Physiological Effects of Green-Colored Food-Derived Bioactive Compounds on Cancer
Abstract
:Featured Application
Abstract
1. Introduction
2. Physiological Effects and Mode of Actions (MoA)
2.1. Sulforaphane
2.2. Catechin Derivatives
2.3. Chlorophyll
2.4. Isoflavone
2.5. Indole Derivatives
2.6. Lutein
| NO | Chemical Name | Structure | Source | Cancer Site | Mechanism or Target Marker | Reference |
|---|---|---|---|---|---|---|
| 1 | Sulforaphane | 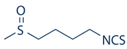 |
|
|
| [22,23,24,26,27] |
| 2 | Catechin derivatives |  |
|
|
| [38,41] |
| 3 | Chlorophyll (Sodium copper salt) |  |
|
|
| [57,59] |
| 4 | Isoflavone (genistein) | 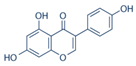 |
|
|
| [74,75,101] |
| 5 | Indole derivatives |  |
|
|
| [82,102] |
| 6 | Lutein |  |
|
|
| [95,98,103] |
| NO | Bioactive Compounds | Green Color Food | Anti-Cancer Type | Reference |
|---|---|---|---|---|
| 1 | Folate |
|
| [104,105] |
| 2 | Riboflavin |
|
| [106] |
| 3 | Retinoic acid |
|
| [107] |
| 4 | Vitamin D3 |
|
| [108] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Medina-Remon, A.; Kirwan, R.; Lamuela-Raventos, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef]
- Marmitt, D.J.; Shahrajabian, M.H.; Goettert, M.I.; Rempel, C. Clinical trials with plants in diabetes mellitus therapy: A systematic review. Expert Rev. Clin. Pharmacol. 2021, 14, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Hackshaw-McGeagh, L.E.; Perry, R.E.; Leach, V.A.; Qandil, S.; Jeffreys, M.; Martin, R.M.; Lane, J.A. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control 2015, 26, 1521–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Mao, Q.; Cao, M.; Xie, L. Cruciferous vegetables intake and risk of prostate cancer: A meta-analysis. Int. J. Urol. 2012, 19, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, S.O.; Pacheco, F.J.; Zapata, G.M.; Garcia, J.M.; Previale, C.A.; Cura, H.E.; Craig, W.J. Food Habits, Lifestyle Factors, and Risk of Prostate Cancer in Central Argentina: A Case Control Study Involving Self-Motivated Health Behavior Modifications after Diagnosis. Nutrients 2016, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Takata, Y.; Xiang, Y.B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. Intakes of fruits, vegetables, and related vitamins and lung cancer risk: Results from the Shanghai Men’s Health Study (2002–2009). Nutr. Cancer 2013, 65, 51–61. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat. Commun. 2020, 11, 1254. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.J.; Lorenzo, T.V. Chlorophylls in foods. Crit. Rev. Food Sci. Nutr. 1990, 29, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Singh, K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis 2012, 33, 1833–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, M.; Singh, N.; Pal, S.; Dev, I.; Ansari, K.M. Chapter Three—Chemopreventive Role of Dietary Phytochemicals in Colorectal Cancer. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 12, pp. 69–121. [Google Scholar]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wade, K.L.; Prestera, T.; Talalay, P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal. Biochem. 1996, 239, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, S.U. Current potential health benefits of sulforaphane. EXCLI J. 2016, 15, 571–577. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral. Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, K.; Davis, M.; Hu, P.; Martin, B.; Baillie, T. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: Identification of phase I metabolites and glutathione conjugates. Chem. Res. Toxicol. 1997, 10, 1228–1233. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Kan, S.-F.; Wang, J.; Sun, G.-X. Sulforaphane regulates apoptosis- and proliferation-related signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 2018, 42, 2447–2458. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhu, J.-Y.; Chen, S.; Qing, Y.; Wu, D.; Lin, Y.-M.; Luo, J.-Z.; Han, W.; Li, Y.-Q. Effects of co-treatment with sulforaphane and autophagy modulators on uridine 5’-diphospho-glucuronosyltransferase 1A isoforms and cytochrome P450 3A4 expression in Caco-2 human colon cancer cells. Oncol. Lett. 2014, 8, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox. Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef] [Green Version]
