Abstract
Extended postoperative mandibular reconstructions due to orofacial disease involving the temporomandibular joint (TMJ) in immature patients remain a challenge as a result of ongoing growth, which is usually affected by the disease and treatment. Current reconstructive techniques based fully on alloplastic total joint replacement fail to meet fully the anatomical and functional requirements for the masticatory system and speech development. Fourteen children aged 12.6 ± 2.6 with tumors or congenital deformities affecting the mandible and TMJ were included in the study. Radical surgical treatment according to our own protocol was performed through microvascular anastomotic flap reconstruction of the soft tissues and bones, together with total TMJ custom replacements. Follow-up lasted 2–6 years. During the follow-up, increases in the mandible body (13% growth) and ramus (12% growth) were observed, both of which were related (p < 0.001). This is the first report concerning the immediate reconstruction of the mandible with ramus and total TMJ in children and adolescents that combines a free vascularized graft and total individual prosthesis of the TMJ. The presented technique enabled optimal function of the TMJ, growth of the mandible, and further rehabilitation of the patients. The technique was demonstrated to be safe, reliable, and provide good functional and cosmetic outcomes.
Keywords:
maxillofacial; pediatric; surgery; cancer; reconstruction; microsurgery; TMJ; custom implants 1. Introduction
Restoring the anatomical shape and function of the temporomandibular joint (TMJ) is one of the most demanding and complex treatments in craniofacial surgery due to the sophisticated and unique structure and physiology. In adults, TMJ reconstruction is most frequently required due to degenerative diseases, accident trauma, or tumors [1]. There are many surgical procedures and techniques for the treatment of a failed TMJ. The most commonly described are:
- Attachment of the resected condyle as a nonvascularized transplant to the end of the bone free flap;
- Insertion of a condylar prosthesis in addition to the vascularized bone graft into the glenoid fossa or the use a free bone graft alone, such as a fibula free flap, second metatarsophalangeal joint free flap, vascularized scapular, or clavicle flap;
- Use of nonvascularized tissues, such as a costochondral rib flap with a deep circumflex iliac artery flap.
At present, the use of alloplastic total joint replacement (TMJR) (with or without bone grafting) is widely accepted and has presented satisfactory long-term outcomes.
While TMJR is the scientifically proven treatment of choice in adults, the optimal protocol for TMJ reconstruction in immature patients remains unclear. The resection of part of the mandible with its condyle and the need for reconstruction of its lost elements in children frequently have different pathological backgrounds and desired treatment needs. In children and adolescents, the TMJ loses its physiological function mostly due to malignancies, congenital deformities, and ankyloses [2]. As a consequence this results in the disturbance of orofacial development, asymmetry, malocclusion, breathing, and food intake problems, which require long-term multidisciplinary dental treatment, rehabilitation, physiotherapy, and psychological support since the disease frequently results in social distancing, depression, and mental health problems [3]. Therefore, there is an undisputable need to develop reliable techniques that enable functional reconstruction of the TMJ in children and adolescents while allowing for subsequent physiological growth of the orofacial skeleton, a reduction in postoperational complications and a reduction in the need for future secondary surgeries. The first reports describing the sole use of alloplastic devices in immature patients were published by Keyser et al. [4], Lypka et al. [5], and Cascone et al. [6]. While these described satisfactory results, they indicated the need for further studies in this area. Additionally, data from the clinical studies have implied the justified use of microvascularized bone grafting techniques in the reconstruction of the resected mandible in children [7,8,9].
Virtual surgical planning (VSP) and custom surgery protocols have found reliable application in contemporary dentistry and oral surgery when plastic, metal, or ceramic materials are used [10,11]. These materials are commonly used in dental implant positioning and peri-implant alveolar surgery (composite, acrylic resins, vacuum-formed thermoplastic matrix) as well as individualized abutment and prosthetic superstructure manufacture (CAD CAM, ceramic). They are also used in the manufacture of individualized maxillofacial osteosynthesis microplates and implants (titanium sinters and solid titanium) and face implants (silicone, high-density polyethylene, e.g., PEEK) [12,13,14].
The general goal of contemporary 3D planning, custom surgery, and prosthetics is to decrease patient morbidity, provide individualized and precise surgical treatment, reduce downtime, and perform body reconstruction with the highest functionality [14,15].
This study presents the first protocol for postresection reconstruction of the mandible with vascularized bone free flaps stabilized with VSP-planned and 3D custom temporomandibular joint replacement in children. The primary purpose of this manuscript is to introduce and further familiarize dentists and surgeons with the techniques of VSP and microsurgery in children, in order to better plan overall dental and orthopedic treatments.
