Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review
Abstract
:1. Introduction
2. Radiation Technologies
2.1. Type of Radiation Sources
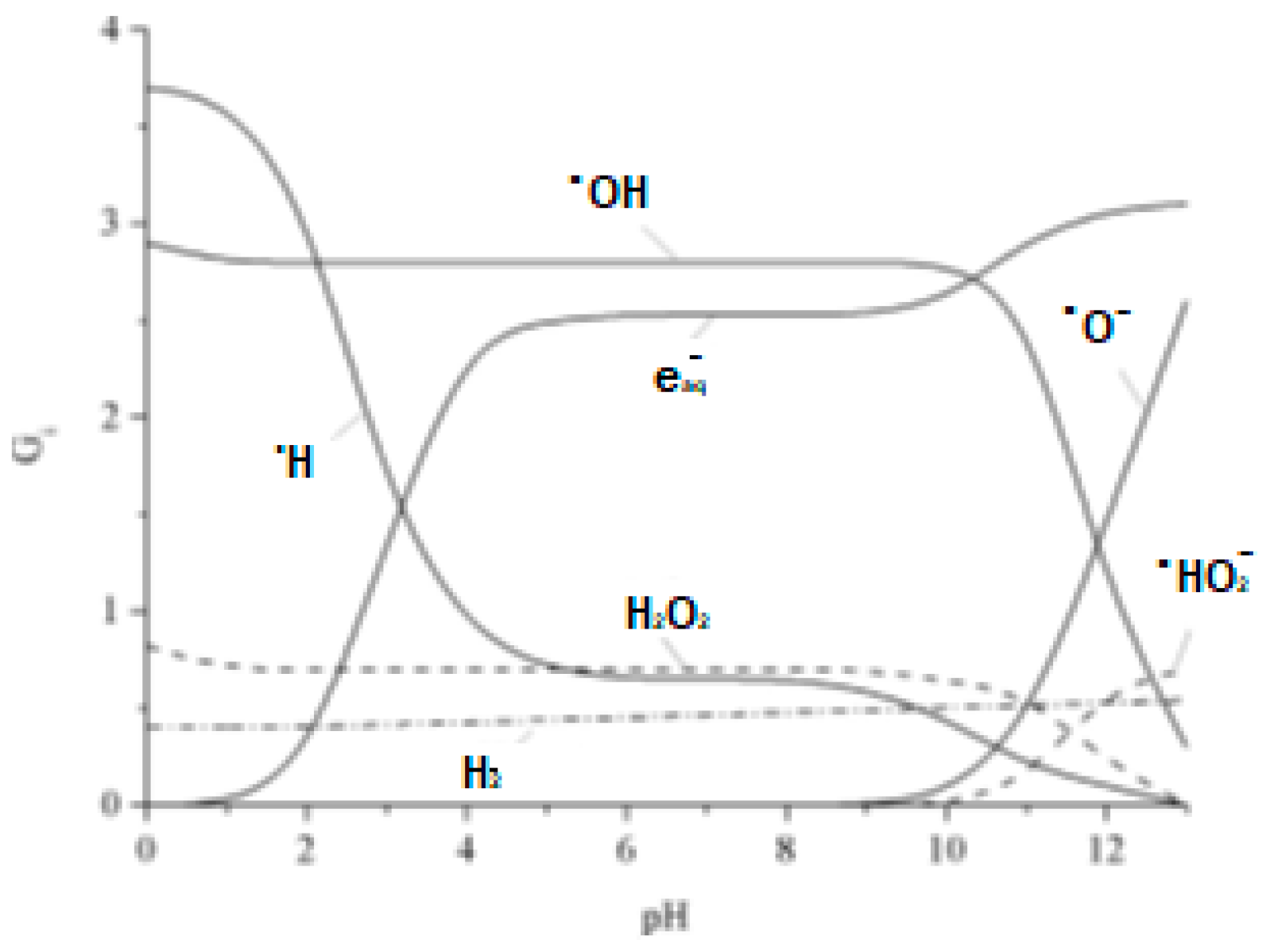
2.2. Water Radiolysis
- − Radical addition;
- − Hydrogen abstraction;
- − Electron transfer;
- − Combination/recombination of radicals.
3. Endocrine-Disrupting Compounds EDCs
- Natural and artificial estrogens;
- Bisphenol A;
- Parabens;
- Perfluorinated compounds PFOA and PFOS;
- Pesticides (e.g., atrazine);
- Psychotropic drugs (carbamazepine and fluoxetine);
- NSADs (DCF and IBU);
- Antibiotics (see Table 1).
| Compound | Initial Concentration/Additives | Dose/Kind of Radiation | Yield of Degradation/Mineralization | Refs. |
|---|---|---|---|---|
| Sulfamethoxazole (SMX) | 0.1 mM | 5 kGy/γ | Decomposition—100% | [29] |
| SMX | 0.1 mM | 4 kGy/γ | Improvement in biodegradability | [30] |
| Lincomycin (LMC) | 17 mg dm−3 | 2 kGy/γ | Improvement in biodegradability of about 18.9% | [22] |
| SMX | 17 mg dm−3 | 2 kGy/γ | Improvement in biodegradability of about 28.33% | [22] |
| Tetracycline (TCN) | 17 mg dm−3 | 2 kGy/γ | Improvement in biodegradability of about 36.62% | [22] |
| SMX | 1 mg dm−3 2 mg dm−3 5 mg dm−3 | 300 Gy/γ 300 Gy/γ 300 Gy/γ | Degradation—100% Degradation—96.2% Degradation—91.3% | [31] |
| SMX | 5 mg dm−3 +2 mM NO2− | 100 Gy/γ | Degradation—44.84% | [31] |
| SMX | 5 mg dm−3 +2 mM NO3− | 100 Gy/γ | Degradation—34.84% | [31] |
| Sulfadiazine (SD) | 25 mg dm−3 +18.5 mM H2O2 +0.3 mmol dm−3 Fe2+ pH 2.6 | 1 kGy/γ | Degradation—100% Mineralization—74% | [32] |
