Abstract
Intervertebral disc (IVD) degeneration is a leading cause of chronic low back pain (LBP) that results in serious disability and significant economic burden. IVD degeneration alters the disc structure and spine biomechanics, resulting in subsequent structural changes throughout the spine. Currently, treatments of chronic LBP due to IVD degeneration include conservative treatments, such as pain medication and physiotherapy, and surgical treatments, such as removal of herniated disc without or with spinal fusion. However, none of these treatments can completely restore a degenerated disc and its function. Thus, although the exact pathogenesis of disc degeneration remains unclear, there are studies examining the effectiveness of biological approaches, such as growth factor injection, gene therapy, and cell transplantation, in promoting IVD regeneration. Furthermore, tissue engineering using a combination of cell transplantation and biomaterials has emerged as a promising new approach for repair or restoration of degenerated discs. The main purpose of this review was to provide an overview of the current status of tissue engineering applications for IVD regenerative therapy by performing literature searches using PubMed. Significant advances in tissue engineering have opened the door to a new generation of regenerative therapies for the treatment of chronic discogenic LBP.
1. Introduction
Intervertebral disc (IVD) degeneration (IVDD) causes chronic low back pain (LBP), including discogenic back pain, significant health problems, and socioeconomic burden [1]. IVDD-induced discogenic LBP accounts for more than 40% of all LBP cases [2] and is considered as one of the top global causes of disability-adjusted life years [3].
IVDD is a process leading to loss of proteoglycans (PGs), destruction of the extracellular matrix (ECM), annular tears, development of disc herniation, and loss of disc height [4]. As a result of these anatomical changes, nerve root compression, spinal canal stenosis, and facet joint arthritis and hypertrophy can occur and can lead to chronic LBP and/or radiating leg pain with or without neurological deficits [5]. Moreover, the inflammatory environment of the degenerative discs and neurite sprouting have been suggested as the cause of discogenic LBP [6]. Thus, discogenic LBP is associated with complex interactions between the mechanical aspects of the IVD, inflammation, and the central or peripheral nervous system [7].
Chronic LBP due to IVDD may be considered for surgical treatments if there is no response to conservative treatments, such as medication and physical therapy. Surgical treatments include discectomy to remove a herniated disc, spinal fusion surgery used to connect two vertebrae to limit the movement of the spinal motion segment, and artificial disc replacements designed to restore and maintain range of motion [8,9]. However, spinal fusion surgery does not restore the previous range of motion and mechanical load-bearing properties of the IVD. Moreover, spinal fusion can lead to adjacent segment disease, which is a typical long-term complication after spinal fusion surgery and further indicates disc herniation, spinal canal stenosis, or spondylosis at levels above or below the index fusion level [10]. Therefore, alternate biological therapies are needed prior to surgery to slow or reverse the progression of IVDD, which usually leads to pain and disability.
The mechanical properties of the IVD are critical for proper functioning. In vivo, the IVD is the load-bearing structure of the spine and is subjected to spinal tension, torsion, compression, and bending [11]. Anatomically, the normal IVD consists of the following three parts: (1) the nucleus pulposus (NP), which contains a highly hydrated gel-like matrix comprising PGs and type II collagen; (2) the annulus fibrosus (AF), which is composed of lamellae in which parallel type I collagen fibers located within each lamella are aligned and help the IVD maintain its integrity from bending, stretching, and twisting; and (3) the end plates (EP), which consist of osseous and two hyaline cartilages [12] (Figure 1). In recent years, the understanding of IVD development, cell biology, and mechanisms of IVD degeneration has significantly advanced, enabling the development of biological approaches for IVD regeneration [4]. Biological approaches include growth factor injection and gene and cell-based therapies, whereas tissue engineering approaches involve the restoration of the mechanical and biological properties of the tissue via the addition of biomaterials to the degenerated disc [13].
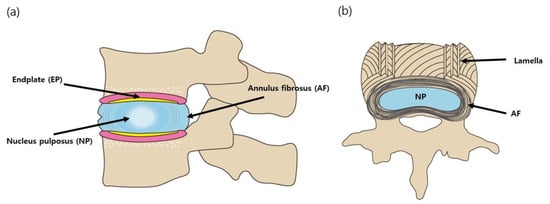
Figure 1.
Schematic diagram of the intervertebral disc (IVD). (a) sagittal cross-section (b) axial cross-section.
Depending on the stage of IVDD, different treatment strategies for managing IVDD have been recommended [14]. Initial IVDD shows change in the NP and AF matrices, while IVDD induces progressive structural changes such as annular fissures, disc herniation, disc height reduction, and disc space collapse. The Pfirrmann Disc Grading is a useful scoring tool for evaluating IVDD on MRI T2-weighted images; Grade I: homogeneous disc with bright high intensity and normal disc height; Grade II: inhomogeneous disc but high intensity signal, clear distinction between NP and AF, and normal disc height; Grade III: inhomogeneous disc with an intermittent gray signal intensity, unclear distinction between NP and AF, and normal or slightly reduced disc height; Grade IV: inhomogeneous disc with low intensity dark gray signal intensity, no more distinction between the NP and AF, and slightly or moderately decreased disc height; Grade V: inhomogeneous disc with a hypointense black signal intensity, no more distinction between the nucleus and annulus, and collapsed disc space [15]. For severe nerve compression due to herniated disc and disc space collapse (Pfirrmann grades IV and V), surgical removal of herniated disc and/or spinal fusion surgery are required. However, patients with discogenic LBP due to Pfirrmann grade II and III IVDD may receive regenerative molecular therapies such as growth factors, genes, and cell therapy with or without biomaterials. In addition, tissue engineered NP and AF could be applied for patients with Pfirrmann grades IV and V.
The aim of this review was to provide an overview of the current status of tissue engineering applications for the treatment of IVD regeneration. To obtain an overview of the current tissue engineering strategies utilized for the repair of degenerated discs, we conducted a literature search using PubMed (https://pubmed.ncbi.nlm.nih.gov/, from 1 January 2000 and 1 December 2020) and the following key words: “intervertebral disc”, “regeneration”, “stem cells”, “biomaterials”, and “tissue engineering”.
