Anticancer Effects of Seaweed-Derived Bioactive Compounds
Abstract
:1. Introduction
2. Categories of Bio-Compounds Isolated from Seaweeds with Anticancer Activity
3. Seaweed Polysaccharide
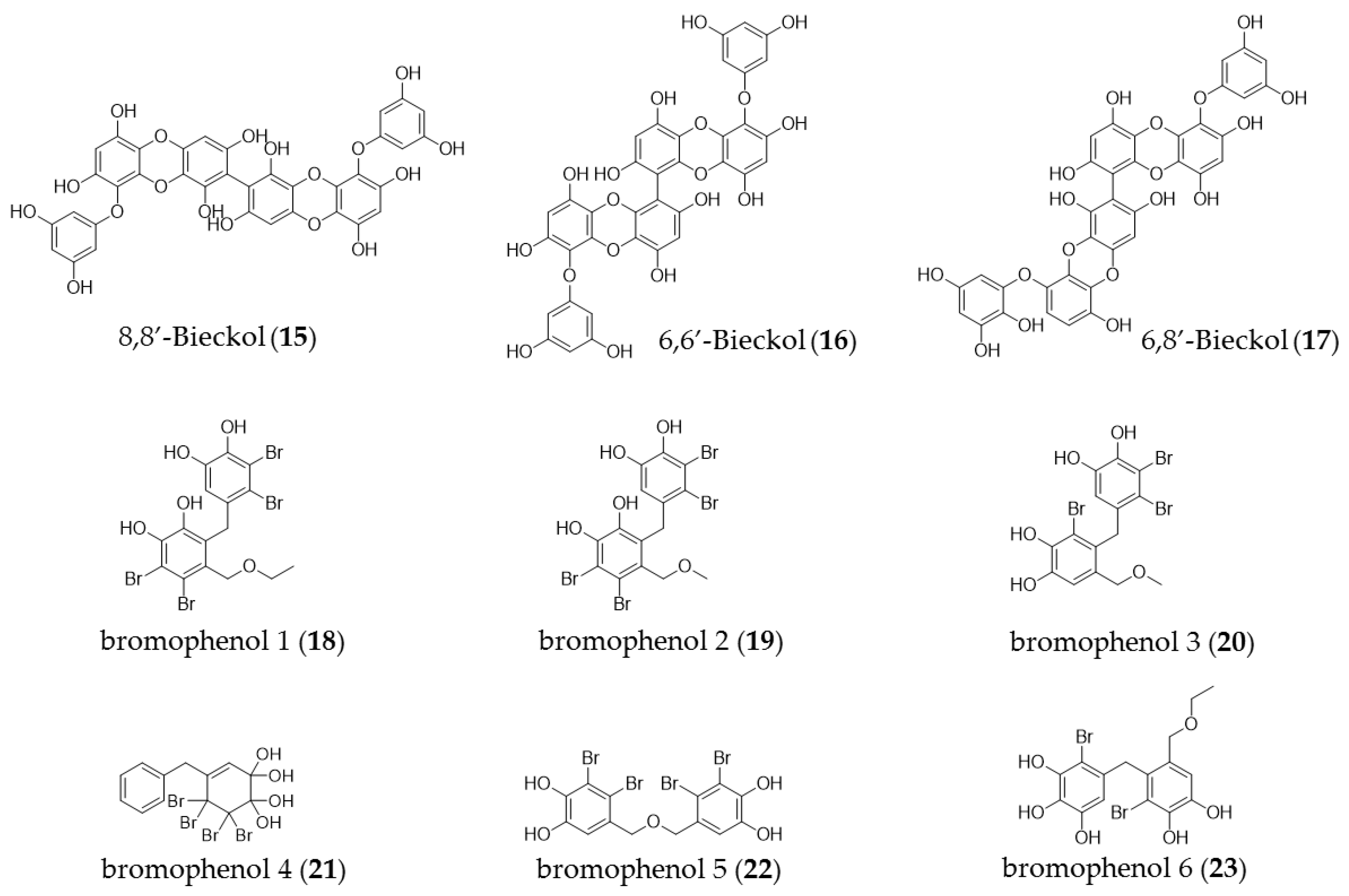
4. Phenolic Compounds
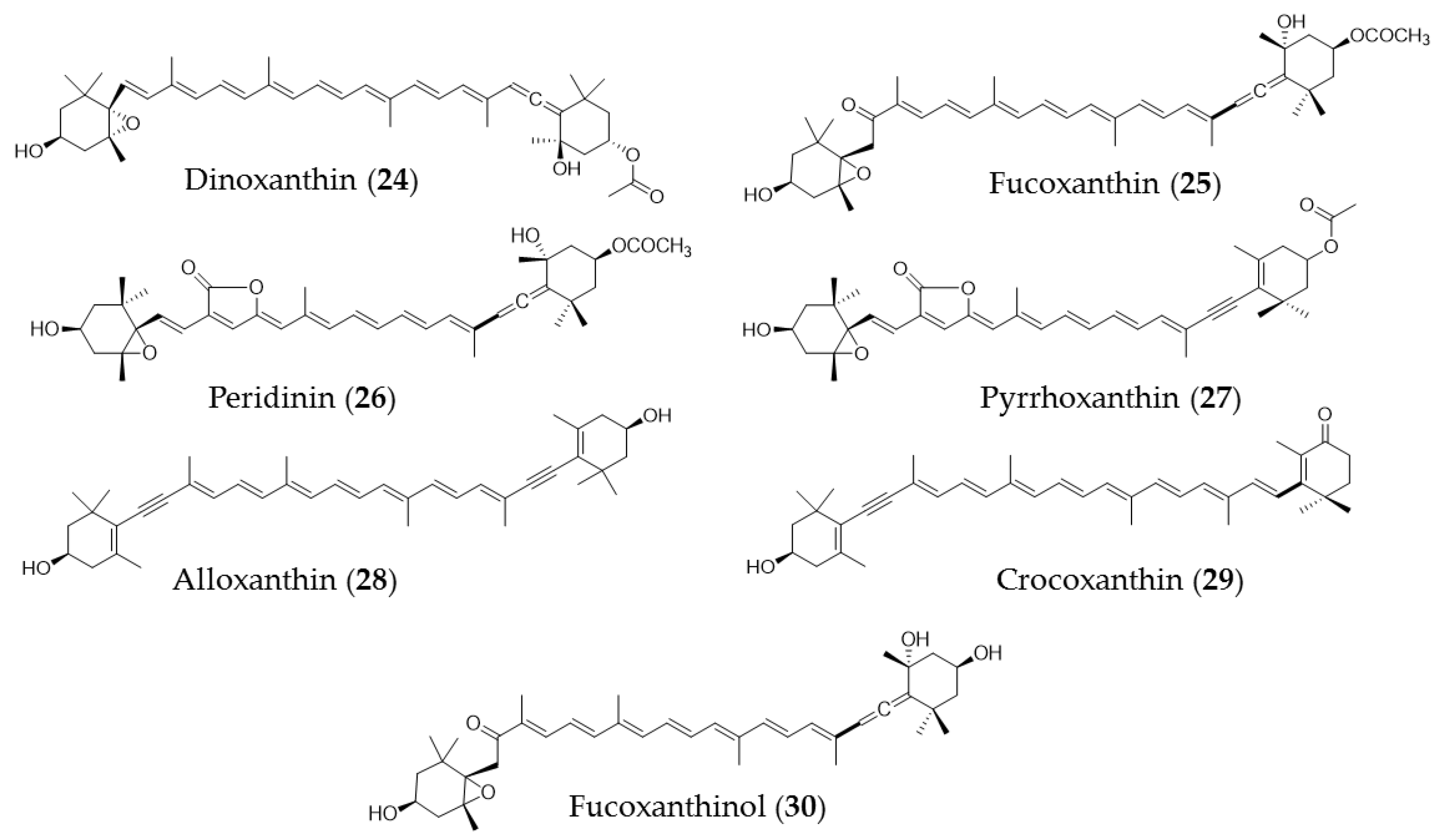
5. Carotenoids
6. Mono-, Sesqui-, and Diterpenes
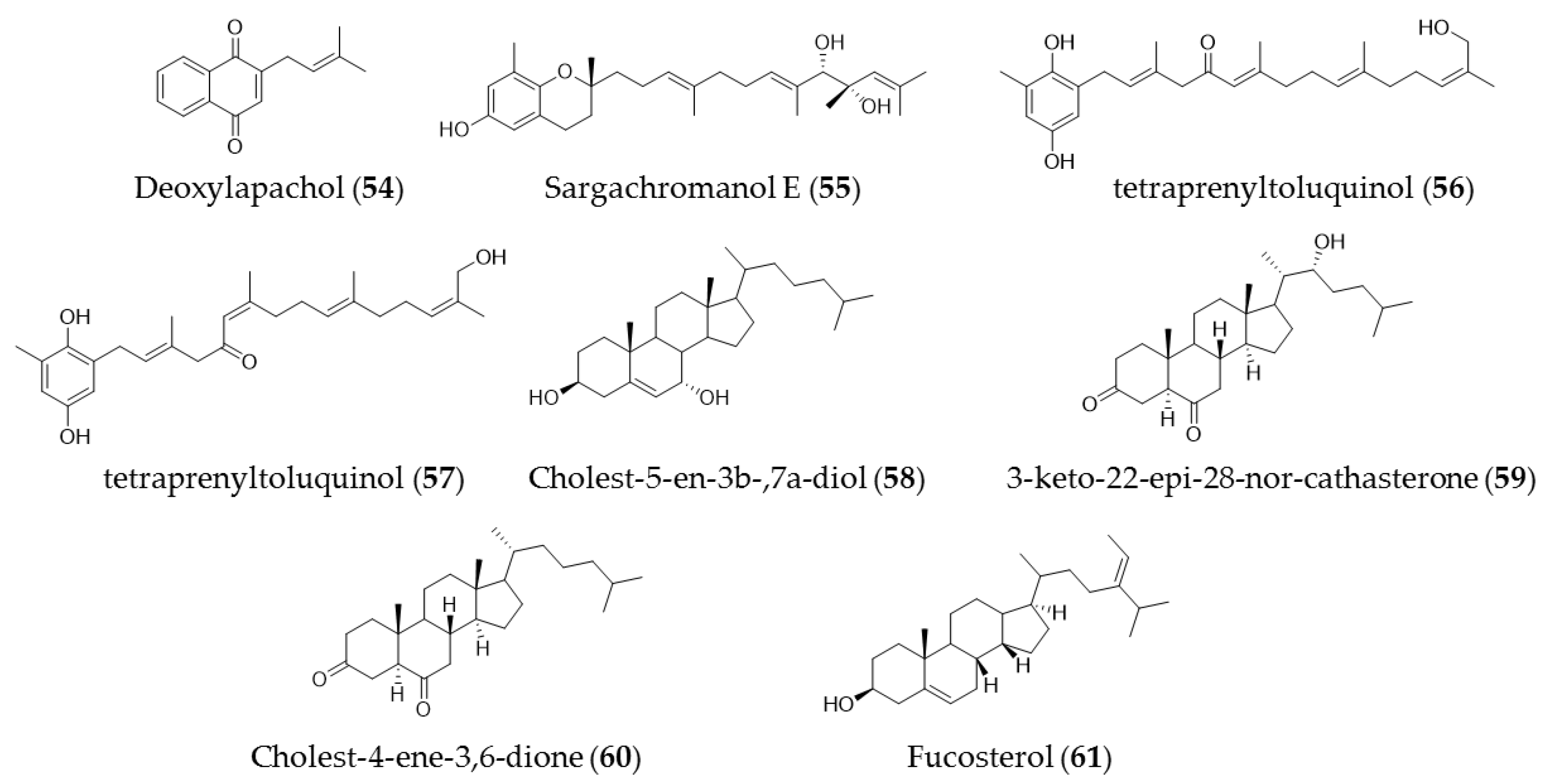
7. Other Compounds (Quinones and Sterols)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinberg, R. The Biology of Cancer, Garland Science, 2nd ed.; W.W.Norton & Company: New York, NY, USA, 2013. [Google Scholar]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef]
- American Cancer Society. What Is Cancer? American Cancer Society: Atlanta, GA, USA, 2020; Available online: https://www.cancer.org/cancer/cancer-basics/what-is-cancer.html (accessed on 30 October 2021).
- Chakravarthi, B.V.S.K.; Nepal, S.; Varambally, S. Genomic and epigenomic alterations in cancer. Am. J. Pathol. 2016, 186, 1724–1735. [Google Scholar] [CrossRef] [Green Version]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 30 October 2021).