- Surh, Y.-J. Nrf2 paradox: Can cancer patients eat broccoli? Food Front. 2021, 2, 25–28. [Google Scholar] [CrossRef]
- Kensler, T.W.; Ng, D.; Carmella, S.G.; Chen, M.; Jacobson, L.P.; Muñoz, A.; Egner, P.A.; Chen, J.G.; Qian, G.S.; Chen, T.Y.; et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis 2012, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bogaards, J.J.; Verhagen, H.; Willems, M.I.; van Poppel, G.; van Bladeren, P.J. Consumption of Brussels sprouts results in elevated alpha-class glutathione S-transferase levels in human blood plasma. Carcinogenesis 1994, 15, 1073–1075. [Google Scholar] [CrossRef]
- Leone, A.; Diorio, G.; Sexton, W.; Schell, M.; Alexandrow, M.; Fahey, J.W.; Kumar, N.B. Sulforaphane for the chemoprevention of bladder cancer: Molecular mechanism targeted approach. Oncotarget 2017, 8, 35412–35424. [Google Scholar] [CrossRef] [Green Version]
- Atwell, L.L.; Zhang, Z.; Mori, M.; Farris, P.; Vetto, J.T.; Naik, A.M.; Oh, K.Y.; Thuillier, P.; Ho, E.; Shannon, J. Sulforaphane Bioavailability and Chemopreventive Activity in Women Scheduled for Breast Biopsy. Cancer Prev. Res. 2015, 8, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Elkashty, O.A.; Tran, S.D. Broccoli extract increases drug-mediated cytotoxicity towards cancer stem cells of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 123, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.S.; Jha, A.K.; Kumari, R.; Rawat, K.; Syeda, S.; Shrivastava, A. Chapter 9—Role of Catechins in Chemosensitization. In Role of Nutraceuticals in Cancer Chemosensitization; Bharti, A.C., Aggarwal, B.B., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 169–198. [Google Scholar]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Aboulaila, M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of (-)-epigallocatechin-3-gallate from green tea on the growth of Babesia parasites. Parasitology 2010, 137, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pai, R. Enhanced Oral Bioavailability of (+)-Catechin by a Self Double-Emulsifying Drug Delivery System (SDEDDS): A New Platform for Oral Delivery of Biopharmaceutics Classification System Class III Drugs. Nanomed. Nanobiol. 2014, 1, 51–56. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H.; Chen, J.X.; Zhang, J. Effects of Tea Catechins on Cancer Signaling Pathways. Enzymes 2014, 36, 195–221. [Google Scholar] [CrossRef] [Green Version]
- Shirakami, Y.; Shimizu, M. Possible Mechanisms of Green Tea and Its Constituents against Cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Fukutomi, Y.; Ninomiya, M.; Nagura, K.; Kato, T.; Araki, H.; Suganuma, M.; Fujiki, H.; Moriwaki, H. Green tea extracts for the prevention of metachronous colorectal adenomas: A pilot study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3020–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; Leis, J.F.; LaPlant, B.; Bowen, D.A.; Roos, M.; Laumann, K.; Ghosh, A.K.; Lesnick, C.; et al. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer 2013, 119, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y. Novel uses of catechins in foods. Trends Food Sci. Technol. 2006, 17, 64–71. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Zheng, H.; Hu, X.; Liao, X.; Zhang, Y. Transformation pathways and metabolic activity of free chlorophyll compounds from chloroplast thylakoid membrane under in vitro gastrointestinal digestion and colonic fermentation in early life. Food Biosci. 2021, 42, 101196. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, C.; Gökmen, V. Chlorophyll. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 37–41. [Google Scholar] [CrossRef]
- Morançais, M.; Mouget, J.-L.; Dumay, J. Chapter 7—Proteins and Pigments. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–175. [Google Scholar] [CrossRef]
- Zuo, Z.; Wu, S.; Qi, X.; Dong, R. Performance enhancement of leaf vegetable waste in two-stage anaerobic systems under high organic loading rate: Role of recirculation and hydraulic retention time. Appl. Energy 2015, 147, 279–286. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Figge, F.H.; Weiland, G.S.; Manganiello, L.O. Cancer detection and therapy; affinity of neoplastic, embryonic, and traumatized tissues for porphyrins and metalloporphyrins. Proc. Soc. Exp. Biol. Med. 1948, 68, 640. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R. Chlorophylls as anticarcinogens (review). Int. J. Oncol. 1997, 10, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S.; Palitti, F.; Natarajan, A.T. Chemopreventive potential of chlorophyllin: A review of the mechanisms of action and molecular targets. Nutr. Cancer 2015, 67, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Li, Y.-F.; Chen, B.-H. Preparation of Chlorophyll Nanoemulsion from Pomelo Leaves and Its Inhibition Effect on Melanoma Cells A375. Plants 2021, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Muñoz, A.; Kensler, T.W. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat. Res. 2003, 523-524, 209–216. [Google Scholar] [CrossRef]