| Sulfametiazol (SMT) | 20 mg dm−3 pH 6.5 | 5 kGy/γ | Degradation—95.5% Mineralization—6.8% | [33] |
| Amoxicillin, Cefradine, LMC, TCN | 30 mg dm−3 30 mg dm−3 30 mg dm−3 30 mg dm−3 | 2 kGy/γ 2 kGy/γ 2 kGy/γ 2 kGy/γ | Degradation—100% Degradation—100% Degradation—100% Degradation—100% | [34] |
| Amoxicillin, Ofloxacin, Cefradine | 50 mg dm−3, pH 9 50 mg dm−3, pH 9 50 mg dm−3, pH 9 | 30 kGy EB 30 kGy EB 30 kGy Ee | Degradation—97% Degradation—97.6% Degradation—96.9% | [35] |
| TCN | 300 mg kg−1 (Livestock samples) | 1 kGy EB 3 kGy EB 5 kGy EB 10 kGy EB | Degradation—42.77% Degradation—62.20% Degradation—77.83% Degradation—90.50% | [36] |
| Norfloxacin, Ciprofloxacin | 0.1mM 0.1 mM | 2 kGy/γ 2 kGy/γ | Degradation—100%/TOC reduction—25% Degradation—100%/TOC reduction—25% | [37] |
| Ornidazole | 50 mg dm−3 | 3 kGy/γ | Degradation—100% | [38] |
3.1. Estrogen Degradation
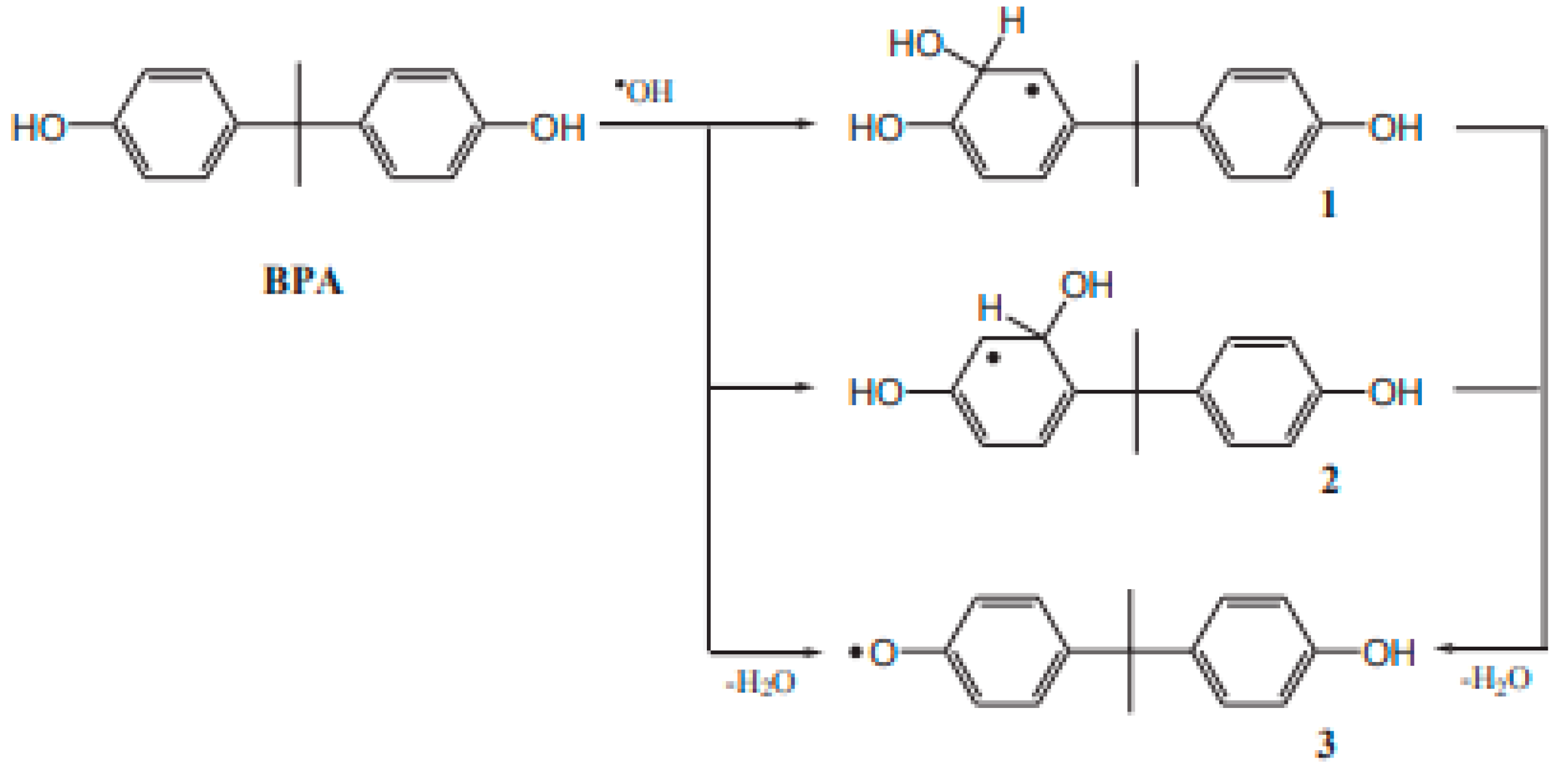
3.2. Bisphenol A Degradation
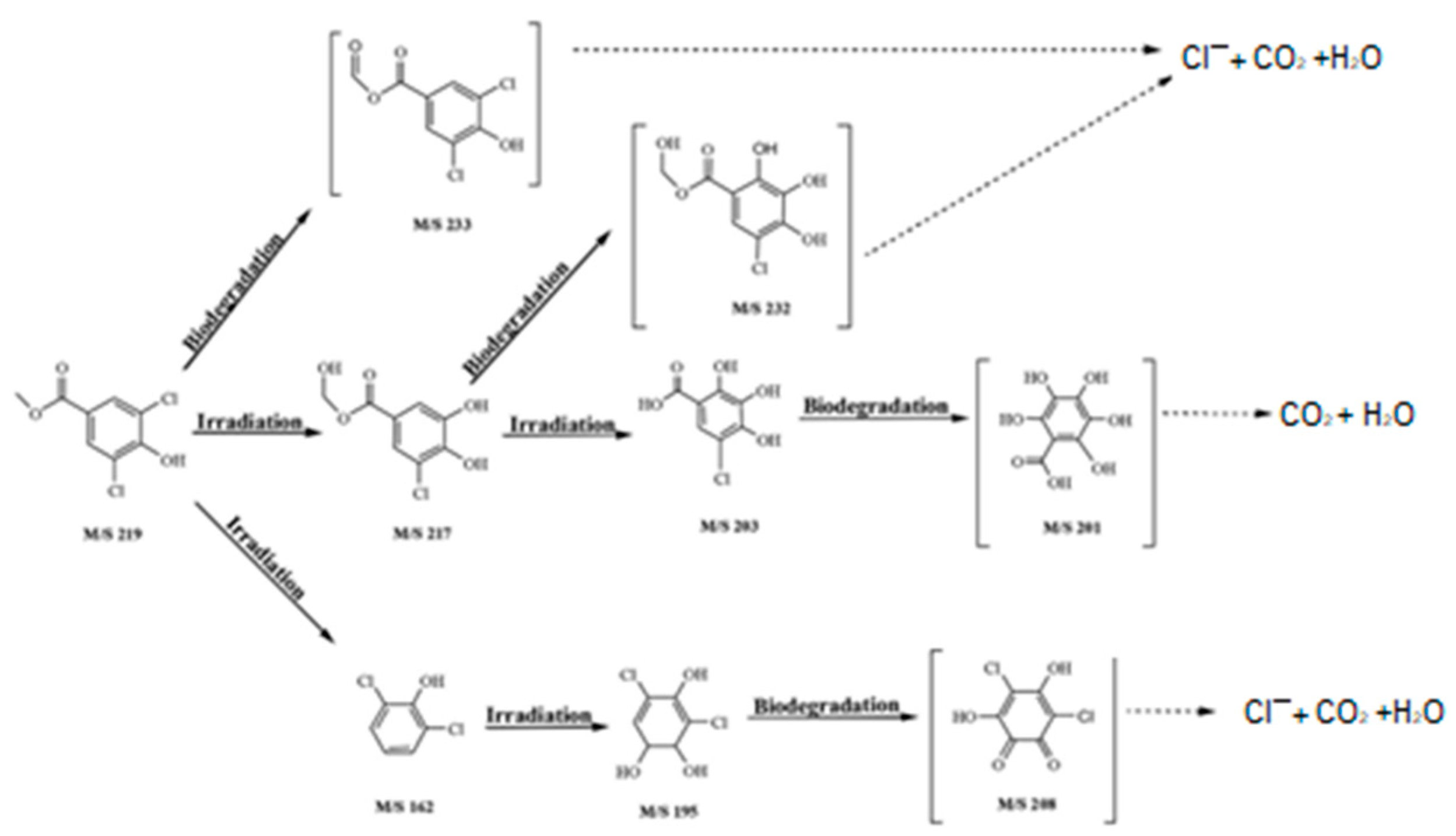
3.3. Paraben Degradation
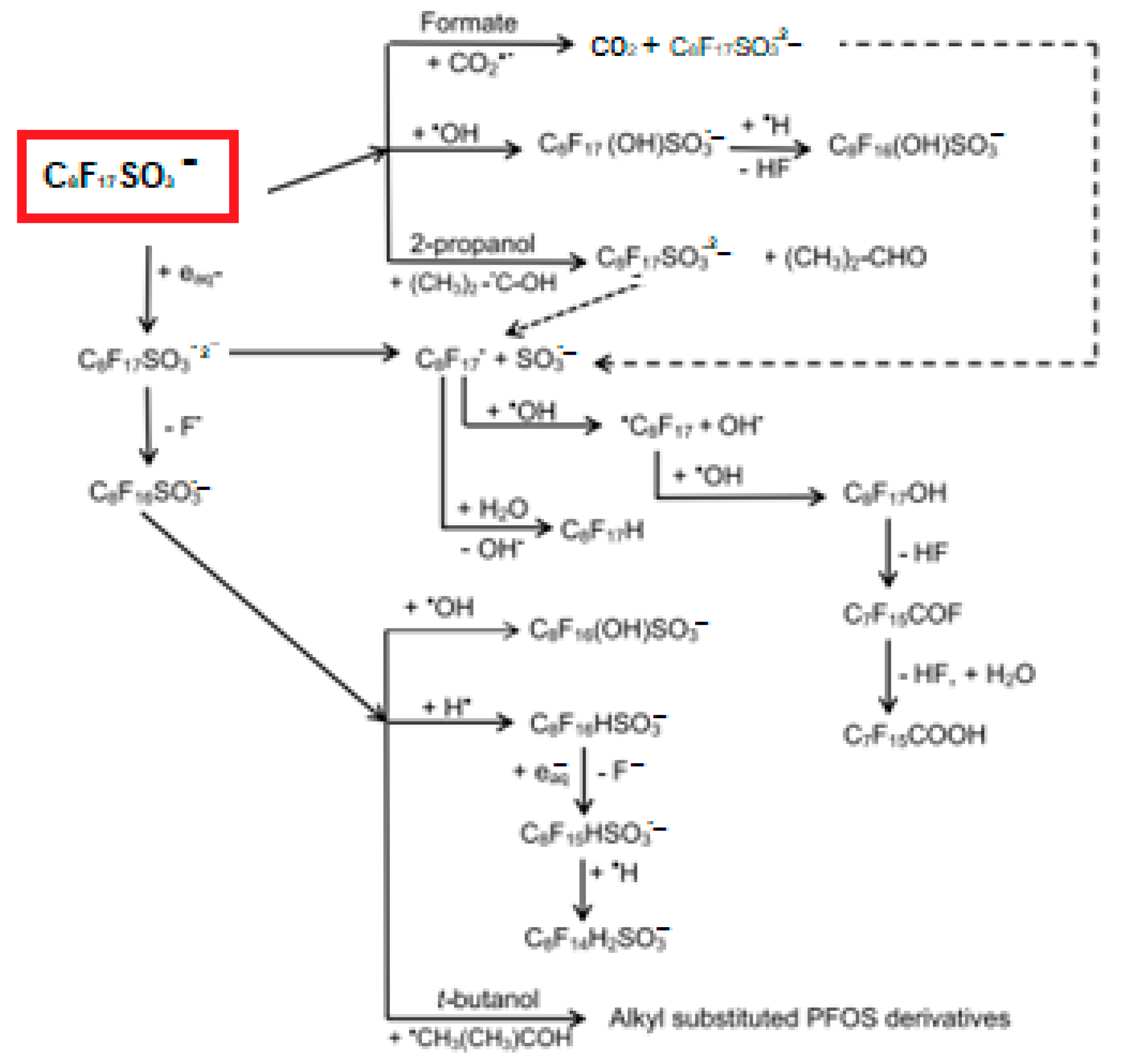
3.4. Perfluorinated Compound Degradation
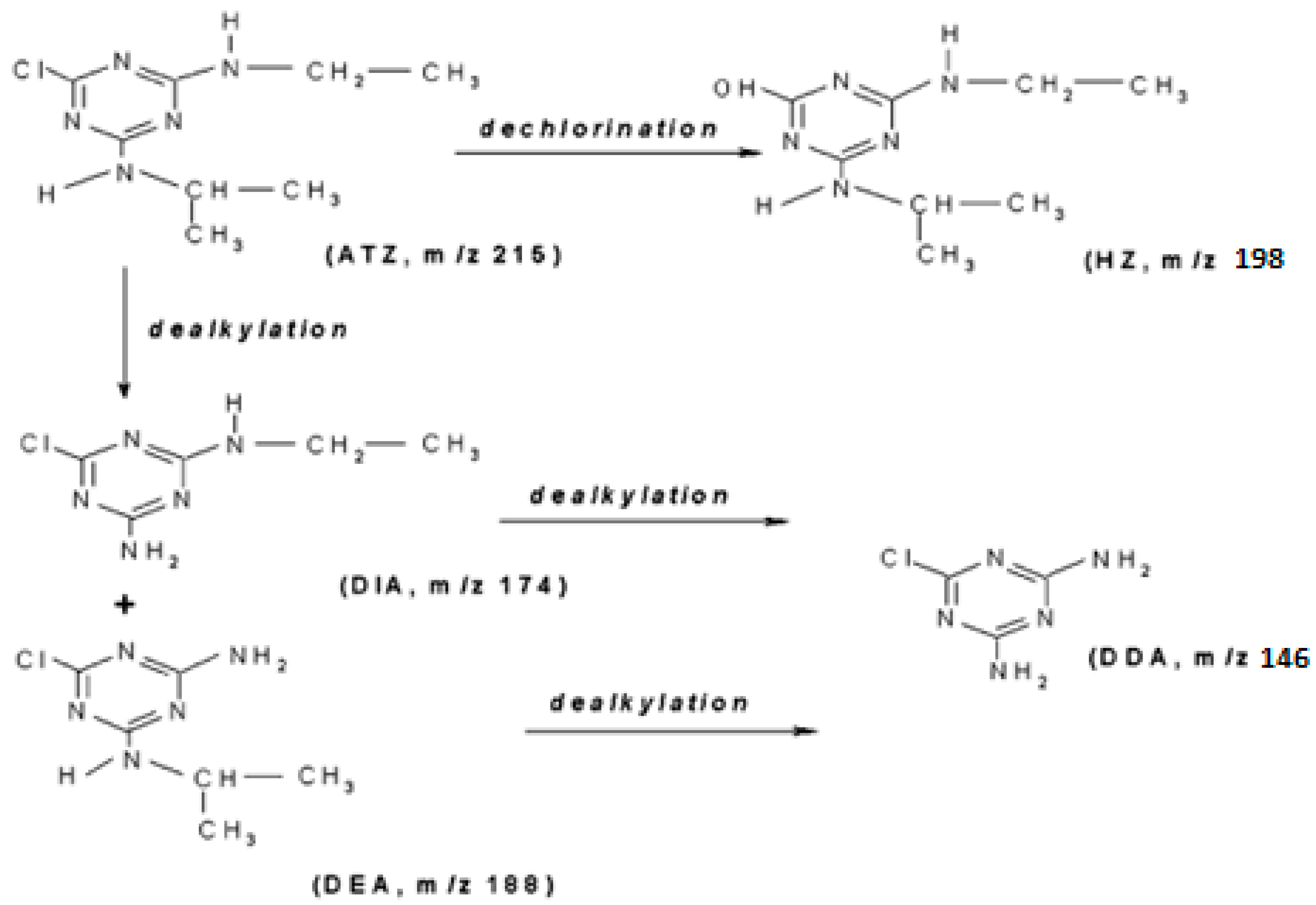
3.5. Atrazine Degradation
3.6. Psychotropic Drug Degradation
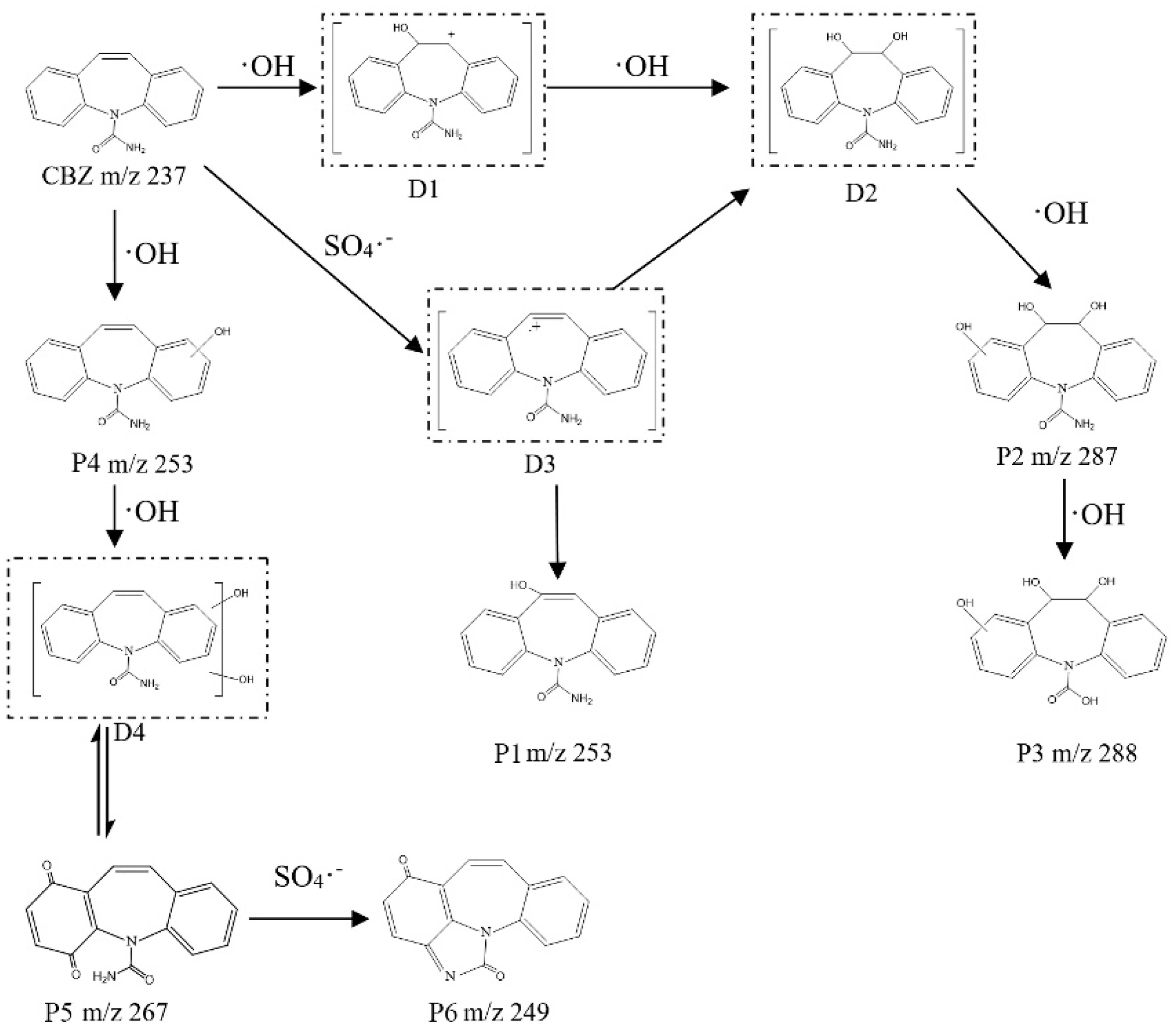
3.6.1. Carbamazepine
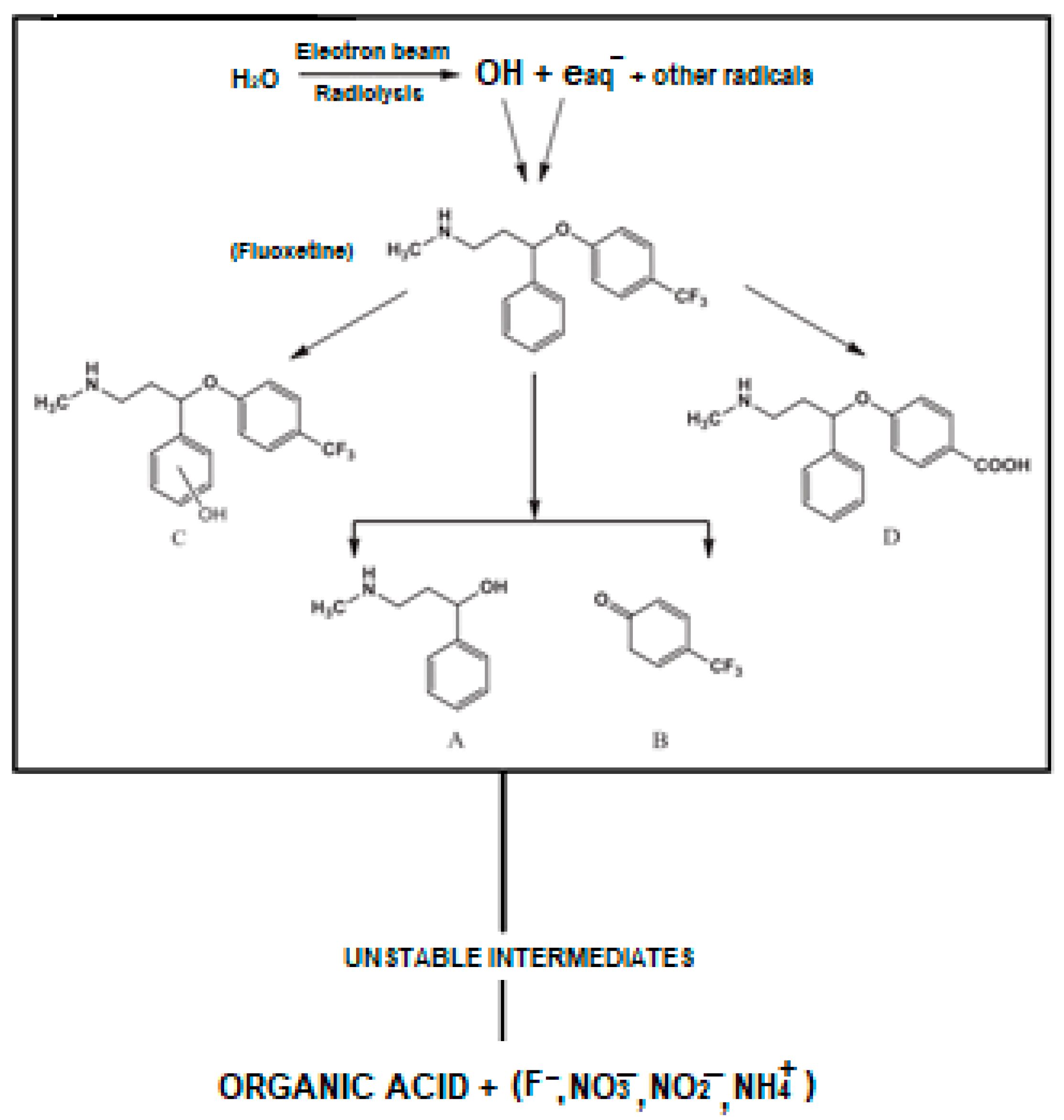
3.6.2. Fluoxetine
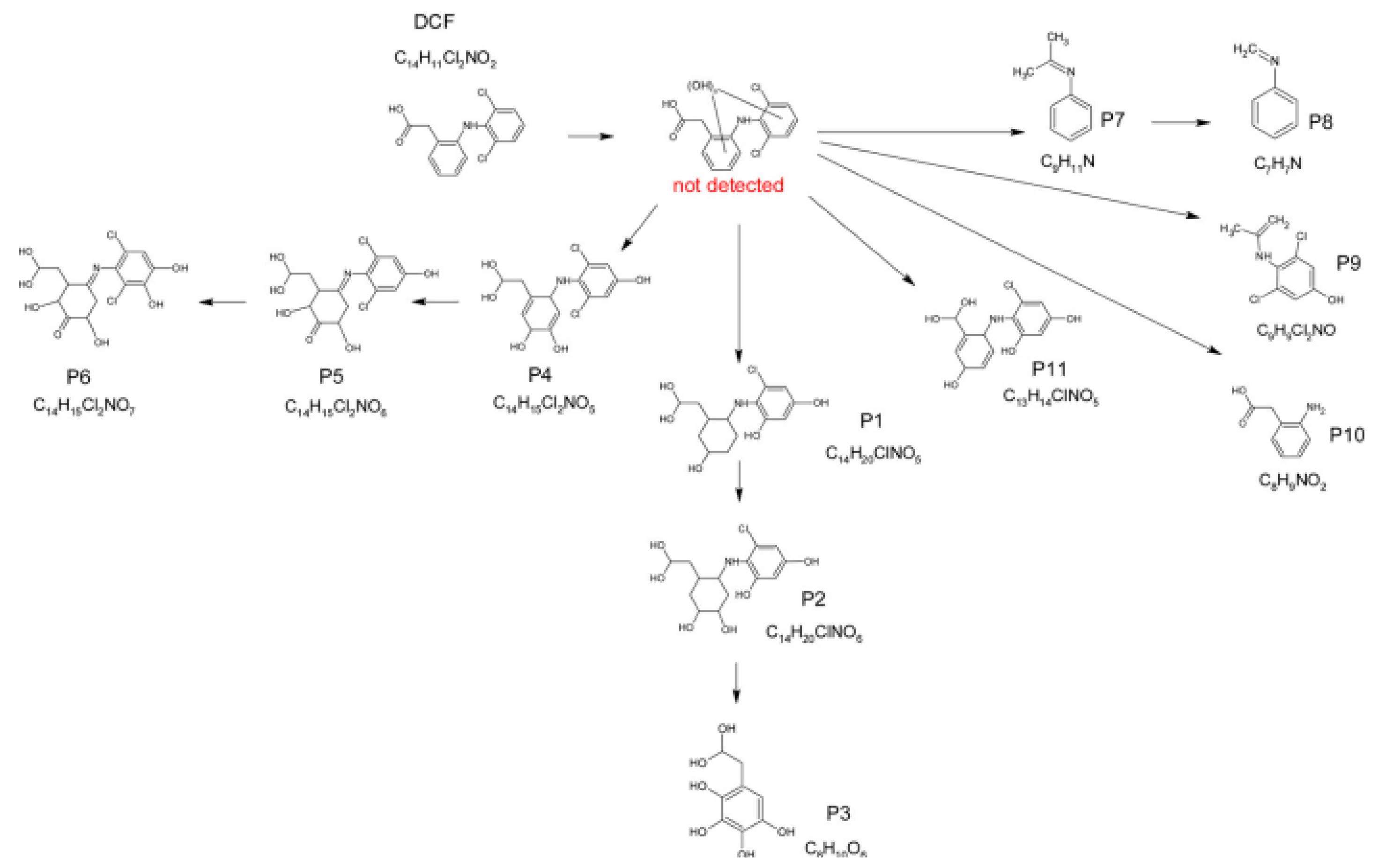
3.7. Non-Steroidal Anti-Inflammatory Drug Degradation
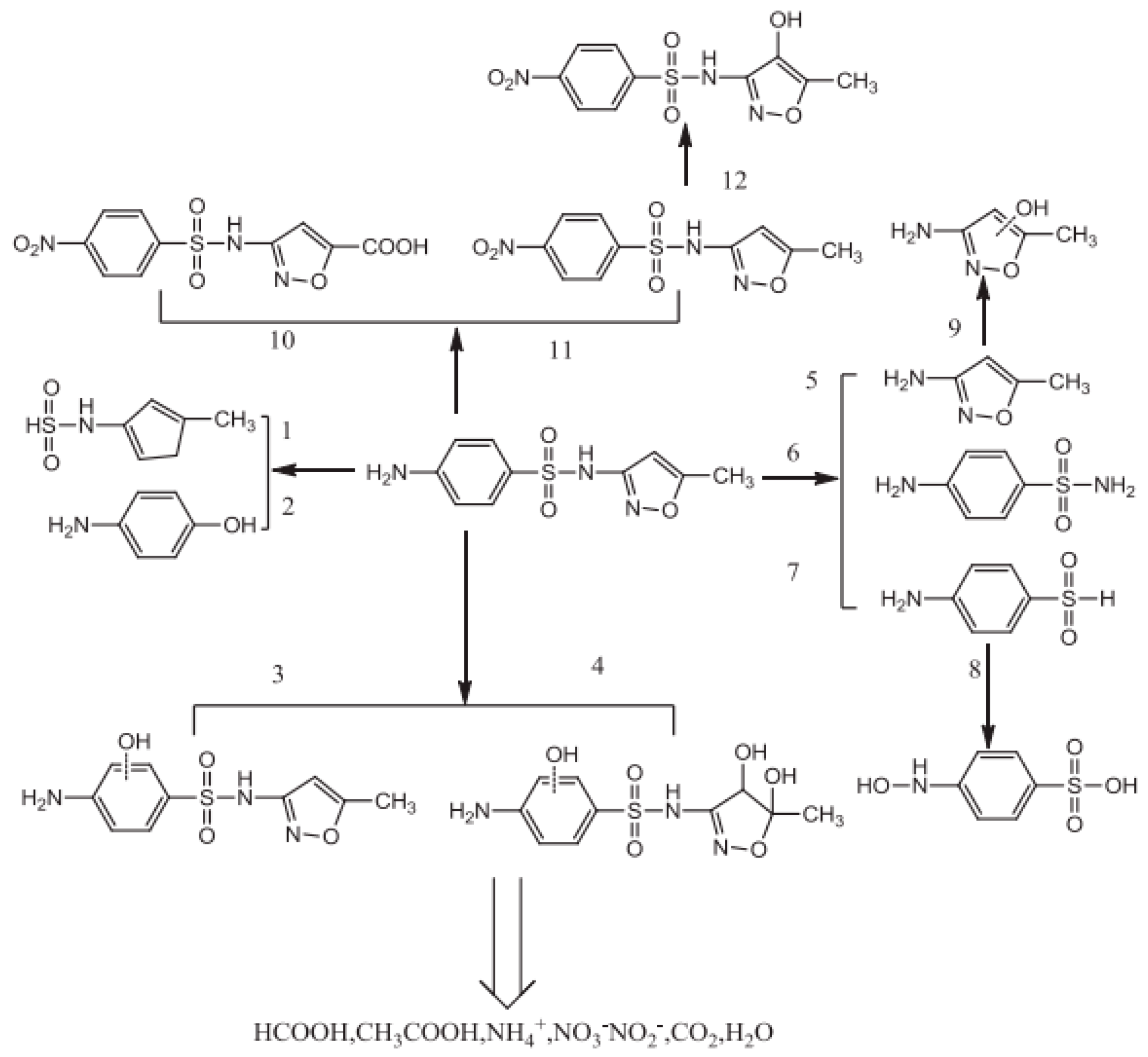
3.8. Antibiotic Degradation
