Featured Application
The voluntary cycle ergometer with novel whole-body neuromuscular electrical stimulation transiently exacerbated blood fluidity ex vivo; however, microvascular flow in vivo was not adversely affected.
Abstract
Vigorous exercise increases blood viscosity and may pose a risk of cardiovascular events in patients with cardiovascular diseases. We recently reported that single-use of novel whole-body neuromuscular electrical stimulation (WB-NMES) can be safely applied in healthy subjects without adversely affecting blood fluidity. We performed a crossover study to explore the effectiveness and safety of a hybrid exercise with ergo-bicycle and WB-NMES; 15 healthy volunteers, aged 23–41 years, participated in this study. No arrhythmias were detected during the hybrid exercise and 20 min recovery, and although blood fluidity was transiently exacerbated immediately after both the exercise programs, in vivo parameters in the sublingual and nailfold microcirculation remained unchanged. There was a significant decrease in blood glucose and increase in lactic acid levels immediately after both exercise programs. Even with the same workload as the cycle ergometer exercise, the oxygen intake during the hybrid exercise remained higher than that during the cycle ergometer exercise alone (p < 0.05, r = 0.79, power = 0.81). Both the hybrid and voluntary cycle ergometer exercises transiently exacerbated blood fluidity ex vivo; however, microvascular flow was not adversely affected in vivo.
1. Introduction
Aerobic exercises, such as walking or cycle ergometer exercises, are highly recommended for the primary and secondary prevention of cardiovascular diseases. However, in people with limited mobility with an underlying cause, the inhibition of skeletal muscle atrophy and motor function is a critical problem. The phenomenon of skeletal muscle contraction induced by neuromuscular electrical stimulation (NMES) is a known treatment for disuse-induced muscle atrophy and is reported to be a safe and effective alternative to exercise therapy in patients with symptomatic heart failure [1,2]. More recently, the effectiveness of whole-body NMES (WB-NMES)—which can provide electrical stimulation to additional areas, including the upper arm, pronotum, abdomen, back, and lordosis—has been reported [3,4]. The combination of NMES with voluntary exercise may also be beneficial; it has been reported that the combination of NMES and voluntary exercise in healthy subjects provides high-intensity dynamic loading to many skeletal muscles [5,6]. Nevertheless, intense exercise transiently reduces microcirculatory flow due to increased hematocrit, sympathetic nerve activation, and leukocytosis [7,8]. Since patients with ischemic heart disease have higher blood viscosity than healthy individuals [9], increased blood viscosity due to strenuous exercise may be a risk factor for cardiovascular events. We previously reported that the single-use of WB-NMES in healthy subjects can be safely performed without any adverse effects on blood fluidity [10].
The aims of this study were to explore the effectiveness and safety of a hybrid exercise with ergo-bicycle exercise and WB-NMES, as well as to examine the effects of the hybrid exercise on microcirculatory dynamics and metabolism.
2. Materials and Methods
2.1. Study Design
This was a single—center, randomized, controlled crossover study.
2.2. Participants
The study protocol was approved by the Ethical Committee of the Nikko Medical Center of Dokkyo Medical University (Approval Number: Nikko 20-020). All participants provided written informed consent, and all procedures complied with the National Ethical Guidelines for Medical and Health Research involving Human Participants and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Participants included 15 healthy Japanese volunteers: 10 males and 5 females (age: 28.9 ± 5.0 years, height: 166.7 cm ± 7.5 cm, BMI 23.2 ± 3.2). No abnormalities were found during routine physical examinations or standard laboratory analyses after overnight fasting. Persons on medication were excluded.
2.3. Measurement
2.3.1. Cardiopulmonary Exercise Test
Cardiopulmonary exercise test (CPX) is a noninvasive standard method for evaluating cardiopulmonary exercise capacity. An incremental, symptom-limited exercise test was performed using an upright, electromagnetically braked cycle ergometer (Strength Ergo 8; Mitsubishi Electric Engineering Co., Ltd., Tokyo, Japan). The exercise test began with a 4 min rest on the ergometer, followed by a 4 min warm-up at 20 W and 50 rpm. The load was then incrementally increased by 20 W/min [11]. Electrocardiograms were continuously monitored during the test using a System ML-9000 (Fukuda Denshi Co., Ltd., Tokyo, Japan). Cuff blood pressure was measured at rest and every minute during exercise testing with an automatic indirect manometer (FB-300; Fukuda Denshi Co., Ltd. Tokyo, Japan). Breath-by-breath VO2, VCO2, and minute ventilation were measured during the test with an AE-310S Respiromonitor (Minato Medical Science Co., Ltd., Osaka, Japan) through a rubber mask attached to the subject’s face, as previously described [12].
2.3.2. Ex Vivo Blood Fluidity Evaluation
Whole blood passage time was measured with a microchannel flow analyzer system (BWA-MCFAN; Kikuchi Microtechnology Co., Ltd., Ibaraki, Japan) using a microchannel array chip (BK 7-7-4.5 microchannels; Kikuchi Microtechnology Co., Ltd. Ibaraki, Japan) for ex vivo microcirculatory evaluation. The BWA-MCFAN has been used in previous studies to assess ex vivo whole blood rheology and leukocyte activity, with detailed procedures and equipment described elsewhere [13,14,15]. Briefly, 0.1 mL of blood was drawn through the BK 7-7-4.5 microchannels as an ex vivo capillary model (7854-parallel, 7 × 4.5 μm equivalent cross-section, 30 μm long) under a constant vacuum of 20 cm H2O (1.96 kPa). The time required for saline to pass through the microchannels was determined before each blood measurement for calibration. The microscopic motion images of blood passing through the microchannels were monitored and stored on a computer system.