- Vaňková, K.; Marková, I.; Jašprová, J.; Dvořák, A.; Subhanová, I.; Zelenka, J.; Novosádová, I.; Rasl, J.; Vomastek, T.; Sobotka, R.; et al. Chlorophyll-Mediated Changes in the Redox Status of Pancreatic Cancer Cells Are Associated with Its Anticancer Effects. Oxid. Med. Cell Longev. 2018, 2018, 4069167. [Google Scholar] [CrossRef] [Green Version]
- TMcQuistan, T.J.; Simonich, M.T.; Pratt, M.M.; Pereira, C.B.; Hendricks, J.D.; Dashwood, R.H.; Williams, D.E.; Bailey, G.S. Cancer chemoprevention by dietary chlorophylls: A 12,000-animal dose-dose matrix biomarker and tumor study. Food Chem. Toxicol. 2012, 50, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their Derivatives Used in Food Industry and Medicine. Mini. Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuiter, A.S. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–26. [Google Scholar]
- Wang, C.; Sherrard, M.; Pagadala, S.; Wixon, R.; Scott, R.A. Isoflavone content among maturity group 0 to II soybeans. J. Am. Oil Chem. Soc. 2000, 77, 483–487. [Google Scholar] [CrossRef]
- Miura, A.; Sugiyama, C.; Sakakibara, H.; Simoi, K.; Goda, T. Bioavailability of isoflavones from soy products in equol producers and non-producers in Japanese women. J. Nutr. Intermed. Metab. 2016, 6, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362s–1375s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ge, X.; Tian, X.; Zhang, Y.; Zhang, J.; Zhang, P. Soy isoflavone: The multipurpose phytochemical (Review). Biomed. Rep. 2013, 1, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; et al. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer 2013, 133, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Kang, H.B.; Li, B.L.; Zhang, R.M. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac. J. Cancer Prev. 2012, 13, 479–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.S.; Gardner, C.; Brooks, J.D.; Peehl, D.M.; Feldman, D. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int. J. Cancer 2009, 124, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Shin, A.; Lee, J.; Lee, J.; Park, M.S.; Park, J.W.; Park, S.C.; Oh, J.H.; Kim, J. Isoflavone and Soyfood Intake and Colorectal Cancer Risk: A Case-Control Study in Korea. PLoS ONE 2015, 10, e0143228. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy Isoflavones in Integrative Oncology: Increased Efficacy and Decreased Toxicity of Cancer Therapy. Integr. Cancer Ther. 2019, 18, 1534735419835310. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, F.H.; Li, Y. Soy isoflavones and cancer prevention. Cancer Investig. 2003, 21, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wong, W.-T.; Wu, R.; Lai, W.-F. Biochemistry and use of soybean isoflavones in functional food development. Crit. Rev. Food Sci. Nutr. 2020, 60, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yuan, F.; Goldberg, I.D.; Rosen, E.M.; Auborn, K.; Fan, S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J. Nutr. 2000, 130, 2927–2931. [Google Scholar] [CrossRef] [Green Version]
- Chadha, N.; Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheng, P.H.; Rao, X.M.; McMasters, K.M.; Zhou, H.S. Indole-3-carbinol (I3C) increases apoptosis, represses growth of cancer cells, and enhances adenovirus-mediated oncolysis. Cancer Biol. Ther. 2014, 15, 1256–1267. [Google Scholar] [CrossRef] [Green Version]
- Maruthanila, V.L.; Poornima, J.; Mirunalini, S. Attenuation of Carcinogenesis and the Mechanism Underlying by the Influence of Indole-3-carbinol and Its Metabolite 3,3’-Diindolylmethane: A Therapeutic Marvel. Adv. Pharmacol. Sci. 2014, 2014, 832161. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-carbinol: A plant hormone combatting cancer. F1000Res 2018, 7, F1000 Faculty Rev-689. [Google Scholar] [CrossRef] [Green Version]
