Index of Body Inflammation for Maxillofacial Surgery Purpose-to Make the Soluble Urokinase-Type Plasminogen Activator Receptor Serum Level Independent on Patient Age
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kramer, F.J.; Baethge, C.; Swennen, G.; Teltzrow, T.; Schulze, A.; Berten, J.; Brachvogel, P. Intra-and perioperative complications of the LeFort I osteotomy: A prospective evaluation of 1000 patients. J. Craniofac. Surg. 2004, 15, 971–979. [Google Scholar] [CrossRef]
- Pereira-Filho, V.A.; Gabrielli, M.F.R.; Gabrielli, M.A.C.; Pinto, F.A.; Rodrigues-Junior, A.L.; Klüppel, L.E.; Passeri, L.A. Incidence of maxillary sinusitis following Le fort I osteotomy: Clinical, radiographic, and endoscopic study. J. Oral. Maxillofac. Surg. 2011, 69, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Cousin, A.S.; Bouletreau, P.; Giai, J.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Severity and long-term complications of surgical site infections after orthognathic surgery: A retrospective study. Sci. Rep. 2020, 10, 12015. [Google Scholar] [CrossRef] [PubMed]
- Eslamipour, F.; Najimi, A.; Tadayonfard, A.; Azamian, Z. Impact of orthognathic surgery on quality of life in patients with dentofacial deformities. Int. J. Dent. 2017, 4103905. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, M.K.; Kang, S.H. Maxillary sinus haziness and facial swelling following suction drainage in the maxilla after orthognathic surgery. Maxillofac. Plast Reconstr. Surg. 2020, 42, 33. [Google Scholar] [CrossRef]
- Dan, A.E.; Thygesen, T.H.; Pinholt, E.M. Corticosteroid administration in oral and orthognathic surgery: A systematic review of the literature and meta-analysis. J. Oral. Maxillofac. Surg. 2010, 68, 2207–2220. [Google Scholar] [CrossRef]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers. 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Eugen-Olsen, J.; Andersen, O.; Linneberg, A.; Ladelund, S.; Hansen, T.W.; Langkilde, A.; Petersen, J.; Pielak, T.; Møller, L.N.; Jeppesen, J.; et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J. Intern. Med. 2010, 268, 296–308. [Google Scholar] [CrossRef]
- Eapen, D.J.; Manocha, P.; Ghasemzadeh, N.; Patel, R.S.; Al Kassem, H.; Hammadah, M.; Veledar, E.; Le, N.A.; Pielak, T.; Thorball, C.W.; et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J. Am. Heart Assoc. 2014, 3e001118. [Google Scholar] [CrossRef]
- Lyngbæk, S.; Andersson, C.; Marott, J.L.; Møller, D.V.; Christiansen, M.; Iversen, K.K.; Clemmensen, P.; Eugen-Olsen, J.; Hansen, P.R.; Jeppesen, J.L. Soluble urokinase plasminogen activator receptor for risk prediction in patients admitted with acute chest pain. Clin. Chem. 2013, 59, 1621–1629. [Google Scholar] [CrossRef]
- Hayek, S.S.; Sever, S.; Ko, Y.A.; Trachtman, H.; Awad, M.; Wadhwani, S.; Altintas, M.M.; Wei, C.; Hotton, A.L.; French, A.L.; et al. Soluble urokinase receptor and chronic kidney disease. N. Engl. J. Med. 2015, 373, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Scolletta, S.; Taccone, F.S.; Covajes, C.; Santonocito, C.; Cortes, D.O.; Grazulyte, D.; Gottin, L.; Vincent, J.L. Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. J. Crit. Care. 2014, 29, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ploug, M.; Rønne, E.; Behrendt, N.; Jemsen; Blasi, F.; Danø, K. Cellular Receptor for Urokinase Plasminogen Activator. J. Biol. Chem. 1991, 266, 1926–1933. [Google Scholar] [CrossRef]