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 2018, 173, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussavou, G.; Kwak, D.H.; Obiang-Obonou, B.W.; Maranguy, C.A.O.; Dinzouna-Boutamba, S.-D.; Lee, D.H.; Pissibanganga, O.G.M.; Ko, K.; Seo, J.I.; Choo, Y.K. Anticancer effects of different seaweeds on human colon and breast cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific t cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Hussain, E.; Wang, L.J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592. [Google Scholar] [CrossRef]
- Gutierrez-Rodriguez, A.G.; Juarez-Portilla, C.; Olivares-Banuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.; Bouvier, T.; Weinbauer, M.G.; Thingstad, T.F. Trade-offs between competition and defense specialists among unicellular planktonic organisms: The “killing tShe winner” hypothesis revisited. Microbiol. Mol. Biol. Rev. 2010, 74, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [Green Version]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement. Altern. Med. 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funaki, M.M.; Nishizawa, M.; Sawaya, V.; Inoue, S.; Yamagishi, V. Mineral composition in the holdfast of three brown algae of the genus Laminaria. Fish. Sci. 2001, 67, 295. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Ahmed, E.F.; Abo-Zeid, M.A. In vitro cancer chemopreventive properties of polysaccharide extract from the brown alga, Sargassum latifolium. Food Chem. Toxicol. 2009, 47, 1378. [Google Scholar]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428. [Google Scholar] [CrossRef]
- Magalhaes, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Camara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352–3365. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, R.; Ermakova, S.; Awada, S.; Zvyagintseva, T.; Kanaan, H. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Compd. 2011, 47, 329. [Google Scholar] [CrossRef]
- Ye, J.; Li, Y.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested fucoidan extracts derived from seaweed mozuku of cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology 2005, 47, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermakova, S.; Sokolova, R.; Kim, S.-M.; Um, B.-H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, Y.S.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.-Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2010, 16, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imbs, T.I.; Ermakova, S.P.; Malyarenko, O.S.; Isakov, V.V.; Zvyagintseva, T.N. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticanceractivity. Carbohydr. Polym. 2016, 135, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-K.; Kim, I.-H.; Kim, J.; Nam, T.-J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 291. [Google Scholar] [CrossRef] [Green Version]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Maria Rizzo, A. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 348. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zuyagintseva, T.N.; Shchelkanov, M.Y. Antiviral effects of polyphenols from marine algae. Biomedicine 2021, 9, 200. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res 2005, 43, 1–91. [Google Scholar]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.C.; Hunter, M.D.; Appel, H.M. Antimicrobial activity of polyphenols mediates plant-herbivore interactions. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 621–637. [Google Scholar]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus Vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T. Variation in natural selection for growth and phlorotannins in the brown alga Fucus vesiculosus. J. Evol. Biol. 2004, 17, 807–820. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from Brown Algae (Fucus vesiculosus) Inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Arg. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef]
- Xu, J.W.; Yan, Y.; Wang, L.; Wu, D.; Ye, N.K.; Chen, S.H.; Li, F. Marine bioactive compound dieckol induces apoptosis and inhibits the growth of human pancreatic cancer cells PANC-1. J. Biochem. Mol. Toxicol. 2021, 35, 22648. [Google Scholar] [CrossRef]
- Wang, C.-H.; Li, X.-F.; Jin, L.-F.; Zhao, Y.; Zhu, G.-J.; Shen, W.-Z. Dieckol inhibits non-small-cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22346. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.-H.; Yang, Y.-I.; Lee, K.-T.; Choi, J.-H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zeng, M.; Dong, S.; Liu, Z.; Li, R. Anti-proliferative activity of phlorotannin extracts from brown algae Laminaria japonica Aresch. Chin. J. Oceanol. Limnol. 2010, 28, 122–130. [Google Scholar] [CrossRef]
- Kang, M.-H.; Kim, I.-H.; Nam, T.-J. Phloroglucinol induces apoptosis via apoptotic signaling pathways in HT-29 colon cancer cells. Oncol. Rep. 2014, 32, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.-K.; Uddin, N.; Hyun, J.-W.; Kim, C.; Suh, Y.; Lee, S.-J. Novel anticancer activity of phloroglucinol against breast cancer stem-like cells. Toxicol. Appl. Pharmacol. 2015, 286, 143–150. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Li, J.; Guo, S.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Oceanol. Limn. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melendez-Martınez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid. Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid. Res. 2007, 46, 328–375. [Google Scholar] [CrossRef]
- Takaichi, S.; Mimuro, M. Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9′-cis, a sole molecular form. Plant Cell Physiol. 1998, 39, 968–977. [Google Scholar] [CrossRef]
- Yoshii, Y.; Takaichi, S.; Maoka, T.; Suda, S.; Sekiguchi, H.; Nakayama, T.; Inouye, I. Variation of siphonaxanthin series among the genus Nephroselmis (Prasinophyceae, Chlorophyta), including a novel primary methoxy carotenoid. J. Phycol. 2005, 41, 827–834. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M. Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Nippon Medical School, Kawasaki, Japan. Unpublished works. 2011.