2.3.3. In Vivo Microcirculatory Evaluation
Sublingual microcirculation and nailfold microvasculature were measured for the in vivo microcirculatory assessments. Sublingual microcirculation was recorded using a handheld video microscope CytoCam (Braedius Medical BV, Huizen, The Netherlands) and analyzed using the microcirculation analysis software CytoCam Tools V2. The blood flow assessment methods have been previously described. The microvascular flow index is a semiquantitative measure of the perfusion quality that distinguishes between no flow (0), intermittent flow (1), sluggish flow (2), and continuous flow (3) [16]. The total vessel density is a measure of vessel density. Perfused vessel density corresponds with functional vessel density, i.e., the density of functionally flowing capillaries. The proportion of perfused vessels is the linear proportion of flowing vessels [17,18].
The nail epithelial capillaries of the fourth left-hand finger were captured on video using a small blood flow scope (TOKU Capillaro; Toku Corporation, Tokyo, Japan) in fingertip blood flow mode 510×. The captured videos were analyzed for blood flow velocity using capillary blood flow analysis software (Capimetrics; Toku Corporation, Tokyo, Japan). For video recording and velocity analysis, the average values of the three vessels were used as the data. This device can locate the same blood vessel, and data from the same vessel were used for measurements at rest, immediately after exercise, and 20 min after exercise.
2.4. Study Protocol
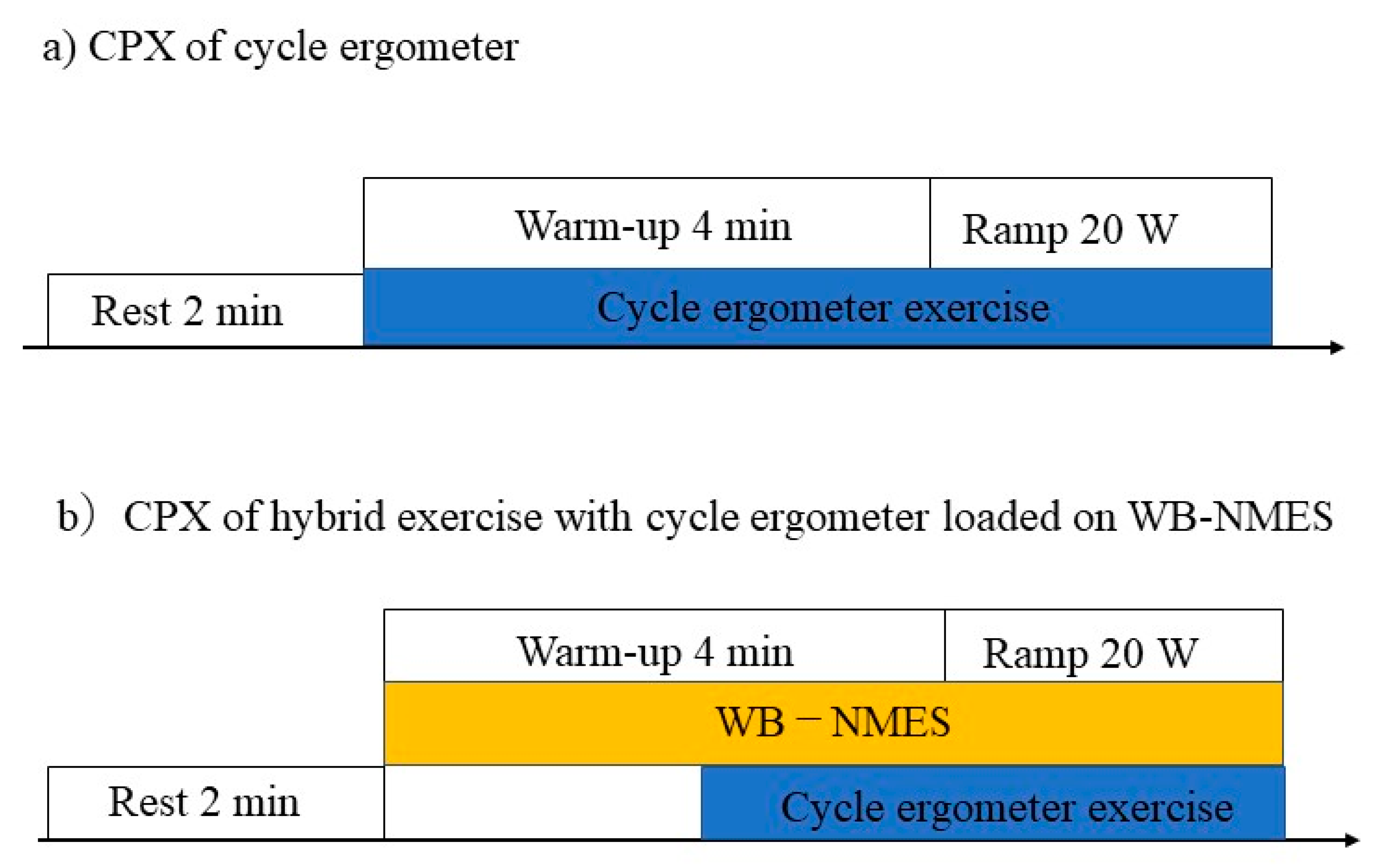
The device (SIXPAD; MTG Co., Ltd., Nagoya, Japan) and WB-NMES protocol have been previously described [6,10]. NMES was applied to the anterior and posterior upper arm, chest, back, abdomen, abdominal oblique, gluteus, and anterior and posterior thigh muscles. The intensity of the electrical stimulation for WB-NMES was determined after three training sessions for WB-NMES. All participants underwent the experiment for a total of three days. NMES was performed at 20 Hz, with 4 s of stimulation followed by 4 s of pause. The output voltage and effective current of the device ranged from level 1 (10.6 V, 1.7 mArms) to level 100 (70.0 V, 11.4 mArms), and the intensity of the stimulation was adjustable for each site. With a numeric rating scale of 7, the stimulus intensity was set to be able to pedal a CPX warm-up electrically braked cycle ergometer (20 W). The stimulation intensity was the same from warm-up to the maximum load. In the second and third sessions, CPX using a cycle ergometer or hybrid exercise with cycle ergometer loaded on WB-NMES were randomly performed. The first and second sessions were conducted over a period of three days; the second and third sessions were conducted one week apart. The protocol for CPX is shown in Figure 1.