- Haupt, T.H.; Rasmussen, L.J.H.; Kallemose, T.; Ladelund, S.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Healthy lifestyles reduce suPAR and mortality in a Danish general population study. Immun. Ageing 2019, 16. [Google Scholar] [CrossRef]
- Tzanakaki, G.; Paparoupa, M.; Kyprianou, M.; Barbouni, A.; Eugen-Olsen, J.; Kourea-Kremastinou, J. Elevated soluble urokinase receptor values in CSF, age and bacterial meningitis infection are independent and additive risk factors of fatal outcome. Eur J. Clin. Microbiol. Infect. Dis. 2012, 31, 1157–1162. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Desmedt, S.; Desmedt, V.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit. Rev. Clin. Lab Sci. 2017, 54, 117–133. [Google Scholar] [CrossRef]
- Lyngbæk, S.; Sehestedt, T.; Marott, J.L.; Hansen, T.W.; Olsen, M.H.; Andersen, O.; Linneberg, A.; Madsbad, S.; Haugaard, S.B.; Eugen-Olsen, J.; et al. CRP and suPAR are differently related to anthropometry and subclinical organ damage. Int. J. Cardiol. 2013, 167, 781–785. [Google Scholar] [CrossRef]
- Bourassa, K.; Rasmussen, L.; Moffitt, T.; Caspi, A. Stressful Life Events and Inflammation in Midlife: Comparing Associations With suPAR, CRP, and IL-6. Innov. Aging. 2020, 4, 250. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Caspi, A.; Moffitt, T. Associations Between a New Biomarker of Elevated Chronic Inflammation and Accelerated Aging. Innov. Aging 2020, 4, 141–142. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Caspi, A.; Ambler, A.; Danese, A.; Elliott, M.; Eugen-Olsen, J.; Hariri, A.R.; Harrington, H.; Houts, R.; Poulton, R.; et al. Association Between Elevated suPAR, a New Biomarker of Inflammation, and Accelerated Aging. J.. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Haupt, T.H.; Kallemose, T.; Ladelund, S.; Rasmussen, L.J.; Thorball, C.W.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Risk factors associated with serum levels of the inflammatory biomarker soluble urokinase plasminogen activator receptor in a general population. Biomark Insights 2014, 16, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wlazel, R.N.; Szwabe, K.; Guligowska, A.; Kostka, T. Soluble urokinase plasminogen activator receptor level in individuals of advanced age. Sci. Rep. 2020, 10, 15426. [Google Scholar] [CrossRef] [PubMed]
- Nocini, P.F.; D’Agostino, A.; Trevisiol, L.; Favero, V.; Pessina, M.; Procacci, P. Is Le Fort I osteotomy associated with maxillary sinusitis? J. Oral. Maxillofac. Surg. 2016, 74, 400–e1. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.S.; Thrash, W.J.; Zysset, M.K. Incidence of maxillary sinusitis following Le fort I maxillary osteotomy. J. Oral. Maxillofac. Surg. 1986, 44, 100–103. [Google Scholar] [CrossRef]
