ. Geol. 2018, 51, 359–370. [Google Scholar]
- ASTM International. ASTM C 128 Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Dweck, J.; Buchler, P.M.; Coelho, A.C.V.; Cartledge, F.K. Hydration of a Portland cement blended with calcium carbonate. Acta 2020, 346, 105–113. [Google Scholar] [CrossRef]
- Collier, N.C. Transition and decomposition temperatures of cement phases—A collection of thermal analysis data. Ceramics-Silikáty 2016, 60, 338–343. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of sample preparation and interpretation of thermogravimetric curves on calcium hydroxide in hydrated pastes and mortars. Transp. Res. Rec. 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- ASTM International. ASTM C 1260 Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. ASTM C 305 Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM International. ASTM C 109 Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- International Organization for Standardization. ISO 679 Cement—Test methods—Determination of Strength; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Deng, M.; Hong, D.; Lan, X.; Tang, M. Mechanism of expansion in hardened cement pastes with hard-burnt free lime. Cem. Concr. Res. 1995, 25, 440–448. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 2017, 97, 15–22. [Google Scholar] [CrossRef]
- Polettni, A.; Pomi, R.; Stramazzo, A. Carbon sequestration through accelerated carbonation of BOF slag: Influence of particle size characteristics. Chem. Eng. J. 2016, 298, 26–35. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. CO2 sequestration through aqueous accelerated carbonation of BOF slag: A factorial study of parameters effects. J. Environ. Manag. 2016, 167, 185–195. [Google Scholar] [CrossRef]
- Wang, H.; Alfredsson, V.; Tropsch, J.; Ettl, R.; Nylander, T. Formation of CaCO3 Deposits on Hard Surfaces-Effect of Bulk Solution Conditions and Surface Properties. Acs Appl. Mater. Interfaces 2013, 5, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.; Thaulow, N. A study of expansion due to alkali—silica reaction as conditioned by the grain size of the reactive aggregate. Cem. Concr. Res. 1974, 4, 591–607. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, W.; Zhou, W.; Byars, E.A. Expansion behaviour of glass aggregates in different testing for alkali-silica reactivity. Mater. Struct. 2009, 42, 485. [Google Scholar] [CrossRef]
- Multon, S.; Cyr, M.; Sellier, A.; Diederich, P.; Petit, L. Effects of aggregate size and alkali content on ASR expansion. Cem. Concr. Res. 2010, 40, 508–516. [Google Scholar] [CrossRef]
- Sanchez, L.F.M.; Fournier, B.; Jolin, M.; Mitchell, D.; Bastien, J. Overall assessment of Alkali-Aggregate Reaction (AAR) in concretes presenting different strengths and incorporating a wide range of reactive aggregate types and natures. Cem. Concr. Res. 2017, 93, 17–31. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).