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Report Genewa/Nowy Jork, 13 lipca 2017 r.—(WHO) i UNICEF. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and Sustainable Development Goal Baselines. Available online: https://www.who.int/mediacentre/news/releases/2017/launch-version-report-jmp-water-sanitation-hygiene.pdf (accessed on 28 October 2021).
- Giulivo, M.; de Alda, M.L.; Capri, E.; Barcelo, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel Rahman, R.O.; Kozak, M.W.; Hung, Y.T. Radioactive pollution and control. In Handbook of Environment and Waste Management; Hung, Y.T., Wang, L.K., Shammas, N.K., Eds.; World Scientific Publishing Co.: Singapore, 2014; pp. 949–1027. [Google Scholar]
- Chmielewski, A.G. Electron Accelerators for environmental protection. Rev. Accel. Sci. Technol. 2011, 4, 147–159. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bartosiewicz, I.; Bojanowska-Czajka, A.; Szreder, T.; Bobrowski, K.; Nałęcz-Jawecki, G.; Męczyńska-Wielgosza, S.; Nichipore, H. Application of ionizing radiation in decomposition of perfluorooctane sulfonate (PFOS) in aqueous solutions. Chem. Eng. J. 2020, 379, 122303. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reaction of hydrated electrons, hydrogen atoms and hydroxyl radical (•OH/•O−) in aqueous solution. J. Phys. Ref. Data 1988, 17, 513–531. [Google Scholar] [CrossRef] [Green Version]