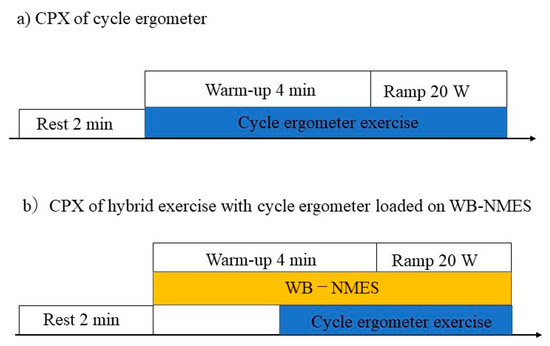
Figure 1.
Protocol for the cardiopulmonary exercise test. (a) Each participant underwent a cycle ergometer exercise and (b) a hybrid of whole-body neuromuscular electrical stimulation with cycle ergometer exercise in a crossover study design.
Participants refrained from drinking alcohol or beverages containing caffeine for 12 h prior to the study. All tests and CPX were performed under the supervision of a doctor. CPX for the cycle ergometer protocol comprised 2 min of rest and 4 min of warm-up at 20 W. After the warm-up exercise, the ergo-load was gradually increased by 20 W per minute. CPX for the hybrid exercise with a cycle ergometer loaded on a WB-NMES device comprised 2 min of rest, followed by 2 min of WB-NMES, and 2 min of warm-up at 20 W; the exercise test was then performed, with a gradual increase in ergo load of 20 W per minute. Participants were instructed to pedal at 50–60 revolutions per minute. Symptom-limited CPX was performed with ramp protocols commonly used in CPX; the session was terminated when they could no longer pedal at more than 50 revolutions per minute. Therefore, exercise time slightly varied depending on the subject. The assessment was conducted such that participants could not see the load on the cycle ergometer. Post-exercise measurements were taken just after symptom—limited CPX. In the hybrid CPX, the same electrical stimulation intensity was used from warm-up to maximum exercise load.
Blood sampling and the observation of sublingual microcirculation and nailfold microvascular flow were performed at three points: at rest, immediately after exercise, and 20 min after exercise. Whole blood passage time, blood glucose level, white blood cells, hematocrit, platelets, blood lactate levels (Lactate Pro 2; Arkray, Kyoto, Japan), plasma levels of derivatives of reactive oxygen metabolites (d-ROMs), and biological antioxidant potential (BAP) were assessed using d-ROMs and BAP test kits (Diacron International, Grosseto, Italy).
2.5. Statistical Analysis
All data were expressed as mean ± standard deviation or standard error. The Friedman test was performed to determine the whole blood passage time, microvascular flow index, and proportion of perfused vessels; the Bonferroni test was used for multiple comparisons. Wilcoxon’s signed rank test was performed on the Δ values for the results that showed significant differences for both the cycle ergometer and hybrid exercises; two-way ANOVA iterative measurements were performed on the other object variables. The Bonferroni test was used to compare each group when there was an interaction, as well as between levels (time) for each factor when there was no interaction. A paired t-test was performed for VO2 during