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Choi, H.-J.; Lee, J.-Y.; Jeong, H.-S.; Kim, C.-H.; Joo, M.; Choi, J.-Y.; Han, C.-W.; Kim, S.-Y.; Choi, J.-S.; et al. Marine algal fucoxanthin inhibits the metastatic potential of cancer cells. Biochem. Biophys. Res. Commun. 2013, 439, 580–585. [Google Scholar] [CrossRef]
- Kim, K.-N.; Ahn, G.; Heo, S.-J.; Kang, S.-M.; Kang, M.-C.; Yang, H.-M.; Kim, D.; Roh, S.W.; Kim, S.-K.; Jeon, B.-T.; et al. Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef]
- Li, Y.-X.; Himaya, S.W.A.; Kim, S.-K. Triterpenoids of marine origin as anti-cancer agents. Molecules 2013, 18, 7886–7909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Rabi, T.; Bishayee, A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res. Treat. 2009, 115, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.L.M.; Seca, A.M.L.; Barreto, M.C.; Neto, A.I.; Kijjoa, A.; Silva, A.M.S. Cytotoxic meroterpenoids from the macroalga Cystoseira abies-marina. Phytochem. Lett. 2013, 6, 593–597. [Google Scholar] [CrossRef]
- Bouaicha, N.; Tringli, C.; Pesando, D.; Mallea, M.; Roussaki, C.; Verbist, J.F. Bioactive diterpenoids isolated from Dipophus ligulatus. Planta Med. 1993, 59, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Sabry, O.M.M.; Andrews, S.; McPhail, K.L.; Goeger, D.E.; Yokochi, A.; LePage, K.T.; Murray, T.F.; Gerwicj, W.H. Neurotoxic meroditerpenoids from the tropical marine brown algae Stypopodium flabelliforme. J. Nat. Prod. 2005, 68, 1022–1030. [Google Scholar] [CrossRef]
- Abatis, D.; Vagias, C.; Galanakis, D.; Norris, J.N.; Moreau, D.; Roussakis, C.; Roussis, V. Atomarianones A and B: Two cytotoxic meroditerpenes from the brown alga Taonia atomaria. Tetrahedron Lett. 2005, 46, 8525–8529. [Google Scholar] [CrossRef]
- De Oliveira, L.S.; Tschoeke, D.A.; de Oliveira, A.S.; Hill, L.J.; Paradas, W.C.; Salgado, L.T.; Thompson, C.C.; Pereira, R.C.; Thompson, F.L. New Insights on the terpenome of the red seaweed Laurencia dendroidea (Florideophyceae, Rhodophyta). Mar. Drugs 2015, 13, 879–902. [Google Scholar] [CrossRef] [Green Version]
- Irie, T.; Suzuki, M.; Masamune, T. Laurencin, a constituent from Laurencia species. Tetrahedron Lett. 1965, 6, 1091–1099. [Google Scholar] [CrossRef]
- Sun, J.; Shi, D.-Y.; Li, S.; Wang, S.-J.; Han, L.-J.; Fan, X.; Yang, Y.-C.; Shi, J.-G. Chemical constituents of the red alga Laurencia tristicha. J. Asian Nat. Prod. Res. 2007, 9, 725–734. [Google Scholar] [CrossRef]
- Zaleta-Pinet, D.A.; Holland, I.P.; Muñoz-Ochoa, M.; Murillo-Alvarez, J.I.; Sakoff, J.A.; van Altena, I.A.; McCluskey, A. Cytotoxic compounds from Laurencia pacifica, Org. Med. Chem. Lett. 2014, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Antunes, E.M.; Afolayan, A.F.; Chiwakata, M.T.; Fakee, J.; Beukes, D.R. Identification and in vitro anti-esophageal cancer activity of a series of halogenated monoterpenes isolated from the South African seaweeds Plocamium suhrii and Plocamium cornutum. Phytochemistry 2011, 72, 769–772. [Google Scholar] [CrossRef]