- Eliason, M.J.; Capra, G.; LaBanc, A.; Braxton, J.; Hamersley, E.; Radabaugh, J.P. A pilot study assessing the incidence of chronic sinusitis following Le fort I osteotomy in maxillofacial surgery. J. Craniofacial. Surg. 2019, 30, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Muire, P.J.; Mangum, L.H.; Wenke, J.C. Time Course of Immune Response and Immunomodulation During Normal and Delayed Healing of Musculoskeletal Wounds. Front. Immunol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Siemionow, M. The past the present and the future of face transplantation. Curr. Opin. Organ. Transplant. 2020, 25, 568–575. [Google Scholar] [CrossRef]
- Demant, S.; Hermann, N.V.; Darvann, T.A.; Zak, M.; Schatz, H.; Larsen, P.; Kreiborg, S. 3D analysis of facial asymmetry in subjects with juvenile idiopathic arthritis. Rheumat 2011, 50, 586–592. [Google Scholar] [CrossRef]
- Lypka, M.; Shah, K.; Jones, J. Prosthetic temporomandibular joint reconstruction in a cohort of adolescent females with juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2020, 18, 68. [Google Scholar] [CrossRef]
- Raggam, R.B.; Wagner, J.; Prüller, F.; Grisold, A.; Leitner, E.; Zollner-Schwetz, I.; Valentin, T.; Krause, R.; Hoenigl, M. Soluble urokinase plasminogen activator receptor predicts mortality in patients with systemic inflammatory response syndrome. J. Intern. Med. 2014, 276, 651–658. [Google Scholar] [CrossRef]
- Wlazeł, R.N.; Migała, M.; Zielińska, M.; Pawlicki, L.; Rośniak-Bąk, K.; Szadkowska, I. Soluble urokinase plasminogen activator receptor in one-year prediction of major adverse cardiac events in patients after first myocardial infarction treated with primary percutaneous coronary intervention. Arch. Med. Sci. 2016, 15, 72–77. [Google Scholar] [CrossRef]
- Wlazeł, R.N.; Szadkowska, I.; Bartncki, P.; Rośniak-Bąk, K.; Rysz, J. Clinical and prognostic usefulness of soluble urokinase plasminogen activator receptor in hemodialysis patients. Int. Urol. Nephrol. 2018, 50, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Dowgierd, K.; Larysz, D.; Szymor, P.; Kozakiewicz, M. Alterations of upper airway volume caused by Le Fort III osteodistraction in children. J. Craniomaxillofac. Surg. 2020, 48, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Sellari-Franceschini, S.; Dallan, I.; Bajraktari, A.; Fiacchini, G.; Nardi, M.; Rocchi, R.; Marcocci, C.; Marinò, M.; Casani, A.P. Surgical complications in orbital decompression for Graves’ orbitopathy. Complicanze chirurgiche in pazienti sottoposti a decompressione orbitaria per oftalmopatia di Graves. Acta Otorhinolaryngol. Ital. 2016, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Duckworth, E.A. Management of infections complicating the orbitocranial approaches: Report of two cases and review of literature. Surg. Neurol. Int. 2015, 6, 89. [Google Scholar] [CrossRef]
- Windfuhr, J.P. Malpractice claims and unintentional outcome of tonsil surgery and other standard procedures in otorhinolaryngology. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2013, 12, Doc08. [Google Scholar] [CrossRef]