warm-up and at the workload of the anerobic threshold (AT) on cycle ergometer exercise. Paired t-test was also performed for exercise workload and VCO2 at the respiratory compensation point. Wilcoxon signed rank test was performed for minute ventilation at the respiratory compensation point; all significance levels were set at p < 0.05. All statistical analyses were performed using SPSS software (SPSS Statistics version 27; IBM, Endicott, New York, NY, USA).
3. Results
Participants
Characteristics of the participants are shown in Table 1.

Table 1.
Characteristics of participants.
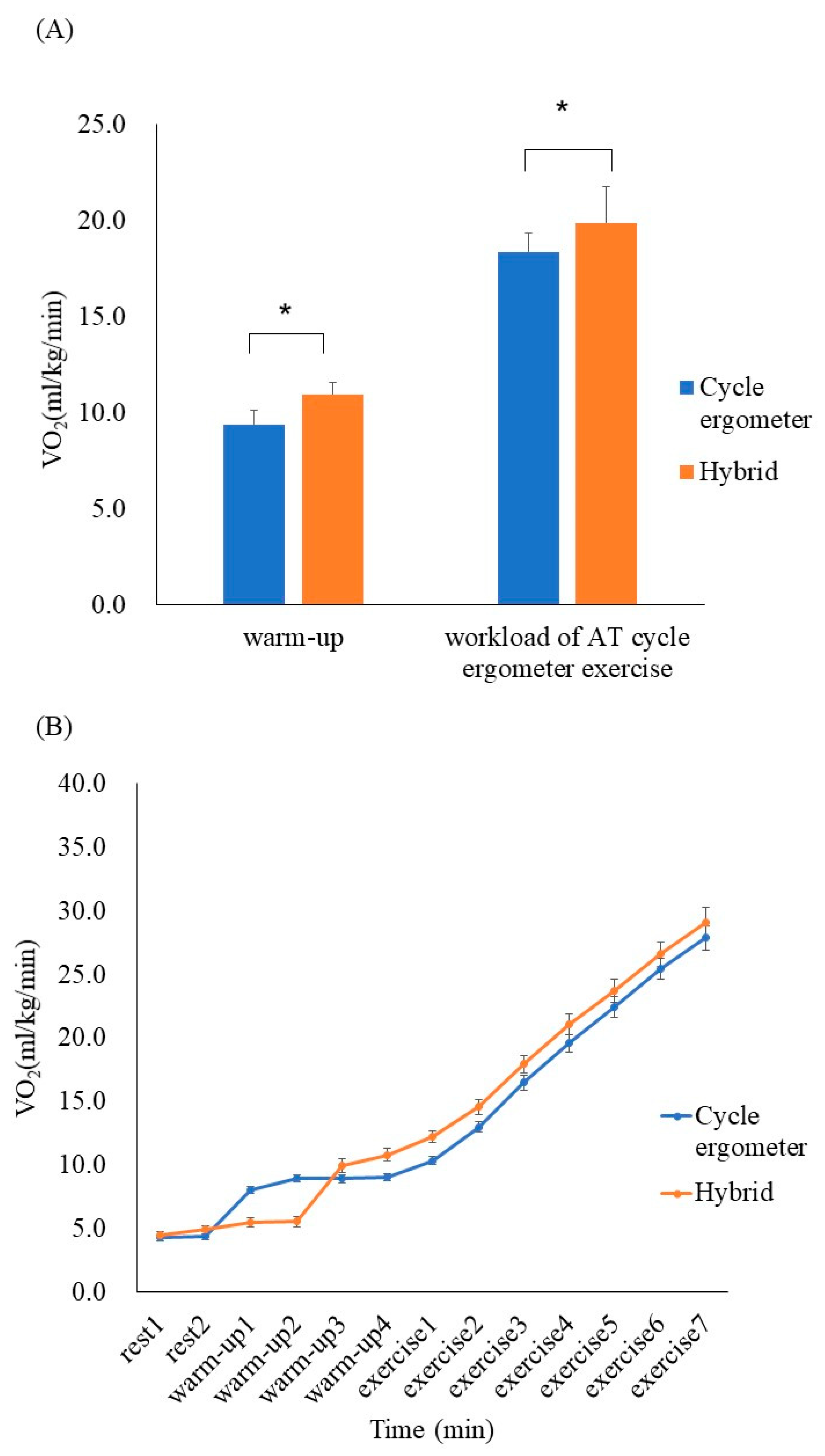
No critical arrhythmias were detected during the exercise protocol, and VO2 increased during the hybrid exercise when compared with the cycle ergometer exercise during the 4 min warm-up (10.9 ± 1.9 mL/kg/min vs 9.4 ± 1.0 mL/kg/min, respectively; p < 0.05, r = 0.72). During the hybrid exercise, the increase in VO2 was greater at the workload of AT (19.9 ± 4.3 mL/kg/min vs 18.4 ± 3.6 mL/kg/min, p < 0.05, r = 0.79); the increase in VO2 was greater up to the maximum load (Figure 2).
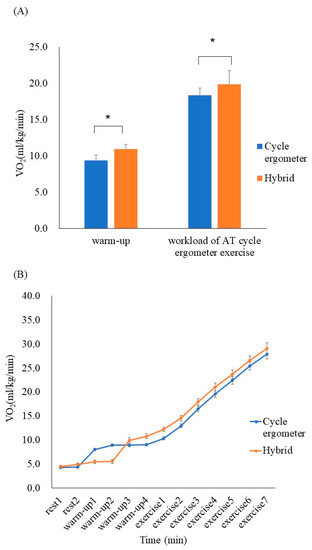
Figure 2.
(A) Comparison of VO2 between the cycle ergometer exercises and hybrid exercises (mean ± SD). During the hybrid exercise, VO2 increased compared with the cycle ergometer exercise during the 4 min warm-up (p < 0.05, r = 0.72). At the workload of anerobic threshold (AT) during the cycle ergometer exercise, the increase in VO2 was greater in the hybrid exercise (p < 0.05, r = 0.79). (B) Serial changes in VO2 every minute during the cycle ergometer and hybrid exercises (mean ± SE) * p < 0.05.
The metabolic responses at the respiratory compensation point of cycle ergometer exercise and hybrid exercise are shown in Table 2. Although there was no significant difference in exercise workload and ventilation at the respiratory compensation point between the two exercise programs, VCO2 in the hybrid exercise at the respiratory compensation point was significantly higher than that in the cycle ergometer exercise (p < 0.05, r = 0.57).

Table 2.
Comparison of exercise load, minute ventilation, and VCO2 at the respiratory compensation point.
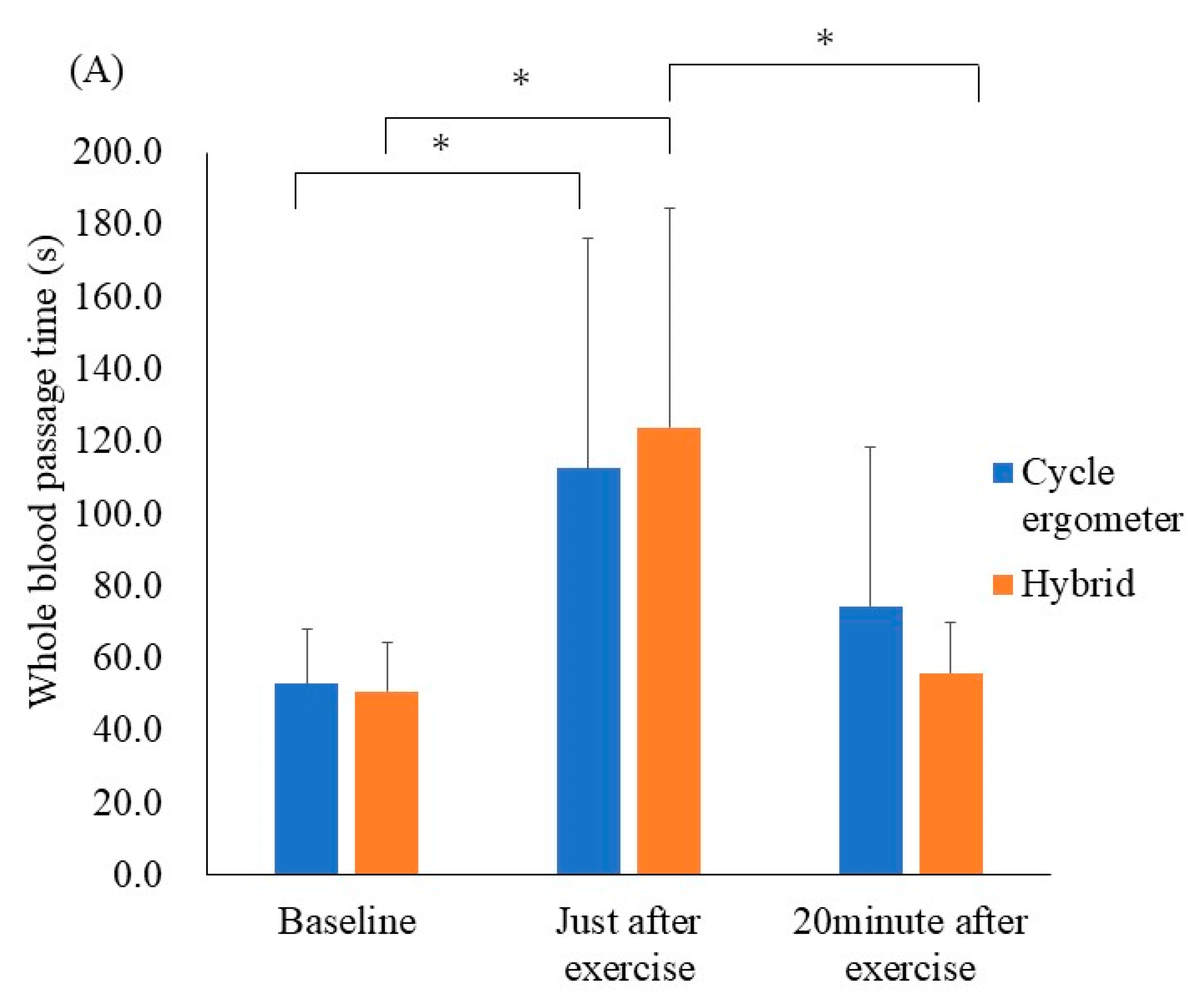
The results of the ex vivo microcirculatory evaluation are shown in Figure 3A. Whole blood passage time—as a parameter of blood fluidity—was significantly prolonged just after performing the cycle ergometer exercise when compared with the resting state (52.8 ± 15.7 s to 112.6 ± 66.0 s, respectively p < 0.05, r = 0.25). Similarly, the whole blood passage time was significantly prolonged just after the hybrid exercise when compared with the resting state (50.8 ± 14.1 s to 123.8 ± 63.1 s, respectively; p < 0.001, r = 0.41). There was no significant difference in the delta values for whole blood passage time (at rest and just after exercise) between the cycle ergometer and hybrid exercises (p = 0.65).