2. Materials and Methods
Ethics approval for this study was received from the Maria Skłodowska-Curie Memorial Cancer Center Ethics Committee in Gliwice (KB 430-15/17). Patients and their legal guardians provided informed consent. A total of 14 patients (10 male/4 female) aged 8–17 years old, treated at our institution between 2015 and 2019 due to malignant/benign tumors or congenital deformities affecting the mandible and TMJ, were enrolled in the study. The protocol for TMJ reconstruction assumed the following: preoperative examination and imaging (orthopantomogram, head and neck, abdomen, pelvis, and lower limb CT scans with contrast), virtual imaging (Figure 1), stereolithographic models, production of 3D custom-made resection templates (Figure 1b,c and Figure 2), and temporomandibular joint implants designed for graft fixation (Figure 1d and Figure 2d) (ChM, Poland, EC Certificate, NO:60099942 0001; ISO 9001:2015, ISO 3485:2016.3).
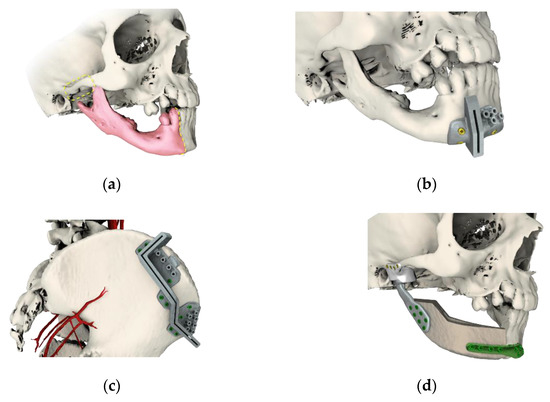
Figure 1.
Virtual imaging and planning, with: (a) planning of the resection area; (b) surgical guideway for resection osteotomies; (c) surgical guideway for graft harvesting osteotomies; and (d) microvascularized graft supported with customized temporomandibular joint (TMJ) prosthesis consisting of polymeric glenoid fossa and titanium, and osteosynthesis trauma plate 2.0 (ChM, Bialystok, Poland).
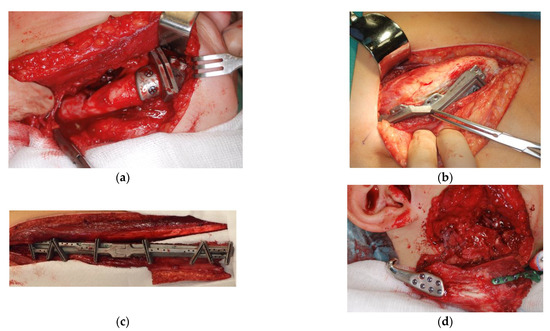
Figure 2.
Intraoperative photographs showing examples of: (a) medial mandible resection template; (b) iliac graft harvesting template; (c) fibular graft harvesting template; and (d) intraoperative view of the graft (blue arrow) connected to the customized prosthesis and reconstruction plate (green) before fixing.
Resection of the mandible ramus within the affected TMJ with/without the body of the mandible was performed through the conventional submandibular approach, and exposure of the TMJ was performed through the conventional preauricular approach. Facial nerve branches were identified and preserved using intraoperative neuromonitoring. Prior to resection osteotomy, customized surgical templates were applied in order to ensure a precise cut and adjust the osteotomy line to the graft (Figure 2a). The microvascularized bone graft was harvested either from the fibula (fibula free flap (FFF)) or iliac crest (iliac crest free flap (ICFF)) with use of customized resection templates to obtain the desired amount and shape of the graft (Figure 2b,c). After resection, the microvascularized graft was conventionally connected to the facial and/or temporal vessels in order to provide sufficient blood supply, connected to the 3D customized TMJ prosthesis and stabilized by the titanium reconstruction plate 2.0 (ChM, Bialystok, Poland) (Figure 2d).
After the surgery, a control orthopantomogram and CT scan were performed in order to confirm the proper positioning of the graft and prosthesis. During the postoperative period the viability of the osteocutaneous free flaps was assessed via clinical monitoring (Doppler imaging and physical examination): the flap was inspected every hour on the first day and every 3–5 h for the next 4 days. Mean postoperative downtime was 10–14 days. All patients were referred to physiotherapy shortly after the surgery. During the preoperative and follow-up periods, maximal interincisal opening (MIO), lateral deflation on the reconstructed side, asymmetry, and growth of the operated and contralateral sides were measured (orthopantomogram, cephalometric X-ray superimposition, CT scans). The patients were functionally assessed by examining masticatory function, speech, and aesthetic appearance. Microplates were removed 3–6 months after the surgery in order to allow further sufficient growth of the mandible. Statgraphics Centurion XVI, StarPoint Technologies INC., The Plains, VA, USA, was used for statistical analyses. Linear regression analysis and one-way analysis of variance were used to evaluate the clinical material. Statistical significance was established at p < 0.05.