- Depix, M.S.; Martínez, J.; Santibañez, F.; Rovirosa, J.; San Martín, A.; Maccioni, R.B. The compound 14-keto-stypodiol diacetate from the algae Stypopodium flabelliforme inhibits microtubules and cell proliferation in DU-145 human prostatic cells. Mol. Cell. Biochem. 1998, 187, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G. A cytotoxic and antifungal 1,4-naphthoquinone and related compounds from a New Zealand brown algae, Landsburgia quercifolia. J. Nat. Prod. 1991, 54, 978–985. [Google Scholar] [CrossRef]
- Sansom, C.E.; Larsen, L.; Perry, N.B.; Berridge, M.V.; Chia, E.W.; Harper, J.L.; Webb, V.L. An antiproliferative bis-prenylated quinone from the new zealand brown alga Perithalia capillaris. J. Nat. Prod. 2007, 70, 2042–2044. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Kim, K.-N.; Yoon, W.-J.; Oh, C.; Choi, Y.-U.; Affan, A.; Lee, Y.-J.; Lee, H.-S.; Kang, D.-H. Chromene induces apoptosis via caspase-3 activation in human leukemia HL-60 cells. Food Chem. Toxicol. 2011, 49, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.M.; Böhm, V.; Wright, A.D.; König, G.M. Antioxidative meroterpenoids from the brown alga Cystoseira crinita. J. Nat. Prod. 2003, 66, 968. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Kornienko, A.; Koutsaviti, A.; Roussis, V.; Newman, D. Algae metabolites: From in vitro growth inhibitory effects to promising anticancer activity. Nat. Prod. Rep. 2019, 36, 810–841. [Google Scholar] [CrossRef]
- Hamdy, A.H.A.; Aboutabl, E.A.; Sameer, S.; Hussein, A.A.; Díaz-Marrero, A.R.; Darias, J.; Cueto, M. 3-Keto-22-epi-28-nor-cathasterone, a brassinosteroid-related metabolite from Cystoseira myrica. Steroids 2009, 74, 927–930. [Google Scholar] [CrossRef]
- Sheu, J.H.; Wang, G.H.; Sung, P.J.; Chiu, Y.H.; Duh, C.Y. Cytotoxic sterols from the formosan brown alga Turbinaria ornata. Planta Med. 1997, 63, 571–572. [Google Scholar] [CrossRef]
- Khanavi, M.; Gheidarloo, R.; Sadati, N.; Ardekani, M.R.; Nabavi, S.M.; Tavajohi, S.; Ostad, S.N. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn. Mag. 2012, 8, 60–64. [Google Scholar] [PubMed] [Green Version]
- Pacheco, B.S.; Dos Santos, M.A.Z.; Schultze, E.; Martins, R.M.; Lund, R.G.; Seixas, F.K.; Colepicolo, P.; Collares, T.; Paula, F.R.; De Pereira, C.M.P. Cytotoxic Activity of Fatty Acids From Antarctic Macroalgae on the Growth of Human Breast Cancer Cells. Front. Bioeng. Biotechnol. 2018, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.H.; Wang, G.H.; Sung, P.J.; Duh, C.Y. New cytotoxic oxygenated fucosterols from the brown alga Turbinaria conoides. J. Nat. Prod. 1999, 62, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Chen, A.; Li, Y.; Xia, M.; Guo, P.; Yao, S.; Chen, S. Fucosterol exhibits selective antitumor anticancer activity against HeLa human cervical cell line by inducing mitochondrial mediated apoptosis, cell cycle migration inhibition and downregulation of m-TOR/PI3K/Akt signalling pathway. Oncol. Lett. 2018, 15, 3458–3463. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Lee, J.-Y.; Song, G.; Lim, W. Fucosterol suppresses the progression of human ovarian cancer by inducing mitochondrial dysfunction and endoplasmic reticulum stress. Mar. Drugs 2020, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysscharide from brown seaweeds sacchrina japonica and undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Park, H.Y.; Park, S.H.; Jeong, J.W.; Yoon, D.; Han, M.H.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Kim, D.H. Induction of p53-independent apoptosis and G1 cell cycle arrest by fucoidan in HCT116 human carcinoma cells. Mar. Drugs 2017, 15, 154–167. [Google Scholar] [CrossRef] [Green Version]
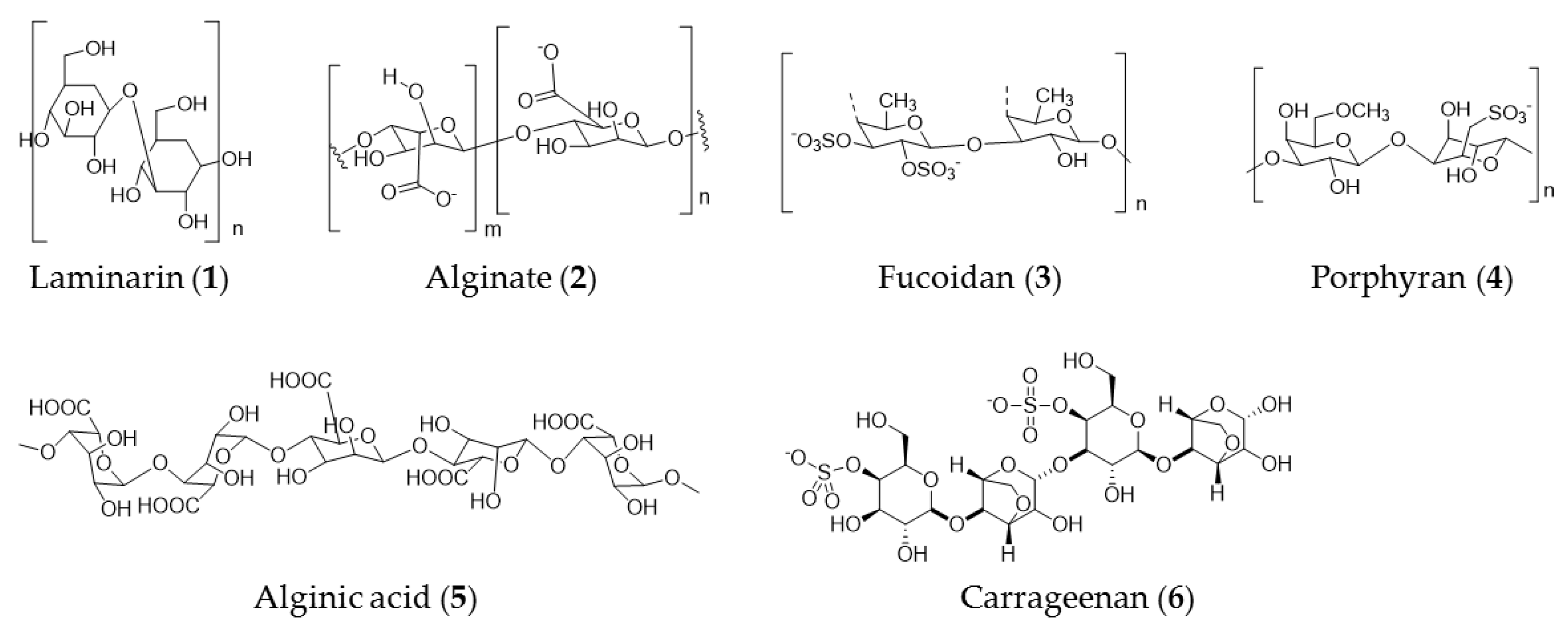

| Seaweed | Active Ingredient(s) | Activity |
|---|---|---|
| Sagassum pallidum | Highly sulfated polysaccharide SP1, SP2, and SP3 | Cytotoxicity against A549, HepG2, and MGC-803 cells |
| Dictyopteris delicatula, Dictyopteris polyodioides | Sulfated polysaccharides | Anticancer activity against HeLa and RPMI-7951 cells |
| Sargassum hornery, Eclonia cava, and Costaria costata | Fucoidan (3) | Inhibiting colony formation of melanoma (SK-MEL-28 cells) and colon cancer cells (DLD-1 cells) |
| Coccophora langsdorfii | Fucoidan (3), laminarin (1), and alginate (2) | Anticancer activity against SK-ML-5 and SK-ML-28 melanoma cells |
| Laminaria difitata | Laminarin (1) | Induction of apoptosis via the ErbB signal pathway in colon cancer cells |
| Ecklonia cava | Dieckol (14) | Inducing apoptosis and inhibited the growth of human pancreatic cancer cells PANC-1, inhibition of non-small-cell lung cancer |
| Laminaria japonica | Phlorotannin-rich fraction | Inhibits BEL-7402 human hepatocellular carcinoma cells (30%) and P388 murine leukemic cells (43%) at 100 μg/mL concentration |
| Ecklonia cava | Phloroglucinol (7) | Induction of apoptosis in HT-29 cells, decreasing CD44 (+) cancer cells and cancer stem cells, inhibition of KRAS and its downstream regulators in breast cancer stem like cells |
| Leathesia nana | Bromo phenol derivatives (18–23) | Cytotoxicity in various human cancer cells, including MCF-7A549, BGC823, HCT-8 cell lines |
| Undaria pinnatifida | Fucoxanthin (25) extracts | Trigger apoptosis in PC-3 cells via caspase-3 |
| Fucus serratus, luminaria digitat, ascophyllm nodsum, pelvetia canaliculata | Astaxanthin, β-carotene, zeaxanthin | Agent for cerebrovascular disease, photoprotection of eye, and anticancer agent |
| Cystoseira abies-marina | Cystoazorones A (30) and B (31) cystoazorols A (32) and B | Anticancer activity against HeLa cells |
| Dilophus ligulatus | Diterpenoids (33–36 and 37) | Cytotoxic activity against murine leukemia cells (p-388) and p-388 doxorubicin-resistant cells with IC50 3.64–13.3 µM and IC50 5.95 to 12.9 µM respectively |