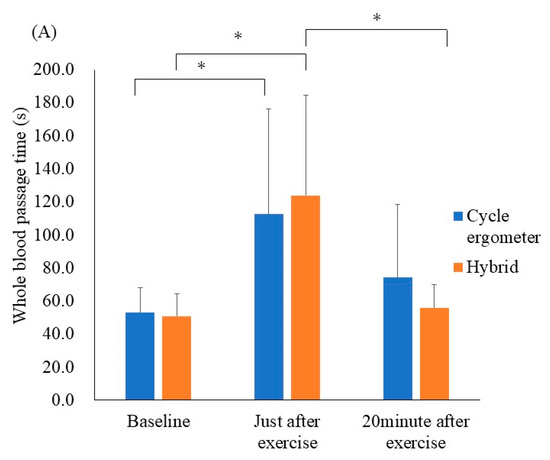
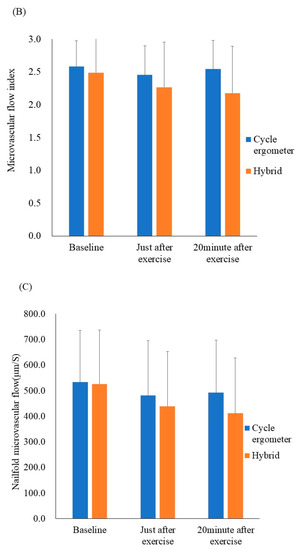
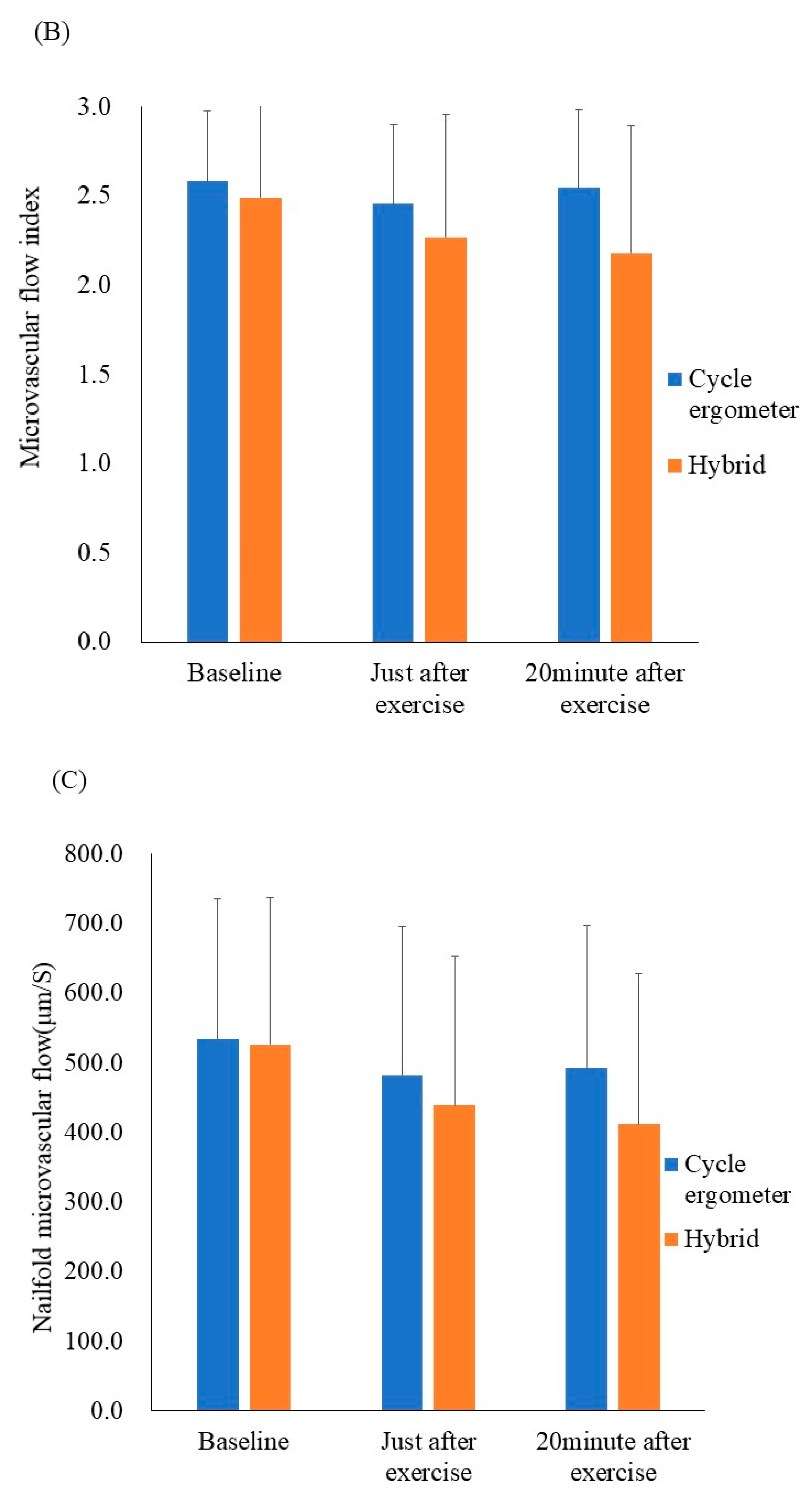
Figure 3.
Results of the ex vivo and in vivo microcirculatory evaluation of the cycle ergometer and hybrid exercises. (A) Whole blood passage time (ex vivo). (B) Microvascular flow index (in vivo). (C) Nailfold microvascular flow (in vivo). Whole blood passage time ex vivo was significantly prolonged just after both exercise protocols when compared with the resting state (p < 0.05). All data were expressed as mean ± SD. * p < 0.05.
The results of the in vivo microcirculatory assessment are shown in Figure 3B,C The microvascular flow index and nailfold microvascular flow remained unchanged. Additionally, other in vivo values—including the total vessel density, perfused vessel density, and proportion of perfused vessels—remained unchanged after the hybrid, as well as cycle ergometer exercises. Still, the ex vivo values for blood fluidity demonstrated transient exacerbation.
Results of the objective variables for the cycle ergometer are shown in Table 3. A significant increase in lactate level was observed just after exercise. A significant decrease in blood glucose level was observed 20 min after exercise. There was no significant difference in d-ROMs and BAP. Total vessel density of small vessels, perfused vessel density, and percentage of perfused small vessels remained changed. The total vessel density of small vessels, perfused vessel density, and percentage of perfused small vessels involved blood flow assessments.

Table 3.
Serial changes in hemodynamics, serum lactate, blood glucose levels, and oxidative stress before and after the cycle ergometer exercise.
The results of the objective variables for the hybrid exercise are shown in Table 4. A significant increase in lactate level was observed just after exercise. A significant decrease in blood glucose level was observed just after exercise and 20 min after exercise. White blood cells, platelets, and hematocrit increased significantly just after exercise and decreased significantly 20 min after exercise. There was a significant increase in BAP just after exercise. Total vessel density of small vessels, perfused vessel density, percentage of perfused small vessels and d-ROMs remained no changed. No synergistic effect was observed between cycle ergometer exercise and hybrid exercise.

Table 4.
Serial changes in hemodynamics, serum lactate, blood glucose levels, and oxidative stress before and after hybrid exercise (cycle ergometer + WB-ENMS).
4. Discussion