2. Biological Approaches
2.1. Molecular Therapies
2.1.1. Growth Factors
In the early stages of IVDD, growth factor injection may rebalance the anabolic and catabolic pathways in the degenerative cascade [16]. Degenerated discs have been reported to be repaired by intradiscal injection of growth factors, including insulin-like growth factor-1(IGF-1), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF or FGF2), transforming growth factor-β (TGF-β), bone morphogenetic protein-2 (BMP-2), BMP-7 (osteogenic protein-1; OP-1), and growth and differentiation factor-5 (GDF-5) by promoting cell proliferation and matrix synthesis in experimental models [17,18,19]. The mitogenic potential of human NP and AF cells can be stimulated by platelet-derived growth factor (PDGF), bFGF, and IGF-1 [20,21]. PDGF, a known angiogenic growth factor, has been shown to inhibit IVD cell apoptosis and promote anabolic gene expression [22]. In several animal experiments, intradiscal injection of BMP-7 has shown improvements in the disc height and NP proteoglycan content [23,24]. GDF-5, also known as BMP-14, is another anabolic protein that promotes cell proliferation and proteoglycan synthesis in degenerated discs [18]. Similar to BMP-7, many animal studies of intradiscal injection of GDF-5 have shown improved disc height, cell proliferation, and matrix synthesis [25,26]. Despite their efficacy, there are many concerns surrounding the clinical use of recombinant growth factors due to their short half-life, limited stability, high cost, and problems associated with binding large molecules to polymers [27]. Thus, the right carrier is a matter to consider. In recent years, the use of biodegradable microspheres for controlled local drug delivery has become a valuable approach to overcome the drawbacks of growth factors. Yan et al. demonstrated that injection of GDF-5 loaded into poly(lactic-co-glycolic acid) (PLGA) microspheres could improve regenerative efficacy of GDF-5 in a rat model [28].
2.1.2. Gene Therapy
A strategy to overcome the short half-life limitation of growth factors is to provide a sustained supply of growth factors within the IVD [29]. The therapeutic effect of gene therapy is based on the induction of target gene upregulation or downregulation. These genes are transferred using viral or non-viral vectors, which are either directly injected into the degenerated discs or transduced into cells [16]. Another strategy used for intradiscal gene therapy is gene expression downregulation, which is detrimental to the physiological balance of the disc and may, thus, lead to IVDD [16]. Hence, if gene therapy is performed properly, it can provide many benefits, including a more sustained target gene expression and long-term biological effects. Promising targets for gene therapy include both anabolic regulators, such as TGF-β, latent membrane protein 1(LMP-1), and SOX-9, and anticatabolic regulators, such as anti-ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)-5, and TIMP (tissue inhibitor of metalloproteinases)-1 [13]. In terms of non-virus vector-mediated gene transfer, ultrasound targeted microbubble transfection method has been reported to improve the transfection efficiency of plasmid DNA in NP cells, and polyplex micelles made from a vector carrying miRNA-25-3p were used in an IVDD rat model [30]. Clustered Regulatory Interspaced Short Palindromic Repeats-Associated Cas9 (CRISPR/Cas9) is an innovative technology that can be used to target other genes for IVDD treatment. Using the CRISPR/Cas9 gene-editing system in AF cells from patients with chronic LBP, knock out of the transient receptor potential vanilloid type 4 (TRPV4) gene induced the reduction in inflammation [30]. However, the use of gene therapy for IVD regeneration is currently limited to in vitro and in vivo animal studies due to safety concerns. There are no ongoing human clinical trials of gene therapies for IVD regeneration.
2.1.3. Summary
In order to overcome the shortcomings of molecular therapy, future studies will focus on the delivery and controlled release to the degenerated discs. In addition, the combination of growth factors, stem cells, and biomaterials should be a focus going forward.
2.2. Cell-Based Therapies
In the intermediate stages of IVDD, cell transplantation can be used to repopulate the disc. Although the NP cell phenotype has not been well defined, the adult NP contains cells similar to chondrocyte [31]. Cell transplantation to a moderately degenerated disc is a possible treatment that promotes disc regeneration by reproliferating cells that can restore the structural and functional properties of the degenerated disc or delay degeneration [27].
An optimal source of cells suitable for cell transplantation in the degenerative disc remains elusive. Several studies have demonstrated that implantation of IVD-derived cells delays the process of progressive degeneration and, in some cases, promotes disc regeneration in an animal model of IVDD [32,33]. However, since these cells are derived from normal NP tissue, they cannot be obtained without damaging the IVD [25].
Stem cells are a promising candidate cell source for use in cell-based therapies for IVD repair. Most of the stem cells used in disc regeneration experiments are derived from the bone marrow, adipose tissue, umbilical cord blood, umbilical cord Wharton’s jelly, and synovium, because these cells are relatively easy to obtain and can differentiate into chondrogenic and IVD-cell lineages [34,35]. Implantation of MSCs into degenerated discs can prevent cellular apoptosis and inflammation (paracrine effect) or differentiate MSCs into NP cells to restore normal homeostasis and prevent or reverse further degeneration [13]. Injecting stem cells into the degenerated disc has been reported to increase the proteoglycan and water contents of the disc ECM (Table 1) [29].

Table 1.
Cell-based therapies for IVD regeneration.
Notochordal cells have been suggested to cause disc degeneration because their loss is associated with the onset of IVDD [33]. Sheyn et al. demonstrated that notochordal-like cells from human induced pluripotent stem cells (iPSCs) reduce IVDD in an injury-induced porcine model [33]. Therefore, the application of iPSCs is a hot topic in the field of IVD regeneration and has several advantages over embryonic stem cells (ESCs), such as fewer ethics and immune rejection issues [33]. However, iPSCs also have drawbacks in clinical applications, such as tumor formation by genomic integration of reprogramming factors [36].
For the clinical application of stem cells for IVD regeneration, MSCs treatment strategies, cell doses, and efficacy are being investigated in various experimental settings of IVDD and clinical trials [37]. The use of MSCs is generally considered safe and effective in preventing IVDD, but the rate of osteophyte formation has been reported to be around 2.7% [37,38]. Osteophyte formation is believed to be the result of implanted MSC leakage. Therefore, the application of scaffolding materials, such as fibrin, hyaluronan, or atelocollagen, is strongly recommended to prevent cell leakage and reduce the risk of ectopic osteoblast differentiation of MSCs [39,40,41,42,43,44,45,46,47,48,49,50].
To summarize, stem cell therapy can be used to induce IVD repair by preventing cellular apoptosis and inflammation, and by increasing the resident population and ECM production, and there is great interest in developing biomaterials for effective cell delivery, increasing cell viability, and inducing differentiation of stem cells into IVD-like cells.