| Stypopodium flabelliforme | Flabellinol (38), flabellinone (39) | Cytotoxicity against NCI-H460 cells (IC50 9 µM and 14 µM, respectively) |
| Taonia atomaria | Atomarianone A (40), atomarianone B (41) | Cytotoxic activity against two lung cancer cell lines (NSCLC-N6 and A549) |
| Plocamium suhrii | Monoterpenes (44–49) | Cytotoxic effects toward esophageal cancer cell lines (WHCO1) with an IC50 value in the range 6.6–15.1 µM |
| Stypopodium flabelliforme | 14-keto-stypodiol diacetate (50) | Cytotoxic effects on the prostate cancer cell line (DU-145) with IC50 of 24 µM |
| Landsburgia quereifolia | Deoxylapachol (51) | Cytotoxicity to P-388 leukemia cells |
| Perithalia capillaris | Bis-prenylated quinone | Antiproliferative effects toward HL-60 cells (IC50 0.34 uM) |
| sargassum siliquastrum | Sargachromanol E (52) | Growth inhibitory effects against HL-60 cells via caspase-3 dependent apoptosis induction |
| Cystoseira crinita | Tetraprenyltoluquinols (53) and (54) | Inhibiting growth of on HMO2, HepG2, and MCF7 cancer cell lines |
| Laurencia tristicha | Cholest-5-en-3b-,7a-diol (55) | Cytotoxic effects against the tested cancer cell lines A549, BGC-823, Bel 7402, HCT-8, and HeLa with IC50 values of 16.8 µM, 5.1 µM, 0.5 µM, 0.5 µM, and 0.3 µM, respectively |
| Cystoseira myrica | 3-Keto-22-epi-28-nor-cathasterone (56), cholest-4-ene-3,6-dione (57) | Antiproliferative effects to HepG2 and HCT116 cancer cells |
| Pelvetica siliquosa, Cystoseira foeniculacea, Sargassum angustifolium | Fucosterol (58) | Cytotoxic effects against various types of cancer cell lines, including HT-29 colon cancer cells, breast cancer, promyelocytic leukemia, lung cancer, and cervical cancer |
| Group | Chemical Name | Structure |
|---|---|---|
| Monomeric unit | Phloroglucinol (7) |  |
| Fucol | Trifucol (8) |  |
| Phlorethol | Tetraphlorethol (9) |  |
| Fucophlorethol | Fucodiphlorethol (10) | 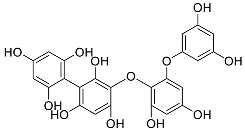 |
| Fuhalol | Pentafuhalol (11) |  |
| Carmalol | Diphlorethohydroxycarmalol (12) | 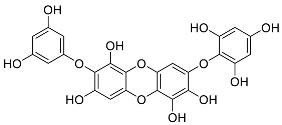 |
| Eckols | Eckol (13) | 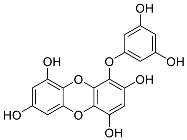 |
| Eckols | Dieckol (14) | 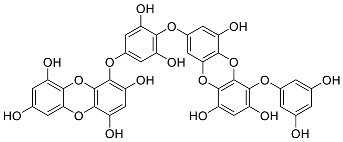 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Selvaraj, B.; Lee, J.W. Anticancer Effects of Seaweed-Derived Bioactive Compounds. Appl. Sci. 2021, 11, 11261. https://doi.org/10.3390/app112311261
Lee H, Selvaraj B, Lee JW. Anticancer Effects of Seaweed-Derived Bioactive Compounds. Applied Sciences. 2021; 11(23):11261. https://doi.org/10.3390/app112311261
Chicago/Turabian StyleLee, Heesu, Baskar Selvaraj, and Jae Wook Lee. 2021. "Anticancer Effects of Seaweed-Derived Bioactive Compounds" Applied Sciences 11, no. 23: 11261. https://doi.org/10.3390/app112311261
APA StyleLee, H., Selvaraj, B., & Lee, J. W. (2021). Anticancer Effects of Seaweed-Derived Bioactive Compounds. Applied Sciences, 11(23), 11261. https://doi.org/10.3390/app112311261