Our major findings revealed that compared with the cycle ergometer exercise, the hybrid exercise with cycle ergometer exercises and WB-NMES can be safely performed with continuously increasing VO2. Additionally, although blood fluidity was transiently exacerbated just after both exercise programs, in vivo sublingual and nailfold microcirculatory parameters remained unchanged, even after the hybrid exercise.
The present study showed that hybrid exercise provided more oxygen uptake at the AT level load of the cycle ergometer exercises. During exercise therapy for cardiac rehabilitation, a 40–60% of peak VO2 or AT level exercise prescription is highly recommended. If hybrid exercises can be performed by cardiac rehabilitation patients, they may be more effective than AT-prescribed exercises. Our results correspond with those of previous studies regarding NMES on the lower extremities [5,11]. Electrical stimulation of the thigh during the cycle ergometer exercise resulted in a significant increase in VO2 when compared with no electrical stimulation [11]; furthermore, the addition of NMES during certain cycle ergometer exercises significantly increased the VO2, HR, and respiratory gas exchange ratios [5]. The difference between this and the previous study is that, here, electrical stimulation was applied to the upper limbs and trunk muscles that were not involved in pedaling over a wide range. We expect that more muscles participated in the exercise, explaining the high VO2 observed during the hybrid exercise.
A previous study reported that the combination of voluntary exercise and electrical stimulation induces metabolic activity and energy expenditure, as well as larger muscle fiber recruitment and motor output, when applied under submaximal conditions [19]. At the respiratory compensation point, carbon dioxide emission was higher in the hybrid exercise, even with the same exercise load. In addition to the accumulation of lactate due to vigorous exercise > AT level, lactate was accumulated by sustained contraction of type II fibers by WB-NMES, resulting in a larger carbon dioxide emission as a buffering response. We believe that WB-NMES may have mobilized more muscle fibers as well as the maximal contraction. The lactate levels just after peak exercise were not significantly different between cycle ergometer exercise and the hybrid exercise. The contraction and relaxation times of NMES were not synchronized with voluntary contraction and relaxation of the muscles. NMES causes the antagonist muscles to contract, causing more muscle fibers in the main action muscles to work. Therefore, it has been shown that a high load can be applied to the skeletal muscle by combining electrical stimulation even with low to moderate load exercise [20].