3. Results
A total of 14 patients aged 8–17 years old (10 male, 4 female) were enrolled in the study. All patients underwent mandibulectomy (ramus and body n = 12; ramus only n = 2) with immediate 3D-assisted TMJR supported by microvascular free flap reconstruction (FFF or ICFF) (Table 1).

Table 1.
Summary of patients enrolled in the study, including: diagnosis, level of malignancy, resection extent, and reconstruction type. Additional surgery performed after the follow-up (2–6 years postoperative): none—orthodontic treatment and implant placement were performed with use of a primary graft after the follow-up; reconstructive—orthodontic treatment and implant placement required secondary bone grafting; orthognathic—additional orthognathic surgery was required in order to treat skeletal deformation at the time of the TMJR surgery or after the follow-up.
Ten patients were operated on due to benign diseases: ameloblastoma (n = 3), fibrous dysplasia (n = 3), central giant cell tumor (n = 1), and fibroma ossificans (n = 1). Mandibulectomy with TMJ was performed due to sarcoma in four patients and due to congenital deformations in two patients: one due to congenital deformations (hemifacial macrosomia) and one for acquired ankylosis of the TMJ. The vast majority of cases were treated with an FFF graft (n = 11) (p < 0.005). Unilateral defects requiring surgical intervention were the most common (p < 0.005). Only two patients required bilateral surgery (also performed immediately): one due to fibrous dysplasia and the other due to severe deformation. In the group of patients operated on unilaterally, mandible growth was evaluated. The distance between reference points on the day of surgery and at the endpoint of the study was measured in each patient. In order to evaluate the growth of the mandible ramus and body, auricular (Ar)–gonion (Go) and gonion (Go)–pogonion (Po) were used as reference points, respectively, on the operated side and the opposite, healthy control side. Additionally, the SNB angles were measured after the surgery and during the follow-up (See Figure 3).
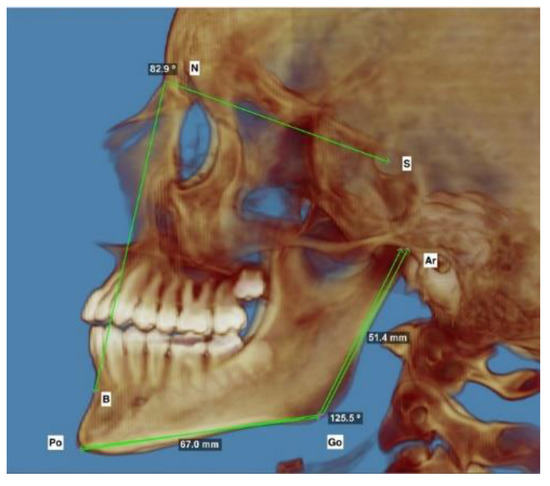
Figure 3.
Representative lateral photograph of 3D imaging of the reference points used for the measurements described in the Table 2. The auricular (Ar)–gonion (Go) sections were used to measure changes in height of the mandible ramus; the gonion (Go)–pogonion (Po) sections were used to measure changes in the length of the mandible body. The SNB angle was used to measure the anterior growth of the mandible.
The measurements enabled straightforward evaluation of the overall mandible growth after the surgery and allowed the growth of the grafted side to be compared with the healthy side in each patient (Table 2).

Table 2.
Measurements of mandible growth on the day of surgery and during the follow-up (2–6 years postoperative), where MIOpre is maximal interincisal opening before resection and reconstruction; MIOpost is maximal interincisal at the final follow-up; Ramus OP is the height of the operated ramus before surgery; Ramus OP’ is the height of the operated ramus after surgery; Body OP is the length of the operated body before surgery; Body OP’ is the length of the operated body after surgery; Ramus C is the height of the nonoperated ramus before surgery; Ramus C’ is the height of the nonoperated ramus after surgery; Body C is the length of the nonoperated body before surgery; and Body C’ is the length of the nonoperated body after surgery. Bilateral N/A: as the procedure was done bilaterally in two patients there was no reference point for estimating the operated vs. nonoperated side for statistical purposes.