- Kiselev, V.I.; Ashrafyan, L.A.; Muyzhnek, E.L.; Gerfanova, E.V.; Antonova, I.B.; Aleshikova, O.I.; Sarkar, F.H. A new promising way of maintenance therapy in advanced ovarian cancer: A comparative clinical study. BMC Cancer 2018, 18, 904. [Google Scholar] [CrossRef]
- Fan, S.; Meng, Q.; Auborn, K.; Carter, T.; Rosen, E.M. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br. J. Cancer 2006, 94, 407–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, S.; Rashid, G.; Klein, A. Indole-3-carbinol inhibits telomerase activity and gene expression in prostate cancer cell lines. Anticancer. Res. 2011, 31, 3733–3737. [Google Scholar] [PubMed]
- Mondal, D.; Kalar, P.; Kori, S.; Gayen, S.; Das, K. Recent Developments on Synthesis of Indole Derivatives Through Green Approaches and Their Pharmaceutical Applications. Curr. Org. Chem. 2020, 24. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Arteni, A.A.; Fradot, M.; Galzerano, D.; Mendes-Pinto, M.; Sahel, J.; Picaud, S.; Robert, B.; Pascal, A. Structure and Conformation of the Carotenoids in Human Retinal Macular Pigment. PLoS ONE 2015, 10, e0135779. [Google Scholar] [CrossRef] [PubMed]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Schwartz, S.J.; Failla, M.L. Assessment of lutein bioavailability from meals and a supplement using simulated digestion and caco-2 human intestinal cells. J. Nutr. 2004, 134, 2280–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Olmedilla-Alonso, B.; Blanco-Navarro, I.; Pérez-Sacristán, B. Lutein bioavailability from lutein ester-fortified fermented milk: In vivo and in vitro study. J. Nutr. Biochem. 2010, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Yamagata, K.; Fujiwara, A.; Onodera, D.; Motoki, T. Lutein Regulates the Expression of Apoptosis-related Genes and Stem Cell Markers in A549 Human Lung Cancer Cells. Nat. Prod. Commun. 2017, 12, 1934578X1701200616. [Google Scholar] [CrossRef] [Green Version]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary lutein modulates growth and survival genes in prostate cancer cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.R.; Swanson, H.M.; Rubin, L.P. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wang, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; et al. Green leafy vegetable and lutein intake and multiple health outcomes. Food Chem. 2021, 360, 130145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Bao, B.; Ahmad, A.; Sarkar, F.H. Induction of Cancer Cell Death by Isoflavone: The Role of Multiple Signaling Pathways. Nutrients 2011, 3, 877–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, G.S.; Tilton, S.; Williams, D.E. Mechanisms of tumor inhibition, and tumor promotion, by dietary indole-3-carbinol. Cancer Res. 2006, 66, 1364–1365. [Google Scholar]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinz-Langenohl, R.; Fohr, I.; Pietrzik, K. Beneficial role for folate in the prevention of colorectal and breast cancer. Eur. J. Nutr. 2001, 40, 98–105. [Google Scholar] [CrossRef]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrubsole, M.J.; Shu, X.O.; Li, H.-L.; Cai, H.; Yang, G.; Gao, Y.-T.; Gao, J.; Zheng, W. Dietary B vitamin and methionine intakes and breast cancer risk among Chinese women. Am. J. Epidemiol. 2011, 173, 1171–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-C.; Hsu, S.-L.; Lin, H.; Yang, T.-Y. Retinoic acid and cancer treatment. Biomedicine 2014, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. Vitamin D as a promising anticancer agent. Indian J. Pharmacol. 2011, 43, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mijan, M.; Sim, W.-J.; Lim, T.-G. Physiological Effects of Green-Colored Food-Derived Bioactive Compounds on Cancer. Appl. Sci. 2021, 11, 11288. https://doi.org/10.3390/app112311288
Al Mijan M, Sim W-J, Lim T-G. Physiological Effects of Green-Colored Food-Derived Bioactive Compounds on Cancer. Applied Sciences. 2021; 11(23):11288. https://doi.org/10.3390/app112311288
Chicago/Turabian StyleAl Mijan, Mohammad, Woo-Jin Sim, and Tae-Gyu Lim. 2021. "Physiological Effects of Green-Colored Food-Derived Bioactive Compounds on Cancer" Applied Sciences 11, no. 23: 11288. https://doi.org/10.3390/app112311288
APA StyleAl Mijan, M., Sim, W.-J., & Lim, T.-G. (2021). Physiological Effects of Green-Colored Food-Derived Bioactive Compounds on Cancer. Applied Sciences, 11(23), 11288. https://doi.org/10.3390/app112311288