Immediately after performing the exercise, the whole blood passage time—as a parameter of ex vivo blood fluidity—was prolonged; additionally, the blood fluidity temporarily deteriorated during both exercise protocols. There was no synergistic effect caused by the load of WB-NMES on blood fluidity. Vigorous exercise transiently impairs blood fluidity, which has been reported to be a result of increased hematocrit, white blood cell, and platelet counts [8,21]. In fact, hematocrit, white blood cell count, and platelets were also increased by cycle ergometer and hybrid exercises, which is considered to be the reason for the prolonged whole blood passage time. During exercise, blood is redistributed to active muscles, and blood volume to the digestive system decreases; even in healthy adults, visceral perfusion decreases immediately after exercise [22]. Decreased visceral blood flow may be reflected in the sublingual blood flow [23]; additionally, it has been reported that high dehydration levels and prolonged exercise may reduce functional capillary density [24].
We predicted that the sublingual and nailfold microcirculatory flow would decrease due to the decrease in visceral blood flow caused by dehydration and blood flow distribution to the motor muscles. Nevertheless, no changes in the sublingual microvascular flow index or nailfold microvascular flow were observed in vivo. Exercise causes shear stress in the vascular endothelium, resulting in vasodilation due to nitric oxide (NO) synthase activity and NO utilization. This may be the reason for the difference between the blood fluidity observed ex vivo, and blood flow observed in vivo. NMES can increase skin perfusion via vasodilation [25]. NMES has been reported to enhance hypoxia-inducible factor (HIF) 1α and vascular endothelial growth factor, which may have accelerated wound healing of diabetic foot ulcers. [26]. HIF-1α is highly expressed in fast-twitch muscle (type II), which has a high glycolytic capacity. Therefore, sustained contraction of fast-twitch muscle by WB-NMES may have elevated HIF-1α and lactate production. Vasodilation due to lactate accumulation in addition to NO-induced vasodilation may have offset the deterioration of sublingual and nailfold microcirculation due to exercise-induced activation of the sympathetic nervous system.
During acute exercise, active oxygen is produced in the body by reperfusion after muscle damage or organ ischemia, causing oxidative stress damage, such as tissue damage and inflammation. Conversely, after acute exercise, antioxidant enzymes increase and suppress the increase in oxidative stress. Creatine kinase and the induction of muscle damage have been reported as side effects of WB-NMES [27]; however, there was no significant increase in oxidative stress due to WB-NMES observed in this study, which was proven to be safe and effective.
5. Limitation
This was a mixed-sex study that did not consider sex differences. Women are generally more prone to anemia and have lower hematocrit levels. Additionally, since blood flow is evaluated on the basis of the movement of red blood cells in the sublingual and nailfold microcirculation, there is a possibility of sex differences in the whole blood passage time and microcirculation in vivo. It is therefore necessary to increase the number of cases for verification.
6. Conclusions
While both cycle ergometer and hybrid exercises transiently impaired blood fluidity ex vivo, they did not affect microcirculation in vivo. Hybrid exercise provides better time-saving training according to oxygen uptake than the cycle ergometer exercise at the same level in healthy young people. Further study is planned to apply the combination therapy to the old people, especially associated with cardiovascular diseases.
Author Contributions
Conceptualization, K.O. and T.Y.; methodology, K.O., M.T., T.T. and Y.T.; software, M.T.; validation M.T., T.T. and Y.T.; formal analysis, K.O.; investigation, K.O.; resources, Y.T.; data curation, M.T.; writing—original draft preparation, K.O.; writing—review and editing, Y.T. and T.Y.; visualization, K.O.; supervision, Y.T.; project administration, T.Y.; funding acquisition, T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to T.Y. No. 17K01463), and by the Vehicle Racing Commemorative Foundation (to T.Y.).
Institutional Review Board Statement
This study was approved by our institutional ethics committee (approval number: Nikko 20-020).