3. Tissue Engineering for IVD Regeneration
Although many studies have reported alternative biological treatments for IVDD, these approaches have certain limitations. Direct administration of growth factors is associated with the short half-life of growth factors and potential lack of IVD cells as therapeutic targets in severe disc degeneration [29]. Gene therapy has several disadvantages, including inefficient gene delivery, unstable long-term expression, and lack of safety. While cell-based therapies have shown more promising therapeutic potential, the best strategies for effectiveness and safety have yet to be addressed [29]. In a clinical setting, stem cells are implanted in a harsh environment consisting of low cellularity, low glucose, low oxygen, low PH due to high lactic acid accumulation, low nutrients, and an inflammatory milieu [6,51,52,53]. Inflammatory mediators are a key component of progressive IVDD. All these factors can affect the differentiation potential, viability, and metabolism of the implanted stem cells [52]. MSCs can function optimally in inflammatory, hypoxic, acidic, and malnourished environments of the degenerated disc and have an immunomodulatory paracrine effect [52,54,55]. Hypoxia-exposed human MSCs (hMSCs) have been reported to improve tissue protection, but exposure of MSCs to inflammatory factors or hypoxic environment may adversely affect MSC differentiation. Therefore, it is important and necessary to design scaffolds for effective cell delivery and induction of stem cell differentiation for tissue engineering applications (Figure 2).
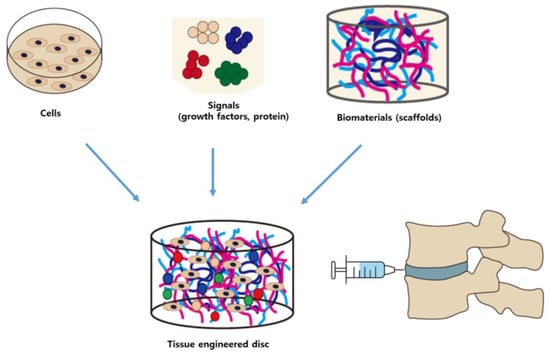
Figure 2.
Combination of cells, signaling molecules (growth factors), and biomaterials for tissue engineering applications for intervertebral disc (IVD) degeneration.
3.1. Biomaterials
In the case of severe IVDD with loss of cell volume and of the physiological disc structure, the disc height must be restored to ensure IVD function [29]. This strategy involves a tissue engineering approach using biomaterials, which may serve as functional alternatives and scaffolds for the IVD tissue [13]. Researchers are using a composite approach that utilizes cell-loaded biomaterials to provide a structural environment for mechanical stability and potential cell regeneration [56]. The biomaterials used include injectable hydrogels and synthetic polymers designed from materials such as alginate, gelatin, polyglycolic acid, polylactic acid, hyaluronic acid (HA), and collagen [57].
Hydrogels can be used as an alternative to NP due to their biophysical properties and ability to absorb water, resist repeated loads, and act as a delivery vehicle [58]. An ideal injectable biomaterial will support cell retention and survival and make it possible to maintain or promote the NP phenotype in vivo. In the absence of biomaterials, cell injection leads to rapid cell death or migration from the injection site [59,60]. Important parameters to consider in the development of biomaterials are material viscosity, gelation rate, final gel stiffness, adhesivity, and degradation time controlled by polymer composition.
HA is a key component of the NP ECM that provides resistance to compression and allows for periodic loading [61,62]. Physiologically, HA has been demonstrated to have cartilage protective and anti-inflammatory properties, which have been shown to be associated with cell-based interventions. Therefore, some clinical trials have used HA as a cell carrier to increase the viscosity of the cell solution and enhance the retention of injected cells [63,64].
Collagen is one of the most widely used materials for tissue regeneration as it has numerous adhesion sites, limited immunogenicity, and is injectable. However, due to its poor degradation and mechanical properties, it has not been widely used for disc repair. Composite collagen hydrogels, on the other hand, have been found to improve the compressive mechanical properties of the scaffold and control the rate of scaffold degradation [27].
Fibrin is a naturally occurring biomaterial that provides intrinsic physical and soluble cues to initiate tissue repair. Biodegradable fibrin hydrogels can be produced as injectable cell carriers and can be mediated by adjusting coagulation protein levels or altering the ionic strength of the system [65,66]. Fibrin-only hydrogels remain vulnerable to cell-mediated remodeling, while fibrin-HA composite hydrogels improve stability by increasing glycosaminoglycan (GAG) synthesis. In addition, silk to fibrin-HA gels significantly improve the mechanical properties and promote chondrogenesis [67]. Silk offers high resistance to compression, and silk-fiber stability, due to its wide range of hydrogen bonds, protein hydrophobicity, and high crystallinity, provides an advantage as a scaffold for IVD bioengineering [68].
Other biomaterials that can be used as a matrix to support AF and NP engineering are chitosan and alginate, which are inexpensive and easily accessible [12]. In addition, these two polymers have a synergistic effect combined with hybrid scaffolding [69]. Chitosan is used as a biodegradable and biocompatible polymer with low toxicity and excellent antibacterial properties. The soft, spongy chitosan-based scaffold has high porosity and pore interconnectivity to support cell adhesion and growth [70]. Alginate, one of the most abundant natural materials, mainly derived from brown algae and some bacteria, is used in a variety of biomedical applications and drug delivery systems due to its excellent biocompatibility, biodegradability, non-antigenicity, and chelation ability [69].
The use of synthetic materials as injectable fillers or cell carriers is a promising strategy to prevent the biomechanical limitations of natural polymer-based hydrogels. Many synthetic biomaterials, such as polyethylene glycol (PEG), PLGA, polyvinyl alcohol (PVA), polyvinylpyrrolidone (PVP), and hydroxyethyl methacrylate (HEMA), have been used as drug delivery and cell carriers [71,72]. The first approach used to restore NP height, function, and motion focused on the use of in situ hydrated synthetic polymers to restore NP hydration, IVD pressure, and disc height. This tactic aimed at mimicking the hydration properties of the NP glycosaminoglycans, which are slowly degraded and modified as they age and degenerate [57]. A copolymeric hydrogel, with the longest history of clinical use, has served as an alternative to NP. However, similar to previously used in situ hydrated polymers, some complications have already been reported, including gel fragmentation during swelling [73]. Other biomaterials, such as NP implant devices, have been developed from an injectable polymer that physically transitions to a gel or solid form. This approach has the advantage of minimizing tissue damage to AF during transplantation. In addition, various strategies, such as chemical cross-linking agents and heat- or pH-induced transition, have been developed. The cross-linked material inhibits proteolysis and induces the stiffness for disc implant [57].