- Tchobanoglous, G.; Burton, F.; Stensel, H. Wastewater Engineering; Matcalf&Eddy Inc.: New York, NY, USA, 2003. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Danton, F.S.; Peterson, D.B. Forms of H and OH produced in the radiolysis of aqueous system. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1962, 267, 443–463. [Google Scholar]
- Christensen, H.; Sehed, K.; Logager, T. Temperature dependence of the rate constant for reactions of hydrated electrons with H, OH and H2O2. Radiat. Phys. Chem. 1994, 43, 527–531. [Google Scholar] [CrossRef]
- Wojnarovits, L.; Takacs, E.; Dajka, K.; Emmi, S.S.; Russo, M.; D’Angelantonio, M. Re-evaluation of the rate constant for the H atom reaction with tert-butanol in aqueous solution. Radiat. Phys. Chem. 2004, 69, 217–219. [Google Scholar] [CrossRef]
- Getoff, N. Radiation-induced degradation of water pollutants—State of the art. Radiat. Phys. Chem. 1996, 47, 581–593. [Google Scholar] [CrossRef]
- Gehringer, P. Advances in radiation processing of wastewater—Basics of the process. In Status of Industrial Scale Radiation Treatment of Wastewater and Its Future; IAEA-TECDOC-1407; IAEA: Vienna, Austria, 2003; pp. 7–17. [Google Scholar]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Szreder, T.; Męczyńska-Wielgosz, S.; Borowski, K.; Fornal, E.; Nichipor, H. Application of ionizing radiation for removal of endocrine disruptor bisphenol A from waters and wastewaters. Chem. Eng. J. 2021, 403, 126169. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of diclofenac in aqueous solution by ionizing radiation in the presence of humic acid. Sep. Purif. Technol. 2020, 234, 116079. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takacs, E. Wastewater treatment with ionizing radiation. J. Radioanal. Nucl. Chem. 2017, 311, 973–981. [Google Scholar] [CrossRef]