Informed Consent Statement
All participants provided written informed consent, and all procedures performed in this study complied with the National Ethical Guidelines for Medical and Health Research involving Human Participants and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Acknowledgments
The authors thank Keiko Yoshizawa for administrative assistance, Yasuko Murakami, and clinical laboratory staff for their technical assistance. The authors also thank MTG for donations of materials (pads, etc.) used for experiments and appropriate technical advice regarding WB-NMES.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Nuhr, M.J.; Pette, D.; Berger, R.; Quittan, M.; Crevenna, R.; Huelsman, M.; Wiesinger, G.F.; Moser, P.; Fialka-Moser, V.; Pacher, R. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur. Heart J. 2004, 25, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Yamada, S.; Tanimura, D.; Kazama, S.; Ishihara, T.; Shimojo, M.; Iwata, E.; Kondo, S.; Hiraiwa, H.; Kato, T.; et al. Neuromuscular electrical stimulation is feasible in patients with acute heart failure. ESC Heart Fail. 2019, 6, 975–982. [Google Scholar] [CrossRef]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; Von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Resultsof the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef]
- Van Buuren, F.; Mellwig, K.P.; Prinz, C.; Körber, B.; Fründ, A.; Fritzsche, D.; Faber, L.; Kottmann, T.; Bogunovic, N.; Dahm, J.B.; et al. Electrical myostimulation improves left ventricular function and peak oxygen consumption in patients with chronic heart failure: Results from the exEMS study comparing different stimulation strategies. Clin. Res. Cardiol. 2013, 102, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Taniguchi, Y.; Moritani, T. Metabolic and cardiovascular responses during voluntary pedaling exercise with electrical muscle stimulation. Eur. J. App. Physiol. 2014, 114, 1801–1807. [Google Scholar] [CrossRef]
- Watanabe, K.; Yoshida, T.; Ishikawa, T.; Kawade, S.; Moritani, T. Effect of the Combination of Whole-Body Neuromuscular Electrical Stimulation and Voluntary Exercise on Metabolic Responses in Human. Front. Physiol. 2019, 10, 291. [Google Scholar] [CrossRef]
- McCarthy, D.A.; Dale, M.M. The Leucocytosis of Exercise. A Review and Model. Sports Med. 1988, 6, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Yasu, T.; Tsuboi, K.; Sugawara, Y.; Kubo, N.; Umemoto, T.; Arao, K.; Kawakami, M.; Momomura, S.-I. Effects of Submaximal Exercise on Blood Rheology and Sympathetic Nerve Activity. Circ. J. 2010, 74, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Mayer, G.A. Blood Viscosity in Healthy Subjects and Patients with Coronary Heart Disease. Can. Med. Assoc. J. 1964, 91, 951–954. [Google Scholar] [PubMed]
- Hoshiai, M.; Ochiai, K.; Tamura, Y.; Tsurumi, T.; Terashima, M.; Tamiya, H.; Maeno, E.; Mizuguchi, S.; Tomoe, T.; Kawabe, A.; et al. Effects of whole-body neuromuscular electrical stimulation device on hemodynamics, arrhythmia, and sublingual microcirculation. Heart Vessel. 2021, 36, 844–852. [Google Scholar] [CrossRef]
- Matsuse, H.; Shiba, N.; Takano, Y.; Yamada, S.; Ohshima, H.; Tagawa, Y. Cycling exercise to resist electrically stimulated antagonist increases oxygen uptake in males: Pilot study. J. Rehabil. Res. Dev. 2013, 50, 545. [Google Scholar] [CrossRef] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Sato, K.; Ohki, H.; Kaneko, T. Optically accessible microchannels formed in a single-crystal silicon substrate for studies of blood rheology. Microvasc. Res. 1992, 44, 226–240. [Google Scholar] [CrossRef]
- Yasu, T.; Mutoh, A.; Wada, H.; Kobayashi, M.; Kikuchi, Y.; Momomura, S.; Ueda, S. Renin-Angiotensin System Inhibitors Can Prevent Intravenous Lipid Infusion-Induced Myocardial Microvascular Dysfunction and Leukocyte Activation. Circ. J. 2018, 82, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, R.; Fukuda, H.; Kikuchi, Y.; Yanaka, H.; Hata, N.; Yamazaki, M.; Nakatani, Y.; Tamura, Y.; Yamakoshi, S.; Kawabe, A.; et al. Clinically feasible method for assessing leukocyte rheology in whole blood. Heart Vessel. 2020, 35, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Boerma, E.C.; Mathura, K.R.; Van Der Voort, P.H.J.; Spronk, P.E.; Ince, C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: A prospective validation study. Crit. Care 2005, 9, R601–R606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Backer, D.; Hollenberg, S.; Boerma, C.; Goedhart, P.; Büchele, G.; Ospina-Tascon, G.; Dobbe, I.; Ince, C. How to evaluate the microcirculation: Report of a round table conference. Crit. Care 2007, 11, R101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, M.J.; Shapiro, N.I. A guide to human in vivo microcirculatory flow image analysis. Crit. Care 2015, 20, 35. [Google Scholar] [CrossRef] [Green Version]
- Paillard, T. Training Based on Electrical Stimulation Superimposed onto Voluntary Contraction Would be Relevant Only as Part of Submaximal Contractions in Healthy Subjects. Front. Physiol. 2018, 9, 1428. [Google Scholar] [CrossRef]
- Wahl, P.; Hein, M.; Achtzehn, S.; Bloch, W.; Mester, J. Acute effects of superimposed electromyostimulation during cycling on myokines and markers of muscle damage. J. Musculoskelet. Neuronal Interact. 2015, 15, 53–59. [Google Scholar]
- Bourey, R.E.; Santoro, S.A. Interactions of Exercise, Coagulation, Platelets, and Fibrinolysis—A Brief Review. Med. Sci. Sports Exer. 1988, 20, 439–446. [Google Scholar] [CrossRef]
- Van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.M.; Buurman, W.A.; DeJong, C.H.C. Exercise-Induced Splanchnic Hypoperfusion Results in Gut Dysfunction in Healthy Men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Weil, M.H.; Sun, S.; Tang, W.; Bisera, J.; Mason, E.J. Decreases in organ blood flows associated with increases in sublingual PCO2 during hemorrhagic shock. J. Appl. Physiol. 1998, 85, 2360–2364. [Google Scholar] [CrossRef]
- Pranskunas, A.; Arstikyte, J.; Pranskuniene, Z.; Bernatoniene, J.; Kiudulaite, I.; Vaitkaitiene, E.; Vaitkaitis, D.; Brazaitis, M. Time Evolution of Sublingual Microcirculatory Changes in Recreational Marathon Runners. BioMed Res. Int. 2017, 2017, 7120785. [Google Scholar] [CrossRef] [Green Version]
- Petrofsky, J.; Schwab, E.; Lo, T.; Cúneo, M.; George, J.; Kim, J.; Al-Malty, A. Effects of electrical stimulation on skin blood flow in controls and in and around stage III and IV wounds in hairy and non-hairy skin. Med. Sci. Monit. 2005, 11, CR309–CR316. [Google Scholar]
- Asadi, M.R.; Torkaman, G.; Hedayati, M.; Mohajeri-Tehrani, M.R.; Ahmadi, M.; Gohardani, R.F. Angiogenic effects of low-intensity cathodal direct current on ischemic diabetic foot ulcers: A randomized controlled trial. Diabetes Res. Clin. Pract. 2017, 127, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Stöllberger, C.; Finsterer, J. Side effects of whole-body electro-myo-stimulation. Wien. Med. Wochenschr. 2018, 169, 173–180. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).