Many scientists have attempted to develop monophasic NP or AF scaffolds such as ECM-based scaffold [74], adipose-derived MSCs (AD-MSCs)-loaded NP tissue-engineered construct [75], ASCs-seeded type II collagen/chondroitin sulfate composite hydrogel [76], and decellularized NP-based scaffold [77] (Table 2). Extensive efforts have also been made to develop biphasic tissue-engineered AF-NP composite scaffolds such as a collagen-GAG co-precipitate (NP-like core)/photochemically crosslinked collagen membranes (AF-like lamellae) composite [78], multiple AF-like lamellae encasing an NP-like core (MSCs-seeded collagen-GAG) [79], engineered nanofibrous disk-like angle-ply structure [80], biomimetic glycosaminoglycan analogues based on sulphonate-containing polymers [81], and multiple HA-PEG composite hydrogel [82] (Table 2). Animal studies using these biphasic scaffolds have shown excellent mechanical and biochemical results, suggesting that mimicking the morphology of the IVD is important for long-term stability and function of the implanted scaffolds [78,79,80,81,82].

Table 2.
Biomaterials for IVD regeneration.
In addition, many studies have demonstrated that differentiation of stem cell into the IVD cell phenotype is promoted by various types of biomaterials; (1) natural biomaterials: collagen type II-chondroitin sulphate hydrogel, gelatin-HA methacrylate hydrogel, silk-protein-based multilayered angle-ply scaffold, chitosan-HA hydrogel, decellularized allogenic IVD, acellular porcine NP hydrogel, NP cell-derived acellular matrix, dextran chitosan and teleostean combined hydrogel, temperature sensitive hydrogel (chitosan-glycerophosphate), chitosan and alginate gel scaffold, alginate and chitosan hydrogels, and self-assembling peptides; (2) Synthetic biomaterials: poly(N-isopropylacrylamide (pNIPAM) hydrogel system, nanofibrous poly(l-lactide) (PLLA) scaffolds, and heparin- poly(ethylene argininylaspartate digylceride) (PEAD) conjugated vehicle; (3) Biosynthetic biomaterials: T1307-fibrinogen hydrogel, HA-pNIPAM hydrogel, and pentosan polysulfate (PEG-HA-PPS) hydrogel [83].
In summary, transplanted stem cells should survive, proliferate, and differentiate into NP-/AF-like cells. The combination of biomaterials and stem cells can provide an effective strategy to enhance effective cell delivery and stem cell differentiation capacity. Although a variety of biomaterials have been studied to investigate the effects of biomaterials on cell delivery and stem cell differentiation, few materials are currently available for clinical application due to the limitations of mechanical properties, immunogenicity, and uncontrollable deviations in inducing stem cells differentiation. In addition to the mechanical properties and biocompatibility of biomaterials, maintaining stem cells activity in a local niche and enhancing the ability of stem cells to differentiate into NP and AF cells facilitates the application of biomaterials in clinical practice [74,75,76,77,78,79,80,81,82,83].
3.2. Tissue Engineering for AF and NP Restoration and Maintenance
The aim of tissue engineering for IVD degeneration is the restoration and maintenance of both AF and NP anatomy and function. Tissue-engineered scaffolds must be able to withstand the physiological IVD loads and have excellent biocompatibility; proper porosity; and shapes, structures, and mechanical properties similar to those of IVD [79].
3.2.1. AF Regeneration and Tissue Engineering
AF is composed of type I collagen and stacked lamellae and is highly organized [84]. AF is needed to transfer stress from the NP, maintain IVD integrity, and protect against damage caused by bending, stretching, and twisting [85,86]. The homeobox protein Mohawk (Mkx) has been reported to be essential for AF development, maintenance, and regeneration. It has been found that Mkx is predominantly expressed in the outer AF, and that removal of Mkx in mice resulted in the loss of numerous tendon- and ligament-related genes in the outer AF. Transplantation of MSCs overexpressing Mkx revealed the AF phenotype and promoted functional AF regeneration [87].
In AF regeneration and tissue engineering, natural materials, such as collagen, HA, chitosan, alginate, silk fibroin, and chondroitin sulfate, as well as natural biologic materials, such as the decellularized matrix from AF, are used to promote tissue regeneration and repair [57,88]. Natural scaffolds have the advantages of having low toxicity and similar properties to those of native tissue, and they can be mass-produced. Synthetic polymer scaffolds can be manufactured and processed based on the desired structural (aligned, angle-ply, hierarchical, bilayer, or biphasic) and mechanical properties of the final engineered tissue [11]. A poly(trimethylene carbonate) (PTMC) scaffold covered with a poly(ester-urethane) (PU) membrane to address AF rupture repair of bovine IVD has been manufactured as a carrier for MSCs. A PTMC scaffold with MSCs and PU membrane has been found to restore the disc height and prevent IVD herniation [89]. Furthermore, biodegradable poly(ether carbonate urethane)urea (PECUU) materials have been produced in AF-derived stem cells (AFSCs) using an electrospinning technique. Moreover, it has been reported that the elasticity of PECUU fibrous scaffolds with AFSCs resembled that of natural AF tissue [90].
3.2.2. NP Regeneration and Tissue Engineering
NP is composed of type II collagen and PGs and contains 77% water. In recent years, bioengineered scaffolds that resemble the native NP structure and its mechanical properties have attracted attention [11]. Easy to inject high molecular weight hyaluronic acid-gelatin-adipic acid dehydrazide (oxi-HAG-ADH) hydrogels with anti-inflammatory and immunosuppressive activities, low viscosity, viscoelasticity similar to that of NP tissue, and expression of NP ECM genes have been fabricated [91]. Choi et al. generated hyaluronic-methylcellulose (HAMC) hydrogels loaded with Wharton’s jelly-derived MSCs (WJ-MSCs), which significantly promoted degenerated disc repair by improving NP cell viability and decreasing ECM degradation [92]. Gan et al. generated a hydrogel with dextran and gelatin as the first network and PEG as the second network to produce hydrogels, forming the optimal 3D interpenetrating network hydrogel. This increased NP cell proliferation, long-term cell retention and survival, and promoted rehydration and regeneration of degenerative NP in animal models [93]. Laminin is the main component of the NP ECM and directly interacts with NP cells to regulate their function. Several laminin mimetic peptides bound to polyacrylamide gels have been reported to be able to support an immature and healthy NP phenotype. These hydrogel scaffolds provided a favorable environment for NP cell proliferation [94]. Wan et al. manufactured a biocompatible self-assembled peptide hydrogel (SAPH) with easily modifiable properties and nanofibrous architecture. They reported that the SAPH scaffold was as strong as native tissue, injectable, and that it restored the IVD cell phenotype and stimulated deposition of aggrecan and type II collagen, which are key NP ECM components [95].