- Yin, Y.N.; Hu, J.; Wang, J.L. Degradation kinetics of 2,4-dichlorophenol by gamma ray irradiation in the presence of ozone. Nucl. Sci. Tech. 2016, 27, 640. [Google Scholar] [CrossRef]
- Bojanowska-Czajka, A.; Drzewicz, P.; Kozyra, C.; Nałęcz-Jawecki, G.; Sawicki, J.; Szostek, B.; Trojanowicz, M. Radiolytic degradation of herbicide 4-chloro-2-methyl phenoxyacetic acid (MCPA) by gamma radiation for environmental protection. Ecotoxicol. Environ. Saf. 2006, 65, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lei, Z.D.; Wang, T.; Wang, J.J.; Zhang, X.D.; Xu, G.; Tang, L. Radiolysis of carbamazepine aqueous solution using electron beam irradiation combining with hydrogen peroxide: Efficiency and mechanism. Chem. Eng. J. 2016, 295, 484–493. [Google Scholar] [CrossRef]
- Ponomarev, A.V.; Ershov, B.G. The green method in water management: Electron beam treatment. Environ. Sci. Technol. 2020, 54, 5331–5344. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, M.M.; Kim, T.H.; Yu, S. Enhanced biodegradability of pharmaceuticals and personal care products by ionizing radiation. Water Environ. Res. 2015, 87, 321. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iaea.org/newscenter/news/started-with-iaea-support-chinas-electron-beam-industry-opens-worlds-largest-wastewater-treatment-facility (accessed on 28 October 2021).