In the follow-up, there were no statistical differences observed in height (p = 0.39) or in length (p = 0.14) (Figure 4) between the graft and the control. Therefore, the operated side exhibited similar growth of the ramus and body in comparison to the control at the endpoint of the study.
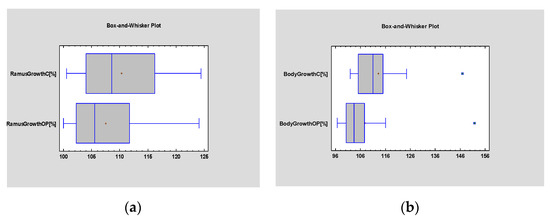
Figure 4.
Student’s t-test evaluating statistical differences in the mean growth of the ramus (a) and body (b) of the graft and healthy control. Statistical significance was established at p < 0.005.
The difference between the mean values of the SNB angles before and after surgery was significant (p = 0.000), which confirms that physiological anterioinferior growth of the mandible occurred after the surgery in all patients (Figure 5).
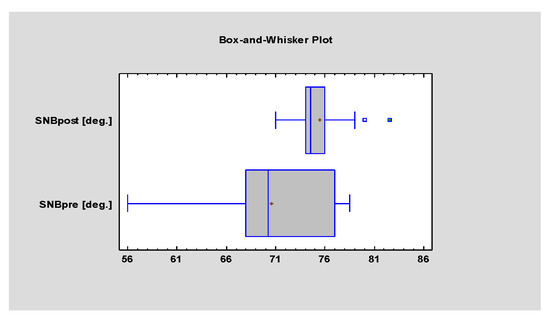
Figure 5.
Student’s t-test evaluating statistical differences in the mean value of the SNB angle before and after surgery. Statistical significance was established at p < 0.005.
The postoperative improvement in the range of mouth opening was statistically significant (p = 0.001) (Figure 6).
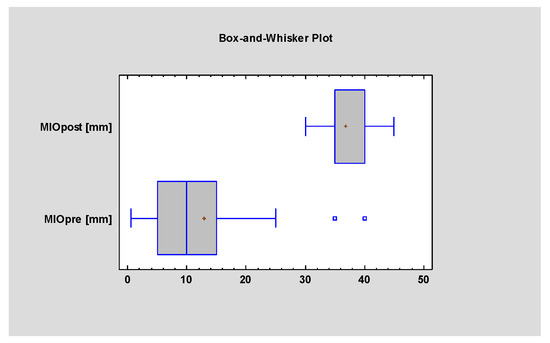
Figure 6.
Student’s t-test evaluating statistical differences in MIO (maximal interincisal opening) before and after the surgery. Statistical significance was established at p < 0.005.
The average postoperative MIO was 38.75 mm (mean improvement of +13.75 mm), which confirms the beneficial effect of the surgery on mouth opening (Figure 7).
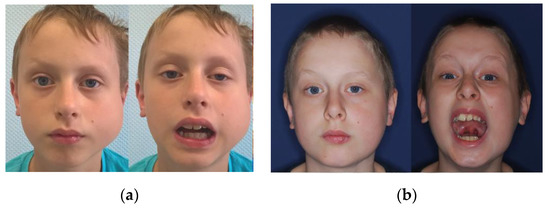
Figure 7.
Photographs of an 8-year-old patient (a) before and (b) one year after the surgery, showing significant improvement in the range of mouth opening and satisfactory aesthetic results.
During the follow-up, genial asymmetry of 3–6 mm was diagnosed in 8 patients (Table 2). The patterns of the ramus and body growth were analyzed using simple regression testing of the subgroups (operated vs. control) (Figure 8).
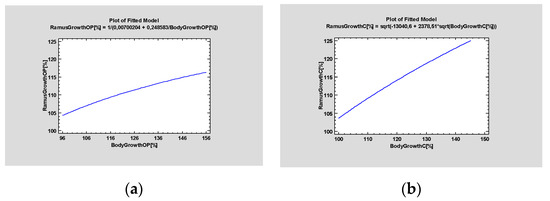
Figure 8.
Simple regression tests evaluating patterns of mandible growth, (a) Operated site, (b) Control site. It can be observed that the operated side exhibited decreased capacity for growth over time when compared with the healthy side. Statistical significance was established at p < 0.005.