3.2.3. NP-AF Regeneration and Tissue Engineering
NP-AF tissue engineering combines two approaches: NP replacement and AF repair. There are three ways to manufacture NP-AF, namely, using NP and AF cell-seeding scaffolds, integrated biphasic NP-AF scaffolds, and scaffolds made with decellularized natural IVD [11]. Scaffolds seeded with NP and AF cells were separately prepared and assembled into a composite construct. Nesti et al. manufactured a biphasic construct using electrospun MSCs seeded on a PLLA scaffold and HA hydrogel [96]. Choy et al. generated a biphasic NP-AF scaffold with integrated collagen and glycosaminoglycans. The biphasic scaffold was composed of collagen-glycosaminoglycan, which coprecipitates as an NP-like core, and encapsulated in multiple lamellae of photochemically cross-linked collagen membranes that made up AF-like lamellae [79]. This scaffold exhibited mechanical characteristics similar to those of native discs with a ring-independent height recovery of 82–89%. Park et al. generated a scaffold consisting of chondrocytes and AF cells, which were respectively seeded into a scaffold consisting of hydrogel in the center and silk protein in the periphery, respectively [97]. Yang et al. manufactured an IVD scaffold by inversely reconstructing the structure of native IVD and bioprinting bacterial cellulose nanofibers using a high-throughput-optimized micropattern screening microchip in rats [98]. Chan et al. made a 70% endogenous cell-removing scaffold that preserved the glycosaminoglycan content, collagen fibril structure, and mechanical properties of the IVD by altering chemical and physical decellularization [99]. Hensley et al. created a natural NP-AF composite scaffold using decellularized bovine tail IVD and confirmed the presence of type II collagen and glycosaminoglycan in the NP region and the native angle-ply collagen microarchitecture in the AF region [100].
3.2.4. Summary
Tissue engineering techniques have emerged as a possible approach to treat IVDD by replacing degenerated discs with appropriate stem cells and biomaterials. Tissue engineered AF and NP can restore their function by repairing or replacing degenerated discs. Therefore, considerable research is underway on the development of scaffolds suitable for AF and NP regeneration. Many natural and synthetic biomaterials can be used as supporting matrices in AF and NP scaffolds [12]. Advances in manufacturing technologies, material processing and development, surface functionalization, drug delivery systems, and cell integration have accelerated the development of tissue engineering therapies for IVDD.
4. Conclusions
Chronic LBP due to IVDD represents a significant health and social burden. Regenerative tactics are being investigated with significant advances in understanding the characteristics of IVDD (Table 3). Current promising strategies include growth factor injection, gene therapy, cell-based therapy, and tissue engineering using biomaterials. In this review, we investigated biological and tissue engineering approaches for the treatment of IVD degeneration and regeneration strategies. Limitations of biological approaches that remain to be overcome include the short half-life and possible lack of IVD endogenous cells associated with growth factor injection therapy, inefficient gene delivery, unstable long-term expression, and safety issues in gene therapy, and the inflammatory environment, low pH, low oxygen tension, and poor nutritional availability in cell-based therapies. Promising tissue engineering strategies using cells, growth factors, and biomaterials could be utilized to overcome these problems. With the development of tissue engineering, scaffolds are considered the ‘holy grail’ of IVD repair [11]. However, tissue engineering therapy remains challenging due to a lack of accurate understanding of the underlying molecular mechanisms and regulation of IVD physiology. To date, there are no FDA approved intradiscal therapies associated with tissue engineering therapy. Therefore, more sophisticated materials and strategies for clinical application need to be developed. In addition, accurate diagnosis of IVDD and evaluation of therapeutic effectiveness are critical to the development of successful biological therapies. Although T2 mapping and diffusion weighted images(DWI) are newly quantified methods for IVDD evaluation [101], the development of improved non-destructive imaging techniques is essential to evaluate IVDD.

Table 3.
Type of regenerative therapies for intervertebral disc regeneration.
Author Contributions
Conceptualization and methodology: I.H.; writing: C.K.L., D.H.H. and I.H.; data acquisition: H.C. (Hungtae Chung), E.J.R., A.D., J.W.K., H.C. (Hyemin Choi), S.Y.K. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Health Technology Research and Development Project, Ministry for Health and Welfare Affairs (HR16C0002, HI20C0579) and a grant of the National Research Foundation of Korea (NRF) (2020R1A2C4001870.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coric, D.; Pettine, K.; Sumich, A.; Boltes, M.O. Prospective study of disc repair with allogeneic chondrocytes Presented at the 2012 Joint Spine Section Meeting. J. Neurosurg. Spine 2013, 18, 85–95. [Google Scholar] [CrossRef]
- de Schepper, E.I.; Damen, J.; van Meurs, J.B.; Ginai, A.Z.; Popham, M.; Hofman, A.; Koes, B.W.; Bierma-Zeinstra, S.M. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine 2010, 35, 531–536. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Measuring the global burden of disease. N. Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.C.; Ulrich, J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: Review of animal model data. J. Bone Jt. Surg. 2006, 88 (Suppl. 2), 76–82. [Google Scholar] [CrossRef]
- Phillips, F.M.; Slosar, P.J.; Youssef, J.A.; Andersson, G.; Papatheofanis, F. Lumbar spine fusion for chronic low back pain due to degenerative disc disease: A systematic review. Spine 2013, 38, E409–E422. [Google Scholar] [CrossRef]
- Geisler, F.H.; McAfee, P.C.; Banco, R.J.; Blumenthal, S.L.; Guyer, R.D.; Holt, R.T.; Majd, M.E. Prospective, randomized, multicenter FDA IDE study of CHARITÉ artificial disc versus lumbar fusion: Effect at 5-year follow-up of prior surgery and prior discectomy on clinical outcomes following lumbar arthroplasty. SAS J. 2009, 3, 17–25. [Google Scholar] [CrossRef]