- Viera, W.T.; Barbosa de Farias, M.; Spaolonzi, M.P.; Carlos de Silva, M.G.; Viera, A.M.G. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Galuke, L.S.; Strand, S.E.; Kalhorn, T.F.; Stensel, H.D. Estrogen biodegradation kinetics and estrogenic activity reduction for two biological wastewater treatment methods. Environ. Sci. Technol. 2009, 43, 7111–7116. [Google Scholar] [CrossRef] [PubMed]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02000L0060-20141120 (accessed on 28 October 2021).
- Garnaga, G. Integrated assessment of pollution in the Baltic Sea. Ekologija 2012, 58, 331–355. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Nałęcz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and tap water in Warsav (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sagi, G.; Csay, T.; Patzay, G.; Csonka, E.; Wojnarovits, L.; Takacs, E. Oxidative and reductive degradation of sulfamethoxazole in aqueous solutions: Decomposition efficiency and toxicity assessment. J. Radioanal. Nucl. Chem. 2014, 301, 475–482. [Google Scholar] [CrossRef]
- Sagi, G.; Kovacs, K.; Bezsenyi, A.; Csay, T.; Takacs, E.; Wojnarovits, L. Enhancing the biological degradability of sulfamethoxazole by ionizing radiation treatment in aqueous solution. Radait. Phys. Chem. 2016, 124, 179–183. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Shen, X.; Guo, Q.; Zhao, Y.; Zhu, S.; Guo, Z. Gamma irradiation-induced decomposition of sulfamethoxazole in aqueous solution: The influence of additives, biological inhibitory, and degradation mechanisms. Environ. Sci. Pollut. Res. 2017, 24, 23658–23665. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Ortiz, I.B.; Cruz-Gonzales, G.; Lastre-Acosta, A.M.; Manduca-Artiles, M.; Rapado-Paneque, M.; Chavez-Ardanza, A.; Teixeira, A.C.S.C.; Jauregui-Haza, U.J. Optimization of radiolytic degradation of sulfadiazine by combining Fenton and gamma irradiation. J. Radioanal. Nucl. Chem. 2017, 314, 2597–2607. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhou, Z.; Zheng, X.; Zhao, L.; Yu, A. The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation. Open Chem. 2020, 18, 1188–1194. [Google Scholar] [CrossRef]
- Nam, J.H.; Shin, J.; Kim, T.H.; Yu, S.; Lee, D.H. Comparison of biological and chemical assay for measuring the concentration of residual antibiotics after treatment with gamma irradiation. Environ. Eng. Res. 2020, 25, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhang, L.; Zhang, G.; Pang, T.; Zhang, X.; Cai, D.; Wu, Z. In situ degradation of antibiotic residues in medical intravenous infusion bottles using high energy electron beam irradiation. Sci. Rep. 2017, 7, 39928. [Google Scholar] [CrossRef]
- Ch, J.Y. Evaluating of degradation of antibiotic tetracycline in pig manure by electron beam irradiation. Bull. Environ. Contam. Toxicol. 2010, 54, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Tezge, A.; Sagi, G.; Kovacs, K.; Homlok, R.; Toth, T.; Mohacsi-Farkas, C.; Wojnarovits, L.; Takacs, E. Degradation of fluoroquinolone antibiotics during ionizing radiation treatment and assessment of antibacterial activity, toxicity and biodegradability of the products. Radiat. Phys. Chem. 2018, 147, 101–105. [Google Scholar]
- Changorta, R.; Guin, J.P.; Dhir, A.; Varshney, L. Decomposition of antibiotic ornidazole by gamma irradiation in aqueous solution: Kinetic and its removal mechanism. Environ. Sci. Pollut. Res. 2018, 25, 32591–32602. [Google Scholar]
- Nazari, E.; Suja, F. Effects of 17β-estra5. diol (E2) on aqueous organisms and its treatment problem: A review. Rev. Environ. Health 2016, 31, 465–491. [Google Scholar] [CrossRef] [PubMed]
- Harries, J.E.; Runnalls, T.; Hill, E.; Harris, C.A.; Maddix, S.; Sumpter, J.P.; Tyler, C.R. Development of a reproductive performance test for endocrine disrupting chemicals using pair-breeding fathead minnows (Pimephales promelas). Environ. Sci. Technol. 2000, 34, 3003–3011. [Google Scholar] [CrossRef]
- Kimura, A.; Taguchi, M.; Arai, H.; Hiratsuka, H.; Namba, H.; Kojima, T. Radiation-induced decomposition of trace amounts of 17 b-estradiol in water. Radiat. Phys. Chem. 2004, 69, 295–301. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Kang, S.W.; Yoo, J.; Kim, W.K.; Bae, P.H.; Jung, J. Identification of estrogenic activity change in sewage, industrial and livestock effluents by gamma-irradiation. Radiat. Phys. Chem. 2012, 81, 1757–1762. [Google Scholar] [CrossRef]
- Ren, L.; Wu, M.; Xu, G.; Liu, N.; Bu, T.; Tang, L.; Wang, L.; Zhou, J. Electron Beam Radiolysis of 17beta-Estradiol in Aqueous Solutions. In Proceedings of the 2011 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 10–12 May 2011. [Google Scholar] [CrossRef]
- Scherr, F.F.; Sarmah, A.K.; Di, H.J.; Cameron, K.C. Degradation and metabolite formation of 17beta-estradiol-3-sulphate in New Zealand pasture soils. Environ. Int. 2009, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Escher, C.; Caminada, D. Estrogenic activity of pharmaceuticals and pharmaceutical mixtures in a yeast reporter gene system. Reprod. Toxicol. 2006, 22, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Li, J.L.; He, X.X.; Xu, G.; Ding, G.J.; Shi, W.Y. Radiation removal of synthetic estrogens in aqueous solution: Influence of reduction or oxidation system and toxicity test. Nucl. Sci. Tech. 2016, 27, 22. [Google Scholar] [CrossRef]
- Fromme, H.; Kuchler, T.; Otto, T.; Pilz, K.; Muller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Nagel, S.C.; Bromfiels, J.J. Bisphenol A: A Model Endocrine Disrupting Chemical with a New Potential Mechanism of Action. Endocrinology 2013, 154, 1962–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peller, J.R.; Mezyk, S.P.; Cooper, W.J. Bisphenol A reactions with hydroxyl radicals: Diverse pathways determined between deionized water and tertiary treated wastewater solutions. Res. Chem. Intermed. 2009, 35, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Peller, J.R.; Cooper, W.J.; Ishida, K.P.; Mezyk, S.P. Evaluation of parameters influencing removal efficiencies for organic contaminant degradation in advanced oxidation processes. J. Water Supply Res. Technol.—AQUA 2011, 60, 69–78. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, Q.; Zhang, C. Gamma radiation for treatment of bisphenol A solution in the presence of different additives. Chem. Eng. J. 2012, 183, 10–14. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Abdel Daiem, M.M.; Sánchez-Polo, M.; Ocampo-Pérez, R.; López-Peñalver, J.J.; Velo-Gala, I.; Mota, A.J. Removal of compounds as plasticizers and herbicides from water by means of gamma irradiation. Sci. Total Environ. 2016, 569–570, 518–526. [Google Scholar] [CrossRef]
- Xu, G.; Ren, H.; Wu, M.H.; Liu, N.; Yuan, Q.; Tang, L.; Wang, L. Electron-beam induced degradation of bisphenol A. Nucl. Sci. Tech. 2011, 22, 277–281. [Google Scholar]
- Alcudia-Leon, M.C.; Lucena, S.; Cardenas, M.; Valcarcel, M. Determination of parabens in waters by magnetically confined hydrophobic nanoparticle microextraction coupled to gas chromatography/mass spectrometry. Microchem. J. 2013, 110, 643–648. [Google Scholar] [CrossRef]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.F. Occurrence, fate and behaviour of parabens in aquatic environments: A review. Water Res. 2015, 68, 1–11. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.J.; Parker, J.; Odum, J.; Ashby, J.; Sumpter, J.P. Some alkyl hydroxyl benzoate preservatives (parabens) are estrogenic. Toxicol. Appl. Pharmacol. 1998, 153, 12–19. [Google Scholar] [CrossRef]
- Angelini, G.; Bucci, R.; Colosimo, M.; Margonelli, A. Stability of methyl p-hydroxybenzoate (methylparaben) to gamma radiolysis. Radiat. Phys. Chem. 1998, 51, 77–83. [Google Scholar] [CrossRef]
- Bartosiewicz, I.; Bojanowska-Czajka, A.; Trojanowicz, M. Application of ionizing radiation for radiolytic decomposition of methylparaben. INCT Annu. Rep. 2020, 62–68. Available online: www.ichtj.waw.pl/ichtj/publ/annual/anrep20.pdf (accessed on 28 October 2021).