During the follow-up period it was observed that, in some cases, the grafted side exhibited decreased potential for growth in comparison to the nonoperated side, but there was no statistically significant difference (p > 0.005). There were no free flap failures in the postoperative period or during the 4–5-year follow-up. All patients exhibited satisfactory function of the TMJ, except for one patient, who had posterior dislocation of the TMJ prosthesis, which was treated by additional rehabilitation. Contralateral excursion was observed in five patients after reconstruction. There were no inflammatory complications or biomechanical problems with the prosthesis itself, such as dislocations or inappropriate positioning of the mandible. Furthermore, there were no incidents of the prosthesis loosening. All patients had orthodontic and dental implant treatment performed as the final stage of oral rehabilitation (Figure 9). In three patients, additional orthognathic surgery had to be undertaken. In another three patients, additional bone grafting was necessary due to the placement of the dental implants.
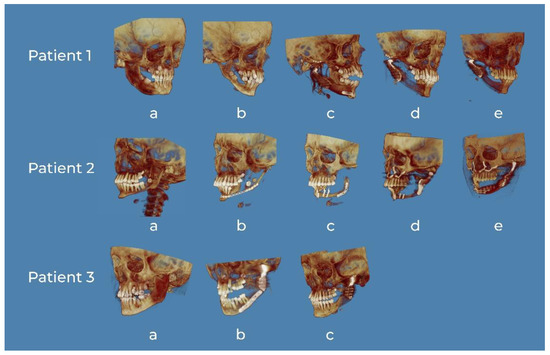
Figure 9.
3D CT reconstructions of representative patients. Patient 1: (a) tumor of the right mandible body and ramus, (b) defect after nonradical resection in another department and secondary deformation, (c) 3D virtual surgical planning (VSP) reconstruction of the mandible and custom TMJ, (d) 6 months postoperative, and (e) 24 months postoperative. Patient 2: (a) tumor of the left mandible body and ramus, (b) defect after nonradical resection in another department and secondary deformation, (c) reconstruction of the mandible with fibula without 3D VSP in another facility—lack of functionality, (d) reconstruction of the TMJ with stock TMJ prosthesis and maxillary advancement in order to improve function and occlusion—failure due to resorption of the previous bone grafting, and (e) 3D VSP reconstruction of the mandible body, ramus and custom TMJ. Patient 3: (a) tumor of the left mandible body and ramus, (b) 3D VSP reconstruction of the mandible and custom TMJ, and (c) 4 years postoperative.
4. Discussion
The reconstruction of mandibular defects in children is technically and aesthetically challenging. Additional TMJ reconstruction protocols that provide long-term functionality in this group of patients are still not fully developed. In most cases of adult patients, simple total joint replacement (TMJR) is usually satisfactory because the requirements related to a growing child do not need to be met. For children the main issues are associated with the development of speech, the masticatory system, and the entire craniofacial region, which are dramatically hampered when the TMJ is lost. The best treatment should restore functionality, including adequate mouth opening, improving the airway patency, preventing the recurrence of the disease, and providing satisfactory aesthetic results [2,4]. To date, there is no proven track for mandible and TMJ grafting techniques that can assist in the postoperative growth of the jaws of pediatric patients. Resnik claimed that the pediatric TMJ may be reconstructed either by distraction osteogenesis, autologous reconstruction (costochondral graft, free fibula flap), or total alloplastic joint replacement [2]. However, the growth potential of the mandible in pediatric patients depends on the preservation of the mandibular epiphyseal growth plates, which are located in the proximal zone of the conical subcondylar ridge [16]. Because of this, in immature patients it is advised that the condyle be preserved at all costs when a mandibulectomy is necessary. Condylar preservation has been shown to positively influence the growth potential of the mandible in the postoperative period [17]. The protocol for the reconstruction of a child’s mandible is more complicated when a condyle must be removed due to malignancy, is deformed, or is absent due to deformation, as was the case in the described population of the patients operated on within this study.
In the literature, costochondral grafts have been reported as the gold standard for mandible and TMJ reconstruction in children, but nonvascularized autological bone grafts have a tendency to induce osteogenesis [18]. This is the reason that they are not the treatment of choice for the ankylosis of the joints [19]. Costochondral graft fractures, hypertrophy of the bone grafts, their unpredictable growth with separation of bone from the chondral part, and resorption in the donor site have been described [20]. Resection of the lower face region without simultaneous reconstruction leads to constrictions of the native soft tissues and dislocations of other bones and, eventually, extreme malocclusion and deformation of the face. These often lead to the need for secondary reconstructions, which are always more difficult and sometimes impossible to conduct. Resection with simultaneous reconstruction of the mandible allows orthodontic treatment and dental rehabilitation for the early restoration of teeth while preventing malocclusion. Indeed, simultaneous microvascular mandibular grafting techniques with satisfactory results have been described by other authors but, again, surgery did not involve the TMJ or enable its sufficient reconstruction when necessary [18,21,22]. Virtual surgical planning and custom biomaterials have already found clinically proven applications in the treatment of orbital trauma [11], cosmetic facial surgery [23], orthognathic surgery [24], free flap reconstruction of midface defects [25], and treatment of facial gunshot wounds and other injuries [26], providing improved efficiency and precision for complex surgical operations and assisting in the restoration of maxillofacial unit functionality, facial volume, symmetry, and harmony [26,27]. However, no data have been reported to date regarding the reconstruction of the pediatric mandible, its implication in further growth, and the possibilities for dental and orofacial treatment.