- Ghiselli, G.; Wang, J.C.; Bhatia, N.N.; Hsu, W.K.; Dawson, E.G. Adjacent segment degeneration in the lumbar spine. J. Bone Jt. Surg. 2004, 86, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, W.; Xia, T.; Yang, L. Disordered mechanical stress and tissue engineering therapies in intervertebral disc degeneration. Polymers 2019, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Stergar, J.; Gradisnik, L.; Velnar, T.; Maver, U. Intervertebral disc tissue engineering: A brief review. Bosn. J. Basic Med. Sci. 2019, 19, 130–137. [Google Scholar] [CrossRef]
- Ju, D.G.; Kanim, L.E.; Bae, H.W. Intervertebral disc repair: Current concepts. Glob. Spine J. 2020, 10 (Suppl. 2), 130S–136S. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. Intervertebral disc diseases PART 2: A review of the current diagnostic and treatment strategies for intervertebral disc disease. Int. J. Mol. Sci. 2020, 21, 2135. [Google Scholar] [CrossRef]
- Griffith, J.F.; Wang, Y.-X.J.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.T.; Leung, P.C. Modified Pfirrmann Grading System for lumbar intervertebral disc degeneration. Spine 2007, 32, E708–E712. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. intervertebral disk degeneration and repair. Neurosurgery 2017, 80, S46–S54. [Google Scholar] [CrossRef]
- Travascio, F.; Elmasry, S.; Asfour, S. Modeling the role of IGF-1 on extracellular matrix biosynthesis and cellularity in intervertebral disc. J. Biomech. 2014, 47, 2269–2276. [Google Scholar] [CrossRef]
- Feng, C.; Liu, H.; Yang, Y.; Huang, B.; Zhou, Y. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell. Physiol. Biochem. 2015, 35, 1–16. [Google Scholar] [CrossRef]
- Cho, H.; Lee, S.; Park, S.H.; Huang, J.; Hasty, K.A.; Kim, S.J. Synergistic effect of combined growth factors in porcine intervertebral disc degeneration. Connect. Tissue Res. 2013, 54, 181–186. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, G.D.; Newman, I.B.; Carapezza, M.A. Effect of long-term osmotic loading culture on matrix synthesis from intervertebral disc cells. BioRes. Open Access 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Sampat, S.R.; O’Connell, G.D.; Fong, J.V.; Alegre-Aguarón, E.; Ateshian, G.A.; Hung, C.T. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng. Part A 2011, 17, 2259–2265. [Google Scholar] [CrossRef]
- Presciutti, S.M.; Paglia, D.N.; Karukonda, T.; Soung do, Y.; Guzzo, R.; Drissi, H.; Moss, I.L. PDGF-BB inhibits intervertebral disc cell apoptosis in vitro. J. Orthop. Res. 2014, 32, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Miyamoto, K.; An, H.S.; Thonar, E.J.-M.A.; Andersson, G.B.J.; Masuda, K. Recombinant human osteogenic protein-1 upregulates proteoglycan metabolism of human anulus fibrosus and nucleus pulposus cells. Spine 2007, 32, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Y.; Yan, J.J.; Hsieh, C.C.; Chang, M.S.; Lin, R.M. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: An animal experiment. Spine 2007, 32, 1174–1180. [Google Scholar] [CrossRef]
- Walsh, A.J.L.; Bradford, D.S.; Lotz, J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine 2004, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ma, S.-Y.; Feng, G.; Shen, F.H.; Li, X.J. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. Spine J. 2010, 10, 32–41. [Google Scholar] [CrossRef]
- O’Connell, G.D.; Leach, J.K.; Klineberg, E.O. Tissue engineering a biological repair strategy for lumbar disc herniation. BioRes. Open Access 2015, 4, 431–445. [Google Scholar] [CrossRef]
- Yan, J.; Yang, S.; Sun, H.; Guo, D.; Wu, B.; Ji, F.; Zhou, D. Effects of releasing recombinant human growth and differentiation factor-5 from poly(lactic-co-glycolic acid) microspheres for repair of the rat degenerated intervertebral disc. J. Biomater. Appl. 2014, 29, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Ropper, A.E.; Konya, D.; Kabatas, S.; Toktas, Z.; Aljuboori, Z.; Zeng, X.; Chi, J.H.; Zafonte, R.; Teng, Y.D. Biological approaches to treating intervertebral disk degeneration: Devising stem cell therapies. Cell Transplant. 2015, 24, 2197–2208. [Google Scholar] [CrossRef]
- Roh, E.; Darai, A.; Kyung, J.; Choi, H.; Kwon, S.; Bhujel, B.; Kim, K.; Han, I. Genetic therapy for intervertebral disc degeneration. Int. J. Mol. Sci. 2021, 22, 1579. [Google Scholar] [CrossRef]
- Risbud, M.V.; Schoepflin, Z.R.; Mwale, F.; Kandel, R.A.; Grad, S.; Iatridis, J.C.; Sakai, D.; Hoyland, J.A. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J. Orthop. Res. 2015, 33, 283–293. [Google Scholar] [CrossRef]
- Sakai, D.; Andersson, G.B.J. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P.; et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506–7524. [Google Scholar] [CrossRef]
- Chen, S.; Emery, S.E.; Pei, M. Coculture of synovium-derived stem cells and nucleus pulposus cells in serum-free defined medium with supplementation of transforming growth factor-beta1: A potential application of tissue-specific stem cells in disc regeneration. Spine 2009, 34, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Leckie, S.K.; Sowa, G.A.; Bechara, B.P.; Hartman, R.A.; Coelho, J.P.; Witt, W.T.; Dong, Q.D.; Bowman, B.W.; Bell, K.M.; Vo, N.V.; et al. Injection of human umbilical tissue–derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013, 13, 263–272. [Google Scholar] [CrossRef]
- Chang, E.-A.; Jin, S.-W.; Nam, M.-H.; Kim, S.-D. human induced pluripotent stem cells: Clinical significance and applications in neurologic diseases. J. Korean Neurosurg. Soc. 2019, 62, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Yim, R.L.-H.; Lee, J.T.-Y.; Bow, C.H.; Meij, B.; Leung, V.; Cheung, K.M.; Vavken, P.; Samartzis, D. A Systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: Insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014, 23, 2553–2567. [Google Scholar] [CrossRef]
- Salzig, D.; Schmiermund, A.; Gebauer, E.; Fuchsbauer, H.-L.; Czermak, P. Influence of porcine intervertebral disc matrix on stem cell differentiation. J. Funct. Biomater. 2011, 2, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Sowa, G.; Hubert, M.; Gilbertson, L.G.; Denaro, V.; Kang, J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 2011, 6, 348–355. [Google Scholar] [CrossRef]