- Guin, J.P.; Bhardwaj, Y.K.; Varshney, L. Efficient degradation of butylparaben by gamma radiolysis. Appl. Radiat. Isot. 2017, 122, 21–27. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Sun, Y. Degradation of chlorinated paraben by integrated irradiation and biological treatment process. J. Environ. Manag. 2017, 189, 29–35. [Google Scholar] [CrossRef]
- Swoboda, F.; Solar, S. Comparative study on the radiolytic degradation of 4-hydroxybenzoate and 4-hydroxybenzoic ethyl ester. Radiat. Phys. Chem. 1999, 56, 291–301. [Google Scholar] [CrossRef]
- Mokra, K. Endocrine disruptor potential of short- and long-chain perfluoroalkyl substances. PFASs—A synthesis of current knowledge with proposal of molecular mechanism. Int. J. Mol. Sci. 2021, 22, 2148. [Google Scholar] [CrossRef] [PubMed]
- Szajdzińska-Piętek, E.; Gębicki, J.L. Pulse radiolytic investigation of perfluorinated surfactants in aqueous solutions. Res. Chem. Intermed. 2000, 26, 897–912. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.J.; Lyu, X.J.; Yin, H.; Sheng, G.P. Complete mineralization of perfluorooctanoic acid (PFOA) by γ-irradiation in aqueous solution. Sci. Rep. 2014, 4, 7418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trojanowicz, M.; Bartosiewicz, I.; Bojanowska-Czajka, A.; Kulisa, K.; Szreder, T.; Bobrowski, K.; Nichipor, H.; Garcia-Reyes, J.F.; Nałęcz-Jawecki, G.; Męczyńska-Wielgosz, S.; et al. Application of ionizing radiation in decomposition of perfluoroctanoate (PFOA) in waters. Chem. Eng. J. 2019, 357, 698–714. [Google Scholar] [CrossRef]
- Kowal, C.; Brorman, E.; Shankar, S.; Klemashevich, C.; Staack, D.; Pillai, S.D. PFOA and PFOS breakdown in experimental sand, laboratory-grade after investigation-derived groundwater and wastewater effluent samples at 50 kGy electron beam dose. Radiat. Phys. Chem. 2021, 180, 109323. [Google Scholar] [CrossRef]
- Wang, L.; Batchelor, B.; Pillai, S.D.; Botlaguduru, V.S.V. Electron beam treatment for potable water reuse: Removal of bromate and perfluorooctanoic acid. Chem. Eng. J. 2016, 302, 58–68. [Google Scholar] [CrossRef]
- Ma, S.H.; Wu, M.H.; Tang, L.; Sun, R.; Zang, C.; Xiang, J.J.; Yang, X.X.; Li, X.; Xu, G. EB degradation of perfluorooctanoic acid and perfluorooctane sulfonate in aqueous solution. Nucl. Sci. Technol. 2017, 28, 137. [Google Scholar] [CrossRef]
- Cimino-Reale, G.; Ferrario, D.; Casati, B.; Brustio, R.; Diodovich, C.; Collotta, A.; Vahter, M.; Gribaldo, L. Combined in utero and juvenile exposure of mice to arsenate and atrazine in drinking water modulates gene expression and clonogenicity of myeloid progenitors. Toxicol. Lett. 2008, 180, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Farombi, E.O.; Pant, A.B. The reproductive toxicity of atrazine, an endocrine disruptor. Toxicol. Lett. 2013, 221, 215. [Google Scholar] [CrossRef]
- Kucka, M.; Pogrmic-Majkic, K.; Fa, S.; Stojilkovic, S.S.; Kovacevic, R. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4M. Toxicol. Appl. Pharm. 2012, 265, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Basfar, A.A.; Mohamed, K.A.; Al-Abduly, A.J.; Al-Shahrani, A.A. Radiolytic degradation of atrazine aqueous solution containing humic substances. Ecotoxicol. Environ. Saf. 2009, 72, 948–953. [Google Scholar] [CrossRef]
- Khan, J.A.; Shah, N.S.; Khan, H.N. Decomposition of atrazine by ionizing radiation: Kinetics, degradation, pathways and influence of radical scavengers. Sep. Purif. Technol. 2015, 156, 140–147. [Google Scholar] [CrossRef]
- Xu, G.; Yao, J.Z.; Tang, L.; Yang, X.Y.; Zheng, M.; Wang, H.; Wu, M.H. Electron beam induced degradation of atrazine in aqueous solution. Chem. Eng. J. 2015, 245, 374–380. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, M.; Lin, K.; Sun, W.; Xiong, B.; Guo, M.; Cui, X.; Fu, R. Eco-toxicological effect of carbamazepine on Scenedesmus obliquus and Chlorell pyrenoidosa. Environ. Toxicol. Pharmacol. 2012, 33, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Keen, O.S.; Baik, S.; Linden, K.G.; Aga, D.S.; Love, N.G. Enhanced biodegradation of carbamazepine after UV/H2O2 advanced oxidation. Environ. Sci. Technol. 2012, 46, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xu, G.; Pei, J.; He, X.; Xu, P.; Liu, N.; Wu, M. EB-radiolysis of carbamazepine: In pure-water with different ions and in surface water. J. Radioanal. Nucl. Chem. 2014, 302, 139–147. [Google Scholar] [CrossRef]
- Zheng, M.; Bao, Y.; Huang, Z.; Qiu, W.; Xu, G.; Wang, Z. Radiolysis of carbamazepine by electron beam: Roles of transient reactive species and biotoxicity of final reaction solutions on rotifer Philodina sp. Sci. Total Environ. 2020, 703, 135013. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, G.; Zhao, I.; Pei, J.C.; Wu, M.H. Comparison of EB-radiolysis and UV/H2O2-degradation of CBZ in pure water and solutions. Nucl. Sci. Tech. 2015, 26, 20302. [Google Scholar]
- Bojanowska-Czajka, A.; Kciuk, G.; Gumiela, M.; Borowiecka, S.; Nałęcz-Jawecki, G.; Koc, A.; Garcia-Reyes, J.F.; Solpan-Ozbay, D.; Trojanowicz, M. Analytical, toxicological and kinetic investigation of decomposition of the drug diclofenac in waters and wastes using gamma radiation. Environ. Sci. Pollut. Res. 2015, 22, 20255–20270. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, J. Oxidative removal of carbamazepine by peroxymonosulfate (PMS)combined to ionizing radiation: Degradation, mineralization and biological toxicity. Sci. Total Environ. 2019, 658, 1367–1374. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Wang, J.; Zhang, Y. Degradation of carbamazepine by combined radiation and persulfate oxidation process. Radiat. Phys. Chem. 2020, 170, 108639. [Google Scholar] [CrossRef]
- Menningen, J.A.; Zamora, J.M.; Chang, J.P.; Trudeau, V.L. Endocrine disrupting effects of waterborne fluoxetine exposure on the reproductive axis of female goldfish Carassius auratus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 202, 70–78. [Google Scholar] [CrossRef]
- Silva, V.H.O.; dos Santos Batista, A.P.; Silva, A.C.; Teixeira, C.; Borrely, S.I. Degradation and acute toxicity removal of the antidepressant Fluoxetine (Prozac®) in aqueous systems by electron beam irradiation. Environ. Sci. Pollut. Res. 2016, 23, 11927–11936. [Google Scholar] [CrossRef] [PubMed]
- Jl, T.Y.; Liu, Y.C.; Zhao, J.F.; Xu, G.; Wang, W.F.; Wu, M.H. Radical-Induced Degradation of Fluoxetine in Aqueous Solution by Pulse and Steady-State Radiolysis Studies. Acta Phys. Chim. Sin. 2017, 33, 823–828. [Google Scholar]
- Shao, H.Y.; Wu, M.H.; Deng, F.; Xu, G.; Liu, N.; Li, X.; Tang, L. Electron beam irradiation induced degradation of antidepressant drug fluoxetine in water matrices. Chemosphere 2018, 190, 184–190. [Google Scholar] [CrossRef]
- Boiani, N.F.; Silva, V.H.O.; Garcia, V.S.G.; Del-Sole, S.V.; Borrely, S.I. Electron beam irradiation of pharmaceuticals aiming at toxicity reduction: A binary mixture of fluoxetine and propranolol. Ecotoxicol. Environ. Contam. 2019, 14, 53–58. [Google Scholar]
- Acuna, V.; Ginebreda, A.; Mor, J.R.; Petrovic, M.; Sabater, S.; Sumpter, J.; Barcelo, D. Balancing the health benefits and environmental risks of pharmaceuticals: Diclofenac as an example. Environ. Int. 2015, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Bojanowska-Czajka, A.; Trojanowicz, M. Comparison of different advanced degradation processes for the removal of the pharmaceutical compounds diclofenac and carbamazepine from liquid solutions. Environ. Sci. Pollut. Res. 2018, 28, 27704–27723. [Google Scholar] [CrossRef]