- Thereza-Bussolaro, C.; Galván Galván, J.; Pachêco-Pereira, C.; Flores-Mir, C. Maxillary osteotomy complications in piezoelectric surgery compared to conventional surgical techniques: A systematic review. Int. J. Oral. Maxillofac. Surg. 2019, 2019. 48, 720–731. [Google Scholar] [CrossRef]
- Steel, B.J.; Cope, M.R. Unusual and rare complications of orthognathic surgery: A literature review. J. Oral. Maxillofac. Surg. 2012, 70, 1678–1691. [Google Scholar] [CrossRef]
- Barrier, A.; Breton, P.; Girard, R.; Dubost, J.; Bouletreau, P. Les infections du site opératoire en chirurgie orthognathique et leurs facteurs de risque. Rev. Stomatol. Chir. Maxillofac. 2009, 110, 127–134. [Google Scholar] [CrossRef]
- Davis, C.M.; Gregoire, C.E.; Davis, I.; Steeves, T.W. Prevalence of Surgical Site Infections Following Orthognathic Surgery: A Double-Blind, Randomized Controlled Trial on a 3-Day Versus 1-Day Postoperative Antibiotic Regimen. J. Oral. Maxillofac. Surg. 2017, 75, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Fama, F.; Cicciu, M.; Sindoni, A.; Nastro-Siniscalchi, E.; Falzea, R.; Cervino, G.; Polito, F.; De Ponte, F.; Gioffre-Florio, M. Maxillofacial and concomitant serious injuries: An eight-year single center experience. Chin. J. Traumatol. 2017, 20, 4–8. [Google Scholar] [CrossRef] [PubMed]
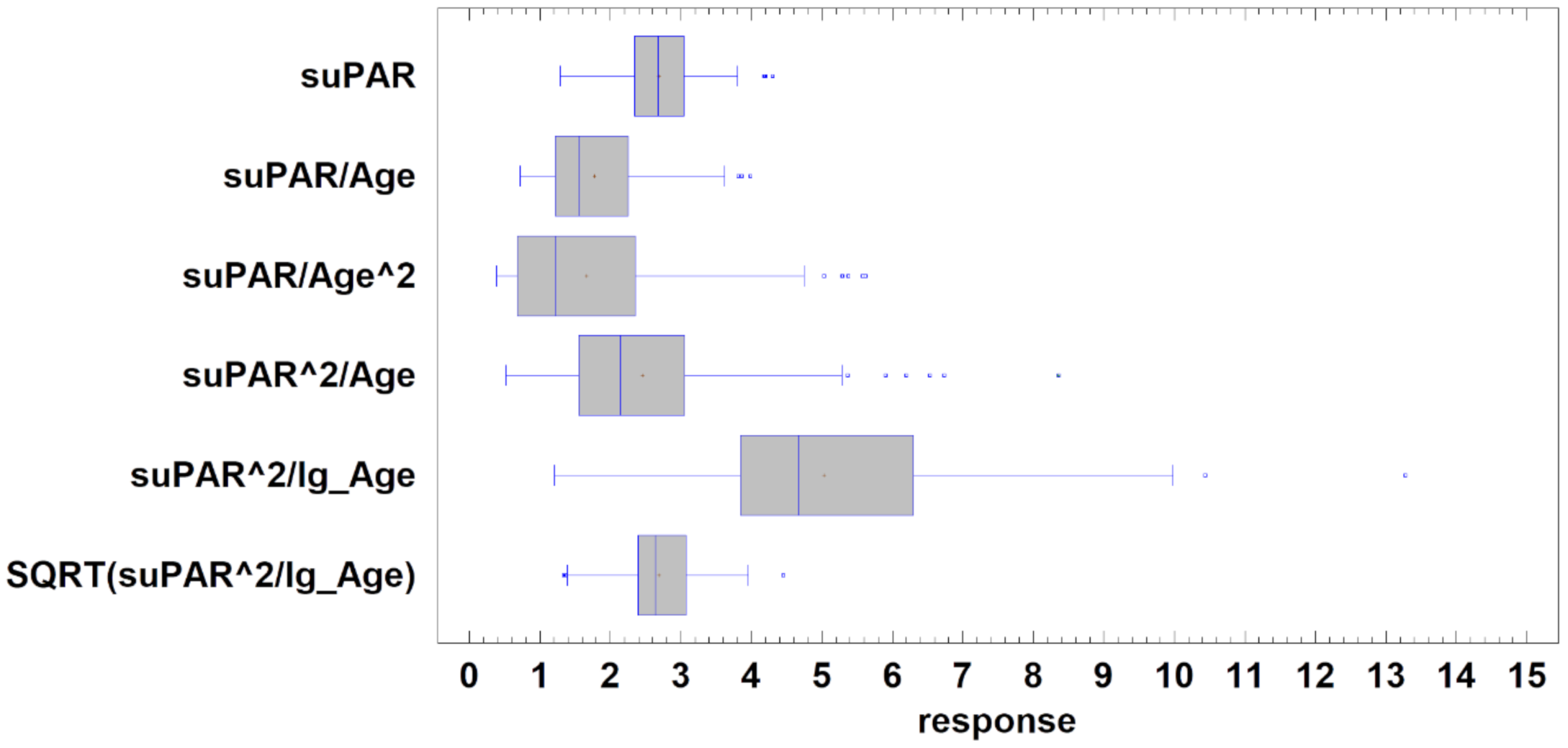



| IBI Modality Name | Avarage ± SD | Stnd. Skewness 1 | Stnd. Kurtosis 2 | Distribution |
|---|---|---|---|---|
| suPAR (raw data) | 2.69 ± 0.57 ng/mL | 0.796 | 0.501 | Normal |
| suPAR/Age | 1.77 ± 0.72 | 5.428 | 0.930 | |
| suPAR/Age^2 | 1.66 ± 1.25 | 7.324 | 3.056 | |
| suPAR^2/Age | 2.45 ± 1.28 | 7.898 | 8.017 | |
| suPAR^2/lg_Age | 5.03 ± 2.03 3 | 3.928 | 2.619 | |
| SQRT(suPAR^2/lg_Age) | 2.69 ± 0.56 3 | 0.141 | 0.503 | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakiewicz, M.; Trzcińska-Kubik, M.; Wlazeł, R.N. Index of Body Inflammation for Maxillofacial Surgery Purpose-to Make the Soluble Urokinase-Type Plasminogen Activator Receptor Serum Level Independent on Patient Age. Appl. Sci. 2021, 11, 1345. https://doi.org/10.3390/app11031345
Kozakiewicz M, Trzcińska-Kubik M, Wlazeł RN. Index of Body Inflammation for Maxillofacial Surgery Purpose-to Make the Soluble Urokinase-Type Plasminogen Activator Receptor Serum Level Independent on Patient Age. Applied Sciences. 2021; 11(3):1345. https://doi.org/10.3390/app11031345
Chicago/Turabian StyleKozakiewicz, Marcin, Magdalena Trzcińska-Kubik, and Rafał Nikodem Wlazeł. 2021. "Index of Body Inflammation for Maxillofacial Surgery Purpose-to Make the Soluble Urokinase-Type Plasminogen Activator Receptor Serum Level Independent on Patient Age" Applied Sciences 11, no. 3: 1345. https://doi.org/10.3390/app11031345
APA StyleKozakiewicz, M., Trzcińska-Kubik, M., & Wlazeł, R. N. (2021). Index of Body Inflammation for Maxillofacial Surgery Purpose-to Make the Soluble Urokinase-Type Plasminogen Activator Receptor Serum Level Independent on Patient Age. Applied Sciences, 11(3), 1345. https://doi.org/10.3390/app11031345