- Shi, P.; Chee, A.; Liu, W.; Chou, P.-H.; Zhu, J.; An, H.S. Therapeutic effects of cell therapy with neonatal human dermal fibroblasts and rabbit dermal fibroblasts on disc degeneration and inflammation. Spine J. 2019, 19, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.Q.; Pereira, C.L.; Ferreira, J.R.; Maia, A.F.; Gomez-Lazaro, M.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Immunomodulation of human mesenchymal stem/stromal cells in intervertebral disc degeneration: Insights from a proinflammatory/degenerative ex vivo model. Spine 2018, 43, e673–e682. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Deng, G.; Ma, J.; Huang, X.; Yu, J.; Xi, Y.; Ye, X. Transplantation of hypoxic-preconditioned bone mesenchymal stem cells retards intervertebral disc degeneration via enhancing implanted cell survival and migration in rats. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maidhof, R.; Rafiuddin, A.; Chowdhury, F.; Jacobsen, T.; Chahine, N.O. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J. Orthop. Res. 2017, 35, 32–40. [Google Scholar] [CrossRef]
- Hang, D.; Li, F.; Che, W.; Wu, X.; Wan, Y.; Wang, J.; Zheng, Y. One-stage positron emission tomography and magnetic resonance imaging to assess mesenchymal stem cell survival in a canine model of intervertebral disc degeneration. Stem Cells Dev. 2017, 26, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Steffen, F.; Smolders, L.A.; Roentgen, A.M.; Bertolo, A.; Stoyanov, J. Bone marrow-derived mesenchymal stem cells as autologous therapy in dogs with naturally occurring intervertebral disc disease: Feasibility, safety, and preliminary results. Tissue Eng. Part C Methods 2017, 23, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: A randomized controlled trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef]
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Williams, C.J.; Hyzy, M.; Ichim, T.; Freeman, M. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: A pilot study on safety and efficacy. J. Transl. Med. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Kumar, H.; Ha, D.-H.; Lee, E.-J.; Park, J.H.; Shim, J.H.; Ahn, T.-K.; Kim, K.-T.; Ropper, A.E.; Sohn, S.; Kim, C.-H.; et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Pettine, K.A.; Suzuki, R.K.; Sand, T.T.; Murphy, M.B. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int. Orthop. 2017, 41, 2097–2103. [Google Scholar] [CrossRef]
- Tschugg, A.; Michnacs, F.; Strowitzki, M.; Meisel, H.J.; Thomé, C. A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART Disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: Study protocol for a randomized controlled trial. Trials 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Grunhagen, T.; Shirazi-Adl, A.; Fairbank, J.C.; Urban, J.P. Intervertebral disk nutrition: A review of factors influencing concentrations of nutrients and metabolites. Orthop. Clin. N. Am. 2011, 42, 465–477. [Google Scholar] [CrossRef]
- Krock, E.; Rosenzweig, D.H.; Haglund, L. The inflammatory milieu of the degenerate disc: Is mesenchymal stem cell-based therapy for intervertebral disc repair a feasible approach? Curr. Stem Cell Res. Ther. 2015, 10, 317–328. [Google Scholar] [CrossRef]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, B.; Rosenzweig, D.H.; Krock, E.; Roughley, P.J.; Beckman, L.; Steffen, T.; Weber, M.H.; Ouellet, J.A.; Haglund, L. Acute mechanical injury of the human intervertebral disc: Link to degeneration and pain. Eur. Cells Mater. 2014, 28, 98–111. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.R.; Chiverton, N.; Cross, A.K.; Le Maitre, C.L. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res. Ther. 2014, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moure, J.; Moore, C.A.; Kim, K.; Karim, A.; Smith, K.; Barbosa, Z.; Van Eps, J.; Rameshwar, P.; Weiner, B. Novel therapeutic strategies for degenerative disc disease: Review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018, 6, 2050312118761674. [Google Scholar] [CrossRef]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Liang, C.-Z.; Li, H.; Tao, Y.-Q.; Peng, L.-H.; Gao, J.-Q.; Wu, J.-J.; Li, F.-C.; Hua, J.-M.; Chen, Q.-X. Dual release of dexamethasone and TGF-β3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013, 9, 9423–9433. [Google Scholar] [CrossRef]
- Francisco, A.T.; Mancino, R.J.; Bowles, R.D.; Brunger, J.M.; Tainter, D.M.; Chen, Y.-T.; Richardson, W.J.; Guilak, F.; Setton, L.A. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials 2013, 34, 7381–7388. [Google Scholar] [CrossRef]
- Henriksson, H.B.; Svanvik, T.; Jonsson, M.; Hagman, M.; Horn, M.; Lindahl, A.; Brisby, H. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine 2009, 34, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Leckie, A.E.; Akens, M.K.; Woodhouse, K.A.; Yee, A.J.; Whyne, C.M. Evaluation of thiol-modified hyaluronan and elastin-like polypeptide composite augmentation in early-stage disc degeneration: Comparing 2 minimally invasive techniques. Spine 2012, 37, E1296–E1303. [Google Scholar] [CrossRef]
- Malhotra, N.R.; Han, W.M.; Beckstein, J.; Cloyd, J.; Chen, W.; Elliott, D.M. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine 2012, 37, E1099–E1105. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 1–18. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Miller, S.; Case, E.; Leach, J. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011, 7, 691–699. [Google Scholar] [CrossRef]
- Park, S.-H.; Cho, H.; Gil, E.S.; Mandal, B.B.; Min, B.-H.; Kaplan, D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng. Part A 2011, 17, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Gil, E.S.; Mandal, B.B.; Cho, H.S.; Kluge, J.A.; Min, B.-H.; Kaplan, D.L. Annulus fibrosus tissue engineering using lamellar silk scaffolds. J. Tissue Eng. Regen. Med. 2012, 6 (Suppl. 3), s24–s33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, H.-J.C.; Meij, B.P.; Onis, D.; Van Der Veen, A.J.; Saralidze, K.; Smolders, L.A.; Huizinga, J.G.; Knetsch, M.L.W.; Luijten, P.R.; Visser, F.; et al. Design, synthesis, imaging, and biomechanics of a softness-gradient hydrogel nucleus pulposus prosthesis in a canine lumbar spine model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gerges, I.; Tamplenizza, M.; Lenardi, C.; Forsyth, N.R.; Liu, Y. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: A potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater. 2014, 10, 3463–3474. [Google Scholar] [CrossRef]
- Durdag, E.; Ayden, O.; Albayrak, S.; Atci, I.B.; Armagan, E. Fragmentation to epidural space: First documented complication of Gelstix(TM.). Turk. Neurosurg. 2014, 24, 602–605. [Google Scholar]