- O’Connor, N.; Dargan, P.I.; Jones, A.L. Hepatocellular damage from non-steroidal anti-inflammatory drugs. Q. J. Med. 2003, 96, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Watson, R.T.; Virani, M.Z.; Oaks, J.L.; Ahmed, A.; Chaudhry, M.J.I.; Arshad, M.; Mahmood, S.; Ali, A.; Khan, A.A. Rapid population declines and mortality clusters in three Oriental white-backed vulture Gyps bengalensis colonies in Pakistan due to diclofenac poisoning. Oryx 2006, 40, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Vergili, I.; Kaya, Y.; Gönder, Z.B.; Boergers, A.; Tuerk, J. Occurrence and Prioritization of Pharmaceutical Active Compounds in Domestic/Municipal Wastewater Treatment Plants. Bull. Environ. Contam. Toxicol. 2019, 102, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Homlok, R.; Takacs, E.; Wojnarovits, L. Elimination of diclofenac from water using irradiation technology. Chemosphere 2011, 85, 603–608. [Google Scholar] [CrossRef]
- Alkhuraiji, T.S. Advanced oxidation process based on water radiolysis to degrade and mineralize diclofenac in aqueous solution. Sci. Total Environ. 2019, 688, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Sayed, M.; Kgan, F.U.; Khan, H.M.; Iqbal, M.; Khan, R.A.; Anas, M. Gamma-irradiation induced degradation of diclofenac in aqueous solution: Kinetics, role of reactive species and influence of natural water parameters. J. Environ. Chem. Eng. 2016, 4, 2573–2584. [Google Scholar] [CrossRef]
- Kimura, A.; Osawa, A.M.; Taguchi, M. Decomposition of persistent pharmaceuticals in waste water by ionizing radiation. Radiat. Phys. Chem. 2012, 81, 1508–1512. [Google Scholar] [CrossRef]
- Yu, H.; Nie, E.; Xu, J.; Yan, S.; Cooper, W.J.; Song, W. Degradation of diclofenac by advanced oxidation and reduction process: Kinetic studies, degradation pathways and toxicity assessments. Water Res. 2013, 47, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Luo, X.; Zheng, Z.; Zheng, B.; Zhang, J.; Zhao, Y.; Yang, X.; Wang, J.; Wang, L. Factors that have an effect on degradation of diclofenac in aqueous solution by gamma ray irradiation. Environ. Sci. Pollut. Res. 2011, 18, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, F.K.; dos Santos Batista, A.P.; Costa Teixeira, A.C.S.; Borrely, S.I. Degradation of diclofenac by electron beam irradiation: Toxicity removal by products identification and effect of another pharmaceutical compound. J. Environ. Chem. Eng. 2018, 6, 4605–4611. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Ye, L.; Zhang, Y.; Yu, J. Removal of diclofenac from surface water by electron beam irradiation combined with a biological aerated filter. Radiat. Phys. Chem. 2014, 105, 104–108. [Google Scholar] [CrossRef]
- Becerril, J.J. Diclofenac Degradation by Radiocatalysis. J. Water Chem. Technol. 2021, 42, 243–248. [Google Scholar] [CrossRef]
- Bezsenyia, A.; Ságic, G.; Makó, M.; Palkó, G.; Tóth, T.; Wojnárovits, L.; Takács, E. The effect of combined cometabolism and gamma irradiation treatment on the biodegradability of diclofenac and sulfamethoxazole. Radiat. Phys. Chem. 2020, 170, 108642. [Google Scholar] [CrossRef]
- Zheng, B.G.; Zheng, Z.; Zhang, J.B.; Luo, X.Z.; Wang, J.Q.; Liu, Q.; Wang, L.H. Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination 2011, 276, 379–385. [Google Scholar] [CrossRef]
- Illes, E.; Takacs, E.; Dombi, A.; Gajda-Schrantz, K.; Racz, G.; Gonter, K.; Wojnarovits, L. Hydroxyl radical induced degradation of ibuprofen. Sci. Total Environ. 2013, 447, 286–292. [Google Scholar] [CrossRef]
- Guin, J.P.; Naik, D.B.; Bhardwaj, Y.K.; Varshney, L. Studies on oxidative radiolysis of ibuprofen in presence of potassium persulfate. Radiat. Phys. Chem. 2014, 100, 38–44. [Google Scholar]
- Deng, W.; Li, N.; Zheng, H.; Lin, H. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol. Environ. Saf. 2016, 125, 121–127. [Google Scholar] [CrossRef]
- Tong, L.; Huang, S.; Wang, Y.; Liu, H.; Li, M. Occurrence of antibiotics in the aquatic environment of Jianghan Plain, central China. Sci. Total Environ. 2014, 497, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Chen, B.; Bai, R.; Song, P.; Lin, H. Contamination of sulfonamide antibiotics and sulfamethazine-resistant bacteria in the downstream and estuarine areas of Jiulong River in Southeast China. Environ. Sci. Pollut. Res. 2015, 22, 12104–12113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Tang, J.; Li, J.; Zheng, Q.; Liu, D.; Chen, Y.; Zou, Y.; Chen, X.; Luo, C.; Zhang, G. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China: Occurrence, distribution and ecological risks. Environ. Pollut. 2013, 174, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Adamek, E.; Sobczak, A.; Makowski, A. Photocatalytic degradation of sulfa drugs with TiO2, Fe salts and TiO2/FeCl3 in aquatic environment-Kinetics and degradation pathway. Appl. Catal. B 2009, 90, 516–525. [Google Scholar] [CrossRef]
- Kim, K.S.; Kam, S.K.; Sun, M.Y. Elucidation of the degradation pathways of sulfonamide antibiotics in a dielectric barrier discharge plasma system. Chem. Eng. J. 2015, 271, 31–42. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Enhanced mineralization of sulfamethoxazole by gamma radiation in the presence of Fe3O4 as Fenton catalyst. Environ. Sci. Pollut. Res. 2019, 26, 27712–27725. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.L. Removal of sulfonamide antibiotics from wastewater by gamma irradiation in presence of iron ions. Nucl. Sci. Tech. 2016, 27, 104. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Li, Z.; Shao, H.; Wu, M.; Lei, J. Radiolytic decomposition of sulfonamide antibiotics: Implications to the kinetics, mechanisms and toxicity. Sep. Purif. Technol. 2018, 202, 259–265. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Yu, S.; Lee, M.J.; Kim, T.H.; Kim, S.D. Radiolysis of selected antibiotics and their toxic effects on various aquatic organisms. Radiat. Phys. Chem. 2009, 78, 267–272. [Google Scholar] [CrossRef]
- Lopez-Penalver, J.J.; Gomez-Pacheco, C.V.; Sanchez Polo, M.; Rivera Utrilla, J. Degradation of tetracycline in different water matrices by advanced oxidation/reduction processes based on gamma radiation. J. Chem. Technol. Biotechnol. 2013, 88, 1096–1108. [Google Scholar] [CrossRef]
- Changotra, R.; Duin, J.P.; Khader, A.A.; Dhir, A. Radiolytic degradation of ornidazole in aqueous solution by electron beam irradiation: Implications to parameters, kinetics, toxicity and cost evaluation. J. Environ. Chem. Eng. 2020, 8, 104423. [Google Scholar] [CrossRef]
- Siwek, M.; Edgock, T. Application of electron beam water radiolysis for sewage sludge treatment-a review. Environ. Sci. Pollut. Res. 2020, 27, 42424–42448. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bobrowski, K.; Szreder, T.; Bojanowska-Czajka, A. Gamma-ray, X-ray and electron beam based processes. In Advanced Oxidation Processes for Wastewater Treatment; Academic Press: Cambridge, MA, USA, 2018; pp. 257–331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojanowska-Czajka, A. Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Appl. Sci. 2021, 11, 12032. https://doi.org/10.3390/app112412032
Bojanowska-Czajka A. Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Applied Sciences. 2021; 11(24):12032. https://doi.org/10.3390/app112412032
Chicago/Turabian StyleBojanowska-Czajka, Anna. 2021. "Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review" Applied Sciences 11, no. 24: 12032. https://doi.org/10.3390/app112412032
APA StyleBojanowska-Czajka, A. (2021). Application of Radiation Technology in Removing Endocrine Micropollutants from Waters and Wastewaters—A Review. Applied Sciences, 11(24), 12032. https://doi.org/10.3390/app112412032