In this study, we describe the first protocol where VSP and alloplastic prosthesis of the TMJ with microvascular free flaps is combined with 3D planning of the resection areas and shape of the graft, and the precision operation was performed using individually designed surgical guides, which are more commonly used in the reconstructive facial surgery of adults [21,25]. This approach minimizes the risk of unwanted trauma and over-resection, reduces the duration of the surgery, and ensures a perfect fit of the graft at the donor site. The alloplastic 3D custom TMJ prosthesis allows full reconstruction of the lost joint and ensured physiological functioning. Similarly to the previously published results, VSP provides improved reconstruction, precision bony segment contact, and anatomical correction of the bone deficiency [25].
No resorption of the bone grafts was observed in the follow-up. The microvascular graft, fixed to the TMJ prosthesis and mandible by a customized microplate, allows further mandible growth and prevents TMJ ankylosis. In the follow-up, growth of the operated side was observed in comparison to the contralateral healthy side. It must be noted that in pediatric patients, reconstruction plates must be removed within 3–4 months of the reconstruction surgery to allow the continued proper and undisturbed growth of the facial skeleton. The surgical procedure described within this study provides significant improvement in TMJ functionality, mouth opening range, food intake effectiveness and satisfactory aesthetic results. It is worth mentioning that in some cases the planning of the surgery and bone reconstruction should include hypercorrection of the grafted side in order to reduce or eliminate genial asymmetry at the follow up.
Three-dimensional VSP has inherent issues that have to be taken into account during the planning stage. Pitfalls resulting from excessive tumor growth, nonfitting of the guides, or misconceived osteotomy lines may lead to incomplete adherence or complete abandonment of VSP-based treatment. Furthermore, it must be noted that communication with the manufacturing engineer is critical for the successful application of 3D-designed biomaterials [28]. Frequently, a multidisciplinary 3D-VSP meeting with a maxillofacial surgeon, orthodontist, oncologic surgeon, and clinical engineer or technician is arranged in order to develop a coherent and prospective surgery and treatment plan [29]. In some cases the design and size of either the surgical guide or the implant itself requires modified and extended operational access in order to properly fit the device. This represents the most disturbing intraoperative factor as 3D-printed biomaterials usually cannot be easily modified, bent, cut, or trimmed, unlike stock solid titanium implants. VSP requires a highly skilled and experienced surgeon and suitable preoperative planning to take full advantage of VSP’s possibilities. The use of 3D custom implants may increase the overall cost of a single hospitalization, but the benefits of limited downtime and fewer subsequent operations balance the costs of this treatment approach [30,31]. By applying this novel concept to TMJ surgery in immature patients, one may reduce the number of operations, such as distraction osteogenesis or secondary bone grafts, which are frequently performed in cases treated with free grafts only or stock biomaterials. Early reconstruction of the defects may support more physiological mandible growth which, in turn, favors logopedic, orthodontic, dental, and pediatric prosthodontics. With special design of the flap and reconstruction it is possible to reconstruct not only the facial bones but also the soft tissues that are crucial for facial aesthetics. Moreover, more physiological growth of the TMJ and mandible ramus after surgery is beneficial for the reduction in the magnitude of secondary reconstructive and orthognathic surgery as the final treatment for the restoration of occlusion and facial balance.
5. Conclusions
This is the first report on the immediate reconstruction of the mandible with the ramus and total TMJ in children and adolescents that combines a free vascularized graft and total individual prosthesis of the TMJ. The presented technique allows the optimal function of the TMJ, growth of the mandible, and further rehabilitation of the patients. It is safe, reliable, and provides good functional and cosmetic outcomes. VSP may present some limitations, such as the increased cost of surgery and extensive, multidisciplinary planning and preparation prior to the surgery. However, a properly designed and performed treatment reduces downtime, morbidity, the surgery time, and the number of subsequent operations. Custom guides and implants may not fit correctly when the osteotomy lines are poorly designed or extensive tumor growth is diagnosed. Therefore, it is crucial to implement reliable, quick, and precise planning to enable rapid intervention, from the primary diagnosis to the surgery itself. There is an undisputable need for further studies to establish a gold standard for TMJ reconstruction in immature patients, which is of great importance in pediatric dentistry and orthodontics. Furthermore, the evaluation of this approach on the active growth of a larger group of patients and a longer follow-up period are required in future studies.