- Penolazzi, L.; Pozzobon, M.; Bergamin, L.S.; D’Agostino, S.; Francescato, R.; Bonaccorsi, G.; De Bonis, P.; Cavallo, M.; Lambertini, E.; Piva, R. Extracellular matrix from decellularized Wharton’s jelly improves the behavior of cells from degenerated intervertebral disc. Front. Bioeng. Biotechnol. 2020, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Kaito, T.; Yarimitsu, S.; Hashimoto, K.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Takenaka, S.; Makino, T.; Sakai, Y.; et al. Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 2019, 87, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018, 71, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Huang, X.; Fang, W.; Tao, Y.; Zhao, T.; Liang, C.; Hua, J.; Chen, Q.; Li, F. Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration. Acta Biomater. 2018, 81, 115–128. [Google Scholar] [CrossRef]
- Choy, A.T.H.; Chan, B.P. A Structurally and Functionally Biomimetic Biphasic Scaffold for Intervertebral Disc Tissue Engineering. PLoS ONE 2015, 10, e0131827. [Google Scholar] [CrossRef] [PubMed]
- Chik, T.K.; Chooi, W.H.; Li, Y.Y.; Ho, F.C.; Cheng, H.W.; Choy, T.H.; Sze, K.Y.; Luk, K.K.D.; Cheung, K.M.C.; Chan, B.P. Bioengineering a Multicomponent Spinal Motion Segment Construct-A 3D Model for complex tissue engineering. Adv. Healthc. Mater. 2014, 4, 99–112. [Google Scholar] [CrossRef]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.; Roberts, S.; Urban, J.; Menage, J.; Bramhill, J.; Campbell, D.; Franklin, V.; Lydon, F.; Merkher, Y.; Maroudas, A.; et al. Injectable hydrogels with high fixed charge density and swelling pressure for nucleus pulposus repair: Biomimetic glycosaminoglycan analogues. Acta Biomater. 2014, 10, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.G.; Francisco, A.T.; Niu, Z.; Mancino, R.L.; Craig, S.L.; Setton, L.A. Screening of hyaluronic acid–poly(ethylene glycol) composite hydrogels to support intervertebral disc cell biosynthesis using artificial neural network analysis. Acta Biomater. 2014, 10, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, D.; Liu, S.; Li, J.; Qing, X.; Shao, Z. Biomaterials-induced stem cells specific differentiation into intervertebral disc lineage cells. Front. Bioeng. Biotechnol. 2020, 8, 56. [Google Scholar] [CrossRef]
- Ishihara, H.; Warensjo, K.; Roberts, S.; Urban, J.P. Proteoglycan synthesis in the intervertebral disk nucleus: The role of extracellular osmolality. Am. J. Physiol. 1997, 272 Pt 1, C1499–C1506. [Google Scholar] [CrossRef]
- Schollum, M.L.; Robertson, P.A.; Broom, N.D. ISSLS prize winner: Microstructure and mechanical disruption of the lumbar disc annulus: Part I: A microscopic investigation of the translamellar bridging network. Spine 2008, 33, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fairbank, J.C.T.; Roberts, S.; Urban, J.P.G. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine 2005, 30, 1815–1820. [Google Scholar] [CrossRef]
- Nakamichi, R.; Ito, Y.; Inui, M.; Onizuka, N.; Kayama, T.; Kataoka, K.; Suzuki, H.; Mori, M.; Inagawa, M.; Ichinose, S.; et al. Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat. Commun. 2016, 7, 12503. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, B.; Yang, Q.; Li, X.; Ma, X.; Xia, Q.; Zhang, Y.; Zhang, C.; Wu, Y.; Zhang, Y. Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS ONE 2014, 9, e86723. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, T.; Blanquer, S.B.; Benneker, L.M.; Grijpma, D.W.; Richards, R.G.; Alini, M.; Eglin, D.; Grad, S.; Li, Z. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials 2015, 42, 11–19. [Google Scholar] [CrossRef]
- Zhu, C.; Li, J.; Liu, C.; Zhou, P.; Yang, H.; Li, B. Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 2016, 29, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Su, W.-Y.; Yang, S.-H.; Gefen, A.; Lin, F.-H. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater. 2013, 9, 5181–5193. [Google Scholar] [CrossRef]
- Choi, U.Y.; Joshi, H.P.; Payne, S.; Kim, K.T.; Kyung, J.W.; Choi, H.; Cooke, M.J.; Kwon, S.Y.; Roh, E.J.; Sohn, S.; et al. An Injectable Hyaluronan–Methylcellulose (HAMC) Hydrogel combined with Wharton’s jelly-derived mesenchymal Stromal cells (WJ-MSCs) promotes degenerative disc repair. Int. J. Mol. Sci. 2020, 21, 7391. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, P.; Wang, L.; Mo, X.; Song, L.; Xu, Y.; Zhao, C.; Ouyang, B.; Tu, B.; Luo, L.; et al. An interpenetrating network-strengthened and toughened hydrogel that supports cell-based nucleus pulposus regeneration. Biomaterials 2017, 136, 12–28. [Google Scholar] [CrossRef]
- Bridgen, D.T.; Fearing, B.V.; Jing, L.; Sanchez-Adams, J.; Cohan, M.C.; Guilak, F.; Chen, J.; Setton, L.A. Regulation of human nucleus pulposus cells by peptide-coupled substrates. Acta Biomater. 2017, 55, 100–108. [Google Scholar] [CrossRef]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.J.; Li, W.-J.; Shanti, R.M.; Jiang, Y.J.; Jackson, W.; Freedman, B.A.; Kuklo, T.R.; Giuliani, J.R.; Tuan, R.S. Intervertebral disc tissue engineering using a Novel Hyaluronic Acid–Nanofibrous Scaffold (HANFS) amalgam. Tissue Eng. Part A 2008, 14, 1527–1537. [Google Scholar] [CrossRef]
- Park, S.-H.; Gil, E.S.; Cho, H.; Mandal, B.B.; Tien, L.W.; Min, B.-H.; Kaplan, D.L. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng. Part A 2012, 18, 447–458. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Zhang, W.; Sun, Z.; Li, Y.; Yang, M.; Zeng, D.; Peng, B.; Zheng, W.; Jiang, X.; et al. Reverse reconstruction and bioprinting of bacterial cellulose-based functional total intervertebral disc for therapeutic implantation. Small 2018, 14, 1702582. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.K.; Leung, V.Y.; Tam, V.; Lu, W.W.; Sze, K.; Cheung, K.M. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater. 2013, 9, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Hensley, A.; Rames, J.; Casler, V.; Rood, C.; Walters, J.; Fernandez, C.; Gill, S.; Mercuri, J.J. Decellularization and characterization of a whole intervertebral disk xenograft scaffold. J. Biomed. Mater. Res. Part A 2018, 106, 2412–2423. [Google Scholar] [CrossRef]
- Da Costa, R.C.; De Decker, S.; Lewis, M.J.; Volk, H.; Canine Spinal Cord Injury Consortium (CANSORT-SCI). Diagnostic imaging in intervertebral disc disease. Front. Vet. Sci. 2020, 7, 588338. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).