Author Contributions
K.D.: conceptualization, methodology, writing—original draft preparation, project administration, funding acquisition, resources; R.P.; writing—original draft preparation, writing—review and editing, visualization, investigation, data curation; M.B.: investigation, software, methodology; M.K.: data curation, formal analysis; D.S.: data curation, validation; Ł.K.: supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ChM SP. Z O.O., Poland.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Maria Sklodowska-Curie Memorial Cancer Center Ethics Committee in Gliwice (KB 430-15/17).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pérez, L.M.G.; Somarriba, B.G.P.; Centeno, G.; Vallellano, C.; Carmona, J.F.M. Evaluation of total alloplastic temporo-mandibular joint replacement with two different types of prostheses: A three-year prospective study. Med. Oral Patol. Oral y Cir. Bucal 2016, 21, e766–e775. [Google Scholar] [CrossRef]
- Resnick, C.M. Temporomandibular Joint Reconstruction in the Growing Child. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 109–121. [Google Scholar] [CrossRef]
- Bender, M.E.; Lipin, R.B.; Goudy, S.L. Development of the Pediatric Temporomandibular Joint. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keyser, B.; Banda, A.; Mercuri, L.; Warburton, G.; Sullivan, S. Alloplastic total temporomandibular joint replacement in skeletally immature patients: A pilot survey. Int. J. Oral Maxillofac. Surg. 2020, 49, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Lypka, M.; Shah, K.; Jones, J. Prosthetic temporomandibular joint reconstruction in a cohort of adolescent females with juvenile idiopathic arthritis. Pediatr. Rheumatol. 2020, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cascone, P.; Basile, E.; Angeletti, D.; Vellone, V.; Ramieri, V.; Giancotti, A.; Castori, M.; Lenzi, J.; Manganaro, L.; Papoff, P.; et al. TMJ replacement utilizing patient-fitted TMJ TJR devices in a re-ankylosis child. J. Cranio-Maxillofac. Surg. 2016, 44, 493–499. [Google Scholar] [CrossRef]
- Abramowicz, S.; Goudy, S.L.; Mitchell, C.E.; Prickett, K.; Marchica, C.; Austin, T.M.; El-Deiry, M.W. A Protocol for Resection and Immediate Reconstruction of Pediatric Mandibles Using Microvascular Free Fibula Flaps. J. Oral Maxillofac. Surg. 2021, 79, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, A.; Moran, S.L. Pediatric Microsurgery. Clin. Plast. Surg. 2020, 47, 561–572. [Google Scholar] [CrossRef]
- Nozari, L.; Simonetti, T.; Bueno, C.; Szydloski, V.; Nsensele, R.; Quevedo, A.; Ponzoni, D.; Freddo, A.L.; Corsetti, A.; Puricelli, E. Reconstruction of jaw using microvascular fibular graft in a pediatric patient. Int. J. Oral Maxillofac. Surg. 2019, 48, 266. [Google Scholar] [CrossRef]
- Fiorillo, L.; Leanza, T. Worldwide 3D Printers against the New Coronavirus. Prosthesis 2020, 2, 9. [Google Scholar] [CrossRef]
- Herford, A.S.; Miller, M.; Lauritano, F.; Cervino, G.; Signorino, F.; Maiorana, C. The use of virtual surgical planning and navigation in the treatment of orbital trauma. Chin. J. Traumatol. 2017, 20, 9–13. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.M.; Aras, M.A. Types of Implant Surgical Guides in Dentistry: A Review. J. Oral Implant. 2012, 38, 643–652. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20. [Google Scholar] [CrossRef]
- Rammos, C.K.; Cayci, C.; Castro-Garcia, J.A.; Feiz-Erfan, I.; Lettieri, S.C. Patient-Specific Polyetheretherketone Implants for Repair of Craniofacial Defects. J. Craniofac. Surg. 2015, 26, 631–633. [Google Scholar] [CrossRef]
- Otte, A. 3D Computer-Aided Design Reconstructions and 3D Multi-Material Polymer Replica Printings of the First “Iron Hand” of Franconian Knight Gottfried (Götz) von Berlichingen (1480–1562): An Overview. Prosthesis 2020, 2, 27. [Google Scholar] [CrossRef]
- Li, J.-S.; Chen, W.-L.; Huang, Z.-Q.; Zhang, D.-M. Pediatric Mandibular Reconstruction after Benign Tumor Ablation Using a Vascularized Fibular Flap. J. Craniofac. Surg. 2009, 20, 431–434. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Liang, T.; Peng, X. Mandibular growth after paediatric mandibular reconstruction with the vascularized free fibula flap: A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 440–447. [Google Scholar] [CrossRef]
- Eckardt, A.M.; Barth, E.-L.; Berten, J.; Gellrich, N.-C. Pediatric Mandibular Resection and Reconstruction: Long-Term Results with Autogenous Rib Grafts. Craniomaxillofac. Trauma Reconstr. 2010, 3, 25–32. [Google Scholar] [CrossRef]
- Felstead, A.M.; Revington, P.J. Surgical Management of Temporomandibular Joint Ankylosis in Ankylosing Spondylitis. Int. J. Rheumatol. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fan, H.; Du, W.; Li, J.; Hu, J.; Luo, E. Overgrowth of costochondral grafts in craniomaxillofacial reconstruction: Rare complication and literature review. J. Cranio-Maxillofac. Surg. 2015, 43, 803–812. [Google Scholar] [CrossRef]
- Nuri, T.; Ueda, K.; Iwanaga, H.; Otsuki, Y.; Nakajima, Y.; Ueno, T.; Kawata, R. Microsurgical mandibular reconstruction using a resin surgical guide combined with a metal reconstructive plate. Microsurgery 2019, 39, 696–703. [Google Scholar] [CrossRef]
- Genden, E.M.; Buchbinder, D.; Chaplin, J.M.; Lueg, E.; Funk, G.F.; Urken, M.L. Reconstruction of the Pediatric Maxilla and Mandible. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 293–300. [Google Scholar] [CrossRef]
- Chang, P.-C. Computer-Assisted Planning and 3D Printing-Assisted Modeling for Chin Augmentation. Aesthetic Surg. J. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Schneider, D.; Kämmerer, P.W.; Hennig, M.; Schön, G.; Thiem, D.G.E.; Bschorer, R. Customized virtual surgical planning in bimaxillary orthognathic surgery: A prospective randomized trial. Clin. Oral Investig. 2019, 23, 3115–3122. [Google Scholar] [CrossRef]
- Swendseid, B.P.; Roden, D.F.; Vimawala, S.; Richa, T.; Sweeny, L.; Goldman, R.A.; Luginbuhl, A.; Heffelfinger, R.N.; Khanna, S.; Curry, J.M. Virtual Surgical Planning in Subscapular System Free Flap Reconstruction of Midface Defects. Oral Oncol. 2020, 101, 104508. [Google Scholar] [CrossRef] [PubMed]
- Khatib, B.; Cuddy, K.; Cheng, A.; Patel, A.; Sim, F.; Amundson, M.; Gelesko, S.; Bui, T.; Dierks, E.J.; Bell, R.B. Functional Anatomic Computer Engineered Surgery Protocol for the Management of Self-Inflicted Gunshot Wounds to the Maxillofacial Skeleton. J. Oral Maxillofac. Surg. 2018, 76, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Fama, F.; Cicciu, M.; Sindoni, A.; Nastro-Siniscalchi, E.; Falzea, R.; Cervino, G.; Polito, F.; De Ponte, F.; Gioffre-Florio, M. Maxillofacial and concomitant serious injuries: An eight-year single center experience. Chin. J. Traumatol. 2017, 20, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Efanov, J.I.; Roy, A.-A.; Huang, K.N.; Borsuk, D.E. Virtual Surgical Planning. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1443. [Google Scholar] [CrossRef]
- Donaldson, C.D.; Manisali, M.; Naini, F.B. Three-dimensional virtual surgical planning (3D-VSP) in orthognathic surgery: Advantages, disadvantages and pitfalls. J. Orthod. 2020, 146531252095487. [Google Scholar] [CrossRef]
- Mazzola, F.; Smithers, F.; Cheng, K.; Mukherjee, P.; Low, T.-H.(H.); Ch’Ng, S.; Palme, C.E.; Clark, J.R. Time and cost-analysis of virtual surgical planning for head and neck reconstruction: A matched pair analysis. Oral Oncol. 2020, 100, 104491. [Google Scholar] [CrossRef]
- Resnick, C.M.; Inverso, G.; Wrzosek, M.; Padwa, B.L.; Kaban, L.B.; Peacock, Z.S. Is There a Difference in Cost Between Standard and Virtual Surgical Planning for Orthognathic Surgery? J. Oral Maxillofac. Surg. 2016, 